Abstract
Background:
The application of neoadjuvant immune checkpoint inhibitors combined with chemotherapy (NICT) in treating locally advanced oesophageal squamous cell carcinoma (ESCC) is a subject of considerable research interest. In light of this, we undertook a comprehensive meta-analysis aiming to compare the efficacy and safety of this novel approach with conventional neoadjuvant chemotherapy (NCT) in the management of ESCC.
Methods:
A systematic search was conducted in PubMed, Embase, Cochrane Library, and Web of Science to gather relevant literature on the efficacy and safety of NICT compared to conventional NCT in locally advanced ESCC published before June 2023. Effect indicators, including odds ratios (ORs) with associated 95% CIs, were employed to evaluate the safety and efficacy outcomes. The risk of bias was assessed using the Cochrane bias risk assessment tool, and subgroup analysis and sensitivity analysis were conducted to investigate the findings further.
Results:
A total of nine studies qualified for the meta-analysis, all of which investigated the efficacy and safety of NICT compared to conventional NCT. The pooled rates of pathologic complete response and major pathologic response in the NICT group were significantly higher compared to the NCT group, with values of 26.9% versus 8.3% (P<0.00001) and 48.1% versus 24.6% (P<0.00001), respectively. The ORs for achieving pathologic complete response and major pathologic response were 4.24 (95% CI, 2.84–6.32, I2=14%) and 3.30 (95% CI, 2.31–4.71, I2=0%), respectively, indicating a significant advantage for the NICT group. Regarding safety outcomes, the pooled incidences of treatment-related adverse events and serious adverse events in the NICT group were 64.4% and 11.5%, respectively, compared to 73.8% and 9.3% in the NCT group. However, there were no significant differences observed between the two groups in terms of treatment-related adverse events (OR=0.67, 95% CI, 0.29–1.54, P=0.35, I2=58%) or serious adverse events (OR=1.28, 95% CI, 0.69–2.36, P=0.43, I2=0%). Furthermore, no significant differences were found between the NICT and NCT groups regarding R0 resection rates, anastomotic leakage, pulmonary infection, and postoperative hoarseness.
Conclusions:
Neoadjuvant immune checkpoint inhibitors combined with chemotherapy demonstrate efficacy and safety in treating resectable oesophageal squamous cell carcinoma. Nevertheless, additional randomized trials are required to confirm the optimal treatment regimen.
Keywords: chemotherapy, efficacy and safety, oesophageal squamous cell carcinoma (ESCC), immune checkpoint inhibitors (ICIs), meta-analysis, neoadjuvant
Introduction
Highlights
Neoadjuvant immunotherapy combined with chemotherapy (NICT) has demonstrated superior pathologic response compared to neoadjuvant chemotherapy (NCT) in patients with locally advanced oesophageal squamous cell carcinoma.
Both NCT and NICT showed high rates of R0 resection, indicating effective tumour resection with negative margins.
Compared with NCT, NICT does not increase perioperative drug toxicity and surgical complications.
Preliminary data suggests that NICT may potentially enhance disease-free survival and overall survival compared to NCT, although long-term survival data are lacking.
Oesophageal cancer (EC) is a digestive system cancer with a high malignancy, incidence, and mortality rate. The total number of new cases and deaths of oesophageal cancer worldwide were 604 000 and 544 000, respectively. Oesophageal cancer ranks the seventh most commonly diagnosed cancer and the sixth most common cause of cancer-related mortality worldwide1. According to the latest data from China National Cancer Center, oesophageal cancer ranked sixth, and mortality ranked fifth2. Over 90% of oesophageal cancers are oesophageal squamous cell carcinoma in Asia3. The treatment efficacy for oesophageal cancer remains poor, with a 5-year survival rate of ~20%4,5. Therefore, oesophageal cancer poses a significant threat to human health.
Surgery remains the primary treatment approach for oesophageal squamous cell carcinoma (ESCC). However, relying solely on surgery has not yielded satisfactory clinical outcomes. Several studies have demonstrated that neoadjuvant therapy offers an effective strategy for improving survival in patients with resectable EC6,7. Currently, neoadjuvant therapy primarily consists of neoadjuvant chemotherapy (NCT) and neoadjuvant chemoradiotherapy (NCRT). NCRT plus surgery as a standard treatment for locally advanced ESCC is based on the CROSS trial and NEOCRTEC5010 trial, which have reported high rates of pathological complete response (pCR) exceeding 40%8,9. Furthermore, the JCOG9907 trial has established neoadjuvant chemotherapy (NCT) as a standard treatment option for locally advanced oesophageal squamous cell carcinoma, particularly in Japan10. Despite these advancements, the long-term survival rates for patients undergoing NCRT or NCT combined with esophagectomy remain unsatisfactory, with a 5-year overall survival (OS) rate of only 47% and a 3-year disease-free survival (DFS) rate of ~49%11. Hence, it is imperative to identify a new neoadjuvant therapy mode that enhances tumour response and survival, minimizes the impact on surgery, and ensures favourable safety profiles.
Programmed cell death protein 1 (PD-1) and its ligands have emerged as crucial regulators of tumour-induced immunosuppression. Inhibiting this signalling pathway using PD-1/PD-L1 inhibitors has shown potential in enhancing T-cell-mediated antitumor activity, leading to effective tumour cell eradication. Consequently, immunotherapy has gained recognition as a promising treatment modality for various malignancies, including oesophageal cancer12,13. The combination of immune checkpoint inhibitors (ICIs) with chemotherapy as a first-line treatment has shown promising survival benefits in patients with advanced/metastatic oesophageal cancer14. This growing significance of immunotherapy in clinical practice has been acknowledged by the National Comprehensive Cancer Network guidelines for the treatment of advanced oesophageal cancer15. Building upon these encouraging findings, numerous clinical trials have investigated the efficacy and safety of neoadjuvant chemoimmunotherapy in locally advanced oesophageal squamous cell carcinoma16–18. Although recent meta-analyses have provided supporting evidence for the use of neoadjuvant ICIs combined with neoadjuvant chemotherapy19,20, comprehensive comparisons with standard neoadjuvant chemotherapy in the form of meta-analyses are currently lacking in the scientific literature. This highlights the need for further research to address this knowledge gap and enhance our understanding of the comparative effectiveness of these treatment approaches.
Hence, the present study aimed to perform a comprehensive systematic review and meta-analysis to assess the outcomes of patients receiving neoadjuvant immunotherapy combined with chemotherapy (NICT) compared to those treated with NCT. By doing so, we aimed to contribute valuable evidence regarding the neoadjuvant treatment approach. As of now, there is a notable absence of published meta-analyses that directly compare the efficacy and safety of NICT with NCT, specifically in the context of locally advanced oesophageal squamous cell carcinoma (ESCC).
Methods
Protocol and registration
The systematic review has been reported in line with PRISMA, Supplemental Digital Content 1, http://links.lww.com/JS9/B158, Supplemental Digital Content 2, http://links.lww.com/JS9/B159 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)21 and AMSTAR, Supplemental Digital Content 3, http://links.lww.com/JS9/B160 (Assessing the methodological quality of systematic reviews) Guidelines22. Furthermore, the protocol for this study has been registered in the PROSPERO database.
Search strategy and study selection
We conducted a comprehensive literature search in various databases published before June 2023 to identify relevant articles comparing the efficacy and safety of neoadjuvant chemotherapy combined with immunotherapy versus conventional neoadjuvant chemotherapy in locally advanced ESCC. The databases searched included PubMed, Embase, Cochrane Library, and Web of Science. Additionally, we thoroughly examined unpublished, updated data from ongoing clinical studies presented at prominent international congresses, such as ASCO, AACR, ESMO, and others, up until June 2023. No language restrictions were applied during the search process, and any potential studies in non-English languages were translated using translation software or with the assistance of translators when necessary. Two reviewers (Jinxin Xu and Yingjie Cai) collaborated to design the search strategies for each database, incorporating the following search terms and keywords: “Neoadjuvant Therapy” or“Neoadjuvant Treatment” or “Neoadjuvant Systemic Treatment” and “esophageal squamous cell carcinoma” or “Esophageal Neoplasms” or “Cancer, Esophagus” and “Immunotherapy” or “Programmed Cell Death 1 Receptorn” or “Immune Checkpoint Inhibitors” and search the appropriate database to identify eligible articles (Supplementary Table 1, Supplemental Digital Content 4, http://links.lww.com/JS9/B161). The search was last updated on June 17, 2023.
The selection of studies was based on the following criteria: (I) inclusion of resectable stage II–IVa ESCC confirmed by histological examination of tissue samples; (II) inclusion of randomized controlled trials (RCTs) or retrospective trials that compared neoadjuvant ICIs, NICT and standard NCT for the treatment of ESCC; (III) evaluation of the efficacy and safety of different neoadjuvant treatment regimens as the main comparator; (IV) assessment of outcomes including the prevalence of major pathological response (MPR), pathological complete response (pCR), treatment-related adverse events (TRAEs), serious adverse events (SAEs), R0 resection rate, and postoperative complications; (V) inclusion of ICIs that are currently under investigation in clinical trials or used in registered practice.
The exclusion criteria were as follows: (I) administration of anti-ESCC therapy prior to neoadjuvant treatment; (II) inclusion of less than ten patients for analysis; (III) studies that did not focus on MPR, PCR, R0 resection rate, the incidence of TRAEs, the incidence of SAEs, and incidence of postoperative complications; (IV) inclusion of reprinted articles, case reports, reviews, expert opinions, and comments; (V) Studies without an eligible control group were also excluded. In cases where studies had overlapping cohorts, the study with the largest cohort or most detailed information was chosen for analysis. The screening process was conducted independently by reviewers Jinxin Xu and Yingjie Cai, who thoroughly assessed the reports based on relevant headings and abstracts containing key terms. Subsequently, the full texts of the identified articles were retrieved to determine their eligibility for inclusion. Additionally, a manual review of references in relevant reports was conducted to identify additional studies. The full texts of all potentially relevant trials and conference abstracts were assessed according to pre-defined eligibility criteria. Any disagreements or discrepancies were resolved through discussion with a third researcher, Zhinuan Hong, ensuring consensus in the final selection process.
Data extraction
Two independent reviewers, Jinxin Xu and Yingjie Cai, conducted data extraction using a pre-defined table to ensure consistency. The extracted information encompassed the following aspects: (I) study characteristics, including first author, publication year, centre location, study design, primary inclusion criteria for patients, neoadjuvant regimen, and sample size; (II) Baseline characteristics of enroled patients, such as gender, age, tumour location, and clinical TNM stage; (III) Endpoint data, comprising MPR, pCR, the incidence of TRAEs, the incidence of SAEs, R0 resection rate, surgical complications, and 1-year OS rate. It is important to note that some studies included in this meta-analysis were conference abstracts, which may result in incomplete epidemiological data, such as the male-female ratio and median age. To ensure the integrity of the analysis, we took measures to avoid duplicating patients if a medical database was utilized by multiple studies within adjacent periods, selecting the dataset with the largest number of participants. Any discrepancies or disagreements during the extraction process were resolved through comprehensive discussions or by involving a third researcher to reach a final decision on study inclusion. In cases where adequate data were not provided in the publication, the researchers made efforts to contact the corresponding authors of the studies to request additional information, if feasible.
Risk of bias and certainty of evidence assessment
Two reviewers (Jinxin Xu and Yingjie Cai) independently evaluated the risk of bias of each study, and in cases of disagreement, decisions were reached through discussion or by seeking the opinion of a third researcher. RCTs were assessed using the Cochrane risk-of-bias tool. The risk of bias in randomized studies was evaluated based on the following criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias. Non-randomized controlled trials were assessed using the ROBINS-I tool (Risk Of Bias In Non-randomised Studies—of Interventions) with respect to the following categories: confounding, selection bias, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of reported results.
Two independent reviewers (Jinxin Xu and Yingjie Cai) assessed the certainty of evidence for each outcome, with any discrepancies resolved by a third reviewer (Zhinuan Hong). The certainty of evidence for each outcome was evaluated using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) framework, categorized as high, moderate, low, or very low23. Initially, the quality of evidence from observational studies was considered low and then adjusted based on pre-defined criteria. Quality can be upgraded for large effect sizes (risk estimates >2 or <0.5 in the absence of plausible confounders), dose-response gradient, or attenuation of the pooled risk estimates by plausible confounders. Conversely, quality could be downgraded for risk of bias (>25% of participants in this comparison were from studies at high risk of bias), inconsistency (substantial unexplained interstudy heterogeneity, I2>50), indirectness (factors limiting generalizability of the results), imprecision (95% CIs for risk estimates are wide or cross a minimally important difference of 10% for outcomes (risk ratio 0.9 to 1.1)), and publication bias (evidence of small study effects).
Definitions of endpoints
The efficacy of neoadjuvant therapy was assessed based on the pathological response and the OSl rate at 1 year. The patients’ pathological responses, including MPR, pCR, and R0 resection rate, were evaluated. Two experienced pathologists independently assessed the pathological responses. pCR was defined as the absence of residual tumour cells in the surgically removed tumour specimen following neoadjuvant therapy and resection. MPR was defined as the presence of less than 10% viable residual tumour cells in the specimen. R0 resection denoted a margin-negative resection under microscopic examination, indicating the absence of tumour cells at the primary tumour site. The R0 resection rate represents the percentage of successful R0 resections. Safety-related endpoints included neoadjuvant TRAEs ,SAEs, and surgical complications. The assessment of TRAEs was conducted according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.024. SAEs were defined as Grade 3-5 TRAEs. Surgical complications occurring within 30 days after surgery were classified using the Clavien–Dindo classification system25.
Statistical analysis
The meta-analysis used Review Manager, version 5.4 (RevMan), a proprietary software provided by the Cochrane Collaboration26, and R statistical language version R 4.2.3. The primary observational endpoints were described previously. To compare the safety and efficacy of neoadjuvant ICIs combined with chemotherapy versus routine neoadjuvant therapy, odds ratios (OR) and 95% CIs were utilized as efficacy indicators. Heterogeneity was assessed using the χ2 test and I2 statistic. In the presence of significant heterogeneity (I2>50%), the random-effects model (Mantel–Haenszel method) was employed; otherwise, the fixed-effects model (Inverse Variance) was used. Subgroup analysis and sensitivity analysis were conducted to identify subgroup differences and potential sources of heterogeneity. The potential for publication bias was evaluated by visually inspecting funnel plots and conducting Egger’s test. All P values were two-sided, and a significance level of 0.05 was considered statistically significant.
Results
Characteristics of included studies
A PRISMA diagram illustrating the study selection process is presented in Figure 1. The initial search strategy yielded 574 records, which were reduced to 306 after removing duplicates. Through a thorough evaluation of titles and abstracts, the full text of 27 articles was subsequently assessed. Ultimately, a total of 9 studies involving 1030 patients with ESCC were included for quantitative meta-analysis. All of the studies originated in China. Among the included studies, three were dual-arm prospective trials27–29, while the remaining six were retrospective30–35. All the studies focused on comparing the efficacy and safety of NICT versus conventional NCT. PD-1 inhibitors were the most commonly investigated immunotherapy agents, with only one prospective study including patients treated with PD-L1 inhibitors27. The primary neoadjuvant chemotherapy regimens employed were Taxol in combination with platinum (TP) or Docetaxel in combination with platinum (DP). Table 1 provides an overview of the key characteristics found in the included articles, and Table 2 presents the main findings. The risk of bias in randomized trials and non-randomized studies was evaluated using the Cochrane risk-of-bias tool and ROBINS-I tool, respectively, as illustrated in Figure 2. The quality of the evidence as assessed by the GRADE tool (Supplementary Table 2, Supplemental Digital Content 5, http://links.lww.com/JS9/B162) was very low to moderate.
Figure 1.
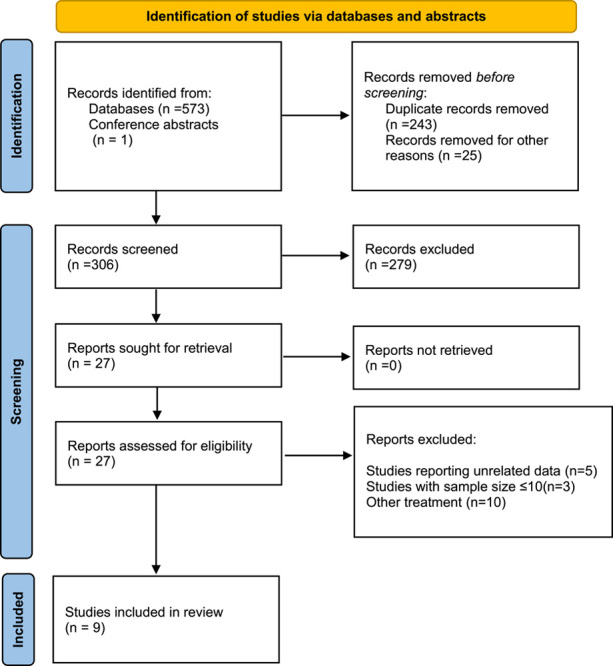
Flow chart of literature search and study selection.
Table 1.
Study characteristics and patient demographics.
| First author | Centre location | Published year | NCT number | Study phase | Study design | Type of article | Clinical staging | Histological type | Intervention | Sample size | chemotherapy regimen | ICI drugs | Neoadjuvant cycle | Proportion of males | Median age,years | Tumor location |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li et al.27 | China | 2023 | NCT04460066 | II | Prospective randomized multicenter |
Full text | cT2N + M0 or cT3-4aN±M0 | ESCC | NICT versus NCT | 64 | Nab-paclitaxel+cisplatin | Socazolimab | 4 cycles | 79.69% | 62 years | Upper 21.9% (14/64) Middle 59.4% (38/64) Lower 18.7% (12/64) |
| Zhang et al.34 | China | 2023 | — | — | Retrospective single-centre observational study |
Full text | cT2-4N+M0 | ESCC | NICT versus NCT | 131 | Paclitaxel +platinum | Camrelizumab | 1–4 cycles | 95.4% | 60.2 years | Upper 9.9% (13/131) Middle 38.2% (50/131) Lower 51.9% (68/131) |
| Zhou et al.33 | China | 2023 | — | — | Retrospective, single-centre, observational study | Full text | Stage II–IVa | ESCC | NICT versus NCT | 59 | Docetaxel + nedaplatin | Camrelizumab | 2 cycles | 81.4% | 64.9 years | Upper 11.9% (7/59) Middle 52.5% (31/59) Lower 35.6% (21/59) |
| Jing et al.31 | China | 2022 | — | — | Retrospective, single-centre, observational study | Full text | Locally advanced resectable stage ESCC |
ESCC | NICT versus NCT | 94 | Platinum-based drugs and 5-flfluorouracil or docetaxel/paclitaxel |
Pembrolizumab; camrelizumab; toripalimab; sintilimab; |
1–3 cycles | 70.2% | — | Upper 5.3% (5/94) Middle 84.0% (79/94) Lower 10.6% (10/94) |
| Qiao et al.32 | China | 2022 | — | — | Retrospective, single-centre, observational study | Full text | cT1-4aN0-3M0 | ESCC | NICT versus NCT | 254 | Paclitaxel, albumin-bound paclitaxel or docetaxel |
Camrelizumab | 2 cycles | 72.8% | 62.6 years | Upper 17.7% (45/254) Middle50.0% (127/254) Lower 32.3% (82/254) |
| Huang et al.30 | China | 2021 | — | — | Retrospective, single-centre, observational study | Full text | Stage II–IVa | ESCC | NICT versus NCT | 54 | Docetaxel + nidaplatin | Pembrolizumab | 2 cycles | 94.4% | — | Upper 11.1% (6/54) Middle64.8% (35/54) Lower 24.1% (13/54) |
| Zhang et al.28 | China | 2023 | ChiCTR2000040330 | IV | Prospective randomized multicenter |
Abstract | cT1-4N1-3M0 or cT3-4N0M0 | ESCC | NICT versus NCT | 150 | Albumin paclitaxel + cisplatin | camrelizumab | — | 85.2% | 65 years | — |
| Hong et al.35 | China | 2023 | — | — | Retrospective, multicenter, observational study | Full text | cT3-4aN0M0 or cN+M0 | ESCC | NICT versus NCT | 164 | Platinum + paclitaxel or platinum + docetaxel |
Camrelizumab; pembrolizumab; sintilimab; tislelizumab; toripalimab | 2–4 cycles | 81.27% | 61 years | Upper 14.0% (23/164) Middle 53.0% (87/164) Lower 32.9% (54/164) |
| Xiao et al.29 | China | 2021 | — | — | Prospective randomized single-centre |
Full text | Stage II-III | ESCC | NICT versus NCT | 60 | oxaliplatin + docetaxel | Camrelizumab | 4 cycles | 93.3% | 43.1 years | — |
ESCC, oesophageal squamous cell cancer; ICI, immune checkpoint inhibitor; NCT number, The National Clinical Trial number; NCT, neoadjuvant chemotherapy; NICT, neoadjuvant immune checkpoint inhibitors combined with chemotherapy.
Table 2.
Research data on endpoints reported in the clinical trials.
| First author | Treatment mode | ITT | Patients with resection | MPR rate, n (%) | pCR rate, n (%) | Incidence of TRAEs, n (%) | Incidence of SAES, n (%) | R0 resection rate, n (%) | Anastomotic leakage, n (%) | Pulmonary infection, n (%) | Postoperative hoarseness, n (%) | Perioperative death, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li et al.27 | NICT | 32 | 29 | 20/29 (69.0) | 12/29 (41.4) | 32/32 (100) | 21/32 (65.6) | 29/29 (100) | 1/29 (3.4) | 1/29 (3.4) | — | 0/29 (0.0) |
| NCT | 32 | 29 | 18/29 (62.1) | 8/29 (27.6) | 32/32 (100) | 20/32 (62.5) | 28/29 (98.6) | 0/29 (0.0) | 1/29 (3.4) | — | 1/29 (3.4) | |
| Zhang et al.34 | NICT | — | 34 | 18/34 (52.9) | 8/34 (23.5) | 16/34 (47.1) | 4/34 (11.8) | — | 2/34 (5.9) | 7/34 (20.6) | 2/34 (5.9) | 0/34 (0.0) |
| NCT | — | 97 | 16/97 (16.5) | 3/97 (3.1) | 37/97 (38.1) | 6/97 (6.2) | — | 8/97 (8.2) | 10/97 (10.3) | 6/97 (6.1) | 1/97 (1.0) | |
| Zhou et al.33 | NICT | — | 19 | — | 5/19 (26.3) | 16/19 (84.2) | 0/19 (0.0) | 19/19 (100) | 1/19 (5.3) | 4/19 (21.1) | 1/19 (5.3) | 1/19 (5.3) |
| NCT | — | 40 | — | 1/40 (2.5) | 35/40 (87.5) | 2/40 (5) | 39/40 (97.5) | 9/40 (22.5) | 15/40 (37.5) | 4/40 (10.0) | 0/40 (0.0) | |
| Jing et al.31 | NICT | — | 47 | 13/46 (28.3) | 8/47 (17.0) | — | 2/47 (4.3) | 41/47 (87.2) | 0/47 (0.0) | 2/47 (4.3) | — | — |
| NCT | — | 47 | 4/44 (9.1) | 1/47 (2.1) | — | 0/47 (0.0) | 43/47 (91.5) | 3/47 (6.4) | 1/47 (2.1) | — | — | |
| Qiao et al.32 | NICT | — | 48 | 29/48 (60.4) | 20/48 (41.7) | 37/48 (77.1) | 4/48 (8.3) | 48/48 (100) | 4/48 (8.3) | 13/48 (27.1) | — | — |
| NCT | — | 206 | 56/206 (27.2) | 22/206 (10.7) | 189/206 (91.7) | 17/206 (8.3) | 206/206 (100) | 10/206 (4.9) | 77/206 (37.4) | — | — | |
| Huang et al.30 | NICT | 23 | 21 | 11/23 (47.8) | 7/23 (30.4) | — | — | 21/21 (100) | — | — | — | — |
| NCT | 31 | 27 | 8/31 (25.8) | 3/31 (9.7) | — | — | 26/27 (96.3) | — | — | — | — | |
| Hong et al.35 | NICT | — | 82 | — | 15/82 (18.3) | — | — | — | 13/82 (15.9) | 27/82 (32.9) | 3/82 (3.7) | 0/82 (0.0) |
| NCT | — | 82 | — | 7/82 (8.5) | — | — | — | 15/82 (18.3) | 32/82 (39.0) | 1/82 (1.2) | 0/82 (0.0) | |
| Zhang et al.28 | NICT | — | 90 | 39/90 (43.3) | 25/90 (27.8) | — | 0/90 (0.0) | — | — | — | — | — |
| NCT | — | 60 | 13/60 (21.7) | 6/60 (10) | — | 0/60 (0.0) | — | — | — | — | — | |
| Xiao et al.29 | NICT | — | 30 | — | — | — | 30/30 (100.00) | 2/30 (6.7) | 1/30 (3.3) | 3/30 (10.0) | — | |
| NCT | — | 30 | — | — | — | 30/30 (100.00) | 4/30 (13.3) | 1/30 (3.3) | 2/30 (6.7) | — |
ICI, immune checkpoint inhibitor; ITT, intention-to-treat; MPR, major pathological response; NCT,neoadjuvant chemotherapy; NICT, neoadjuvant immune checkpoint inhibitors combined with chemotherapy; pCR, complete pathological response; SAE, severe adverse event; TRAE, treatment-related adverse event.
Figure 2.
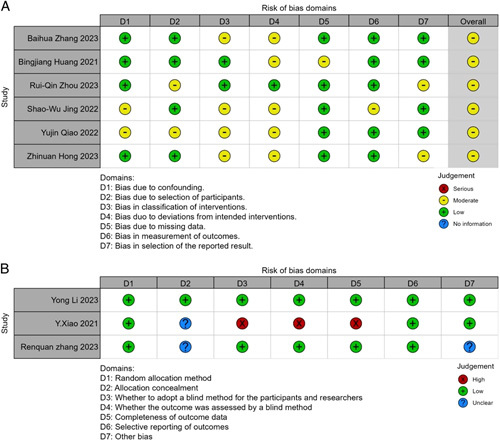
The risk of bias of the included studies. (A) Risk of bias of non-randomized trials; (B) Risk of bias of randomized trials.
Pooled analysis of efficacy-related endpoint
We first conducted a comprehensive meta-analysis to evaluate the pCR , MPR, and R0 resection in all included studies. Eight studies27,28,30–35 were included in the pCR meta-analysis, revealing a pooled pCR rate of 8.3% for the NCT group and 26.9% for the NICT group (P<0.00001) (Fig. 3A). Similarly, six studies27,28,30–32,34 were included in the MPR meta-analysis, demonstrating pooled MPR rates of 24.6% for the NCT group and 48.1% for the NICT group (P<0.00001) (Fig. 3B). Additionally, six studies27,29–33 were included in the R0 resection rate meta-analysis, indicating R0 resection rates of 98.2% for the NCT group and 96.6% for the NICT group (P=0.98) (Fig. 3E). From the data provided, it is evident that the NICT group exhibited significantly higher pCR and MPR rates compared to the NCT group (OR=4.24; 95% CI, 2.84–6.32; OR=3.30; 95% CI, 2.31–4.71, respectively). However, no significant differences were observed in the R0 resection rates between the two groups (OR=1.01; 95% CI, 0.36–2.84). Notably, there was no significant heterogeneity observed in the pCR rate (P=0.32, I2=14%), MPR rate (P=0.28, I2=0%), or R0 resection rate (P=0.74, I2=0%). Thus the fixed-effects model was applied.
Figure 3.
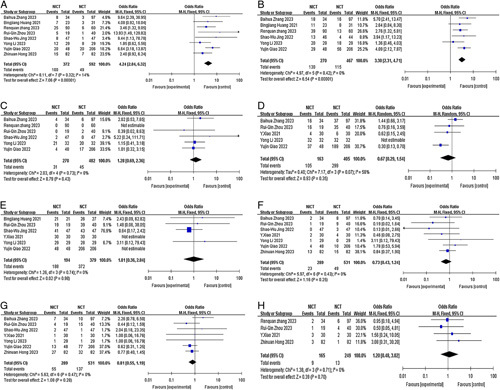
Forest plot of the efficacy and safety of neoadjuvant immune checkpoint inhibitors combined with chemotherapy and neoadjuvant chemotherapy. (A) pCR rate; (B) MPR rate; (C) incidence of SAEs; (D) incidence of TRAEs; (E) R0 resection rate; (F) incidence of anastomotic leakage; (G) incidence of pulmonary infection; (H) incidence of postoperative hoarseness. MPR, major pathological response; NICT, neoadjuvant immune checkpoint inhibitors combined with chemotherapy; NCT, neoadjuvant chemotherapy; pCR, complete pathological response; SAE, severe adverse event; TRAE, treatment-related adverse event.
Pooled analysis of safety-related endpoint
The safety-related endpoints included the incidence of TRAEs and SAEs and the incidence of surgical complications and perioperative mortality. Five studies27,29,32–34 were included in the meta-analysis of TRAEs. Due to the observed heterogeneity between studies (I2=58%, P=0.07), the random-effects model was employed. The pooled incidence of TRAEs in the NCT group and NICT group was found to be 73.8% and 64.4%, respectively (P=0.35) (Fig. 3D). The most frequently reported TRAEs in the NICT group were leukopenia (31.6–82.6%), neutropenia (5.3–78.3%), anaemia (10.5–100.0%), decreased platelet count (5.3–100.0%), rash (4.2–43.8%), fatigue (37.5–91.3%), and loss of appetite (26.3–53.1%). In the NCT group, the most commonly reported TRAEs were leukopenia (35.0–68.8%), neutropenia (7.5–78.1%), anaemia (12.5–84.4%), decreased platelet count (7.5–84.4%), fatigue (25.0–71.0%), and loss of appetite (22.5–28.1%). Six studies27,28,31–34 were included in the meta-analysis of SAEs. Due to the absence of heterogeneity between studies (I2=0.0%, P=0.73), the fixed-effects model was utilized. The pooled incidence of SAEs in the NCT and NICT groups was 9.3% and 11.5%, respectively, (P=0.43) (Fig. 3C). Most of the reported grade 3 or higher TRAEs were haematologic. In the NICT group, the incidence of grade 3 or higher TRAEs included leukopenia (5.3–43.8%), neutropenia (0.0–59.4%), decreased platelet count (0.0–12.5%), and anaemia (0.0–12.5%). In the NCT group, the incidence of grade 3 or higher TRAEs consisted of leukopenia (0.0–25.0%), neutropenia (2.5–56.3%), thrombocytopenia (0.0–6.3%), and anaemia (3.2–6.3%). No significant differences were observed in the incidence of TRAEs and SAEs between the two groups (OR=0.67, 95% CI, 0.29–1.54; OR=1.28, 95% CI, 0.69–2.36).
The incidence of surgical complications included anastomotic leakage, pulmonary infection, and postoperative hoarseness. The incidence of anastomotic leakage was found to be comparable between the NCT and NICT groups, with an odds ratio (OR) of 0.73 (95% CI, 0.43–1.24; I2=0.0%, P=0.43). Similarly, the incidence of pulmonary infection showed no significant difference between the two groups, with an OR of 0.81 (95% CI, 0.55–1.19; I2=0.0%, P=0.47). The incidence of postoperative hoarseness also exhibited comparable rates in the NCT and NICT groups, with an OR of 1.20 (95% CI, 0.48–3.02; I2=0.0%, P=0.71). These findings are presented in Figure 3F-H. There were few reports of surgical mortality. Our results indicated that two patients in the NCT group (Li et al.27 and Zhang et al.34) died due to pneumonia and sudden death, respectively. In the NICT group, one patient (Zhou et al.33) died as a result of severe septic shock following anastomotic leakage.
Survival
Survival data were predominantly incomplete or insufficient for a comprehensive pooled analysis. However, we did find some relevant research reports regarding the OS and DFS rates. One study34 reported that in the NICT group, the 1-year OS rate was 82.4% and the 3-year OS rate was 73.3%, which were not significantly different from the NCT group (77.3% and 46.1%, respectively) (P<0.05). Another study31 provided information on the 1-year and 2-year DFS rates, which were 95.7% and 80.7% for the NICT group and 76.1% and 63.8% for the NCT group, respectively (P=0.001 and P=0.046). The 1-year OS rates were reported as 95.7% for the NICT group and 84.8% for the NCT group (P=0.074).
Sensitivity analysis and publication bias
We conducted sensitivity analyses to assess the robustness of the pooled results and ensure that individual trials did not unduly influence them. In these analyses, one study was excluded at a time to evaluate the potential impact of any single study on the overall results. The sensitivity analyses confirmed the overall stability and reliability of the meta-analysis results (Fig. 4). To examine the possibility of publication bias, we performed Egger regression tests for various endpoints, including pCR, MPR, the incidence of TRAEs, and SAEs, R0 resection rate, anastomotic leakage, pulmonary infection, and postoperative hoarseness. The results indicated a potential publication bias in the R0 resection rate (P=0.043). However, no significant publication bias was detected in the other groups (P>0.05) (Fig. 5). The funnel plots for all the analyzed items are provided in Figure 6, illustrating the distribution of the included studies and their potential bias.
Figure 4.
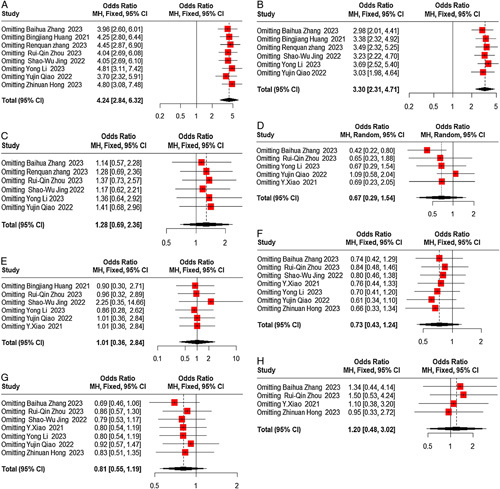
Sensitivity analysis.(A) pCR rate;(B) MPR rate; (C) incidence of SAEs; (D) incidence of TRAEs; (E) R0 resection rate; (F) incidence of anastomotic leakage;(G) incidence of pulmonary infection; (H) incidence of postoperative hoarseness. MPR, major pathological response; pCR, complete pathological response; SAE, severe adverse event; TRAE, treatment-related adverse event.
Figure 5.
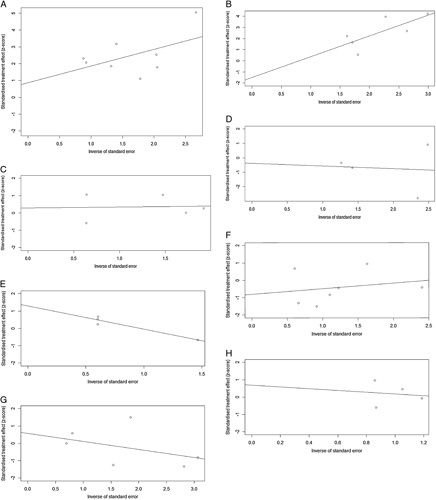
Publication bias of included studies was conducted based on Egger regression tests for (A) pCR rate; (B) MPR rate; (C) incidence of SAEs; (D) incidence of TRAEs; (E) R0 resection rate; (F) incidence of anastomotic leakage; (G) incidence of pulmonary infection; (H) incidence of postoperative hoarseness. MPR, major pathological response; pCR, complete pathological response; SAE, severe adverse event; TRAE, treatment-related adverse event.
Figure 6.
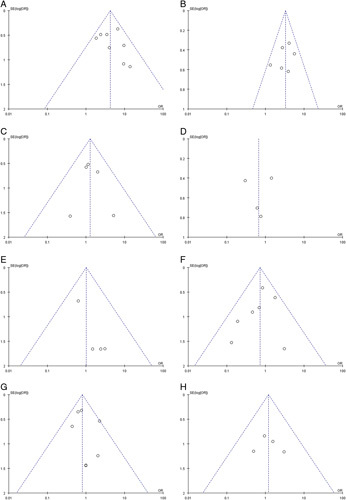
Publication bias of included studies was conducted based on funnel plot for (A) pCR rate; (B) MPR rate; (C) incidence of SAEs; (D) incidence of TRAEs; (E) R0 resection rate; (F) incidence of anastomotic leakage; (G) incidence of pulmonary infection; (H) incidence of postoperative hoarseness. MPR, major pathological response; pCR, complete pathological response; SAE, severe adverse event; TRAE, treatment-related adverse event.
Exploratory subgroup analysis
By conducting subgroup analysis, we aimed to gain further insights into the potential influences of various factors on the observed outcomes. This approach contributes to a more nuanced understanding of the study findings and enhances the overall scientific rigour of our analysis. The analysis was based on the study type (prospective versus retrospective), neoadjuvant treatment cycles (2 cycles versus≥2 cycles), and chemotherapy regimen (TP versus DP), provided that there were a sufficient number of studies available in each subgroup. In cases where multiple chemotherapy regimens or different neoadjuvant treatment cycles were represented in the included articles, subsequent subgroup analyses were not conducted to avoid potential confounding factors. The results of our subgroup analysis revealed that different study types were identified as one of the main sources of heterogeneity for pCR and MPR (I2=61.1%; I2=61.0%) but not for SAEs and TRAES (both I2 =0.0%)(Fig. 7). Nevertheless, these findings do not alter the overall conclusion derived from the previous analyses. Regarding the chemotherapy regimen subgroup, it did not contribute to heterogeneity in pCR, TRAES, R0 resection rate, or anastomotic leakage (I2<50.0%). However, it may have contributed to heterogeneity in pulmonary infection (I2=68.4%), although this did not impact the previous conclusion (Fig. 8). Similarly, the neoadjuvant treatment cycles subgroup did not contribute to heterogeneity in pCR, TRAES, R0 resection rate, anastomotic leakage, or pulmonary infection (I2<50.0%) (Fig. 9).
Figure 7.
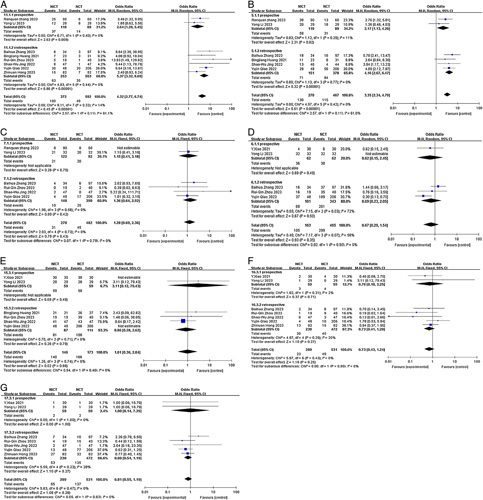
Forest plot of the efficacy and safety of subgroup analysis based on study type. (A) pCR rate; (B) MPR rate; (C) incidence of SAEs; (D) incidence of TRAEs; (E) R0 resection rate; (F) incidence of anastomotic leakage; (G) incidence of pulmonary infection. MPR, major pathological response; pCR, complete pathological response; SAE, severe adverse event; TRAE, treatment-related adverse event.
Figure 8.
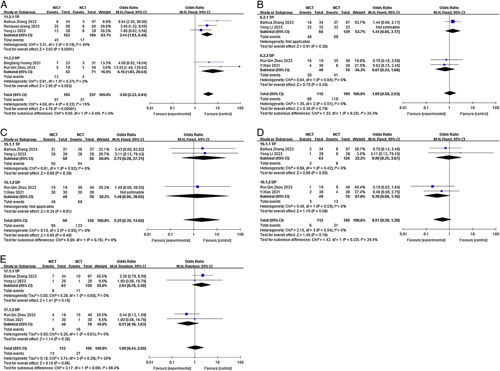
Forest plot of the efficacy and safety of subgroup analysis based on chemotherapy regimen. (A) pCR rate; (B) incidence of TRAEs; (C) R0 resection rate; (D) incidence of anastomotic leakage; (E) incidence of pulmonary infection. DP, Docetaxel in combination with platinum; pCR, complete pathological response; RAE, treatment-related adverse event; TP, Taxol in combination with platinum.
Figure 9.
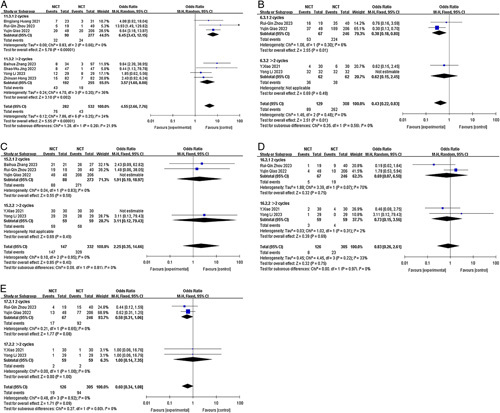
Forest plot of the efficacy and safety of subgroup analysis based on neoadjuvant treatment cycles. (A) pCR rate; (B) incidence of TRAEs; (C) R0 resection rate; (D) incidence of anastomotic leakage; (E) incidence of pulmonary infection. pCR, complete pathological response; RAE, treatment-related adverse event; TRAE, treatment-related adverse event.
Discussion
Neoadjuvant immunotherapy has demonstrated significant improvement in pCR rates with manageable toxicity in patients with oesophageal squamous cell carcinoma19,36,37. However, the optimal neoadjuvant treatment strategy for ESCC remains inconclusive. This meta-analysis systematically compared the antitumor efficacy and safety of NICT versus standard NCT in patients with locally advanced ESCC. Our findings support the superior pathologic response achieved with NICT compared to NCT. Furthermore, the two treatment approaches had no significant differences in R0 resection rates, drug toxicity, or surgical complications.
Surgery-based combination therapy is currently the standard treatment for nonmetastatic ESCC38. Neoadjuvant concurrent chemoradiotherapy has been established as a crucial component of preoperative treatment, significantly improving R0 resection rates and survival outcomes, as demonstrated in the CROSS trial and NEOCRTEC5010 trial8,9. However, this approach is associated with increased postoperative complications and higher postoperative mortality. The results of two multicenter, prospective, randomized controlled Phase III clinical studies (JCOG9907 and JCOG1109) suggest that preoperative neoadjuvant chemotherapy can also lead to a significant improvement in the long-term survival of patients with operable locally advanced oesophageal squamous cell carcinoma10,39, making it more popular in Asian populations. Several pivotal trials, including KEYNOTE590, CheckMate 649, ESCORT-1, and ATTRACTION-0314,40–42, have established immunotherapy, either alone or in combination with chemotherapy, as the standard first-line treatment for advanced or metastatic ESCC. The CheckMate-577 trial has provided evidence of enhanced disease-free survival in patients with residual disease following neoadjuvant chemoradiotherapy and subsequent R0 resection, who received adjuvant nivolumab43. These studies collectively support the exploration of immunotherapy in the neoadjuvant setting. While many trials have evaluated the clinical outcomes and safety of neoadjuvant immunotherapy in locally advanced oesophageal cancer patients19,36, few articles have reported on the efficacy and safety of direct comparisons between neoadjuvant immunochemotherapy and neoadjuvant chemotherapy. Therefore, conducting a meta-analysis incorporating newly updated research data is crucial to address this knowledge gap.
In terms of efficacy, our meta-analysis revealed a significant advantage of NICT over NCT in achieving favourable pathological outcomes. The NICT group exhibited substantially higher rates of pCR and MPR than the NCT group. The pooled analysis of eight studies demonstrated a remarkable pCR rate of 26.9% in the NICT group, which was more than three times higher than the observed rate of 8.3% in the NCT group [odds ratio (OR)=4.24; 95% CI, 2.84–6.32]. Similarly, the NICT group displayed a significantly higher MPR rate (48.1%) compared to the NCT group (24.6%) (OR=3.30; 95% CI, 2.31–4.71). These findings suggest that the addition of immunotherapy to chemotherapy in the neoadjuvant setting improves the likelihood of achieving complete pathological response and significant tumour regression. The achievement of R0 resection serves as a pivotal criterion for evaluating the efficacy of surgical interventions in oesophageal cancer, as it is associated with improved patient prognosis and serves as a benchmark for successful treatment outcomes44. The two groups had no significant differences in the R0 resection rates. Both NCT and NICT demonstrated high rates of R0 resection, with 98.2% for the NCT group and 96.6% for the NICT group (OR =1.01; 95% CI, 0.36–2.84). These results indicate that both neoadjuvant treatment strategies are effective in achieving complete tumour resection with negative margins. However, since the follow-up time was short and complete survival data have not been published, it is difficult to illustrate the benefits of neoadjuvant chemoimmunotherapy on extended survival; clinical benefits in survival outcomes can be expected based on the association of MPR or pCR with improved survival. Some studies provided preliminary information on OS and DFS rates. Notably, one study34 reported similar 1-year and 3-year OS rates between the NCT and NICT groups, although a more favourable prognosis was observed in the NICT group. Another study31 reported higher DFS rates at 1 and 2 years in the NICT group compared to the NCT group. These findings imply that neoadjuvant immunotherapy combined with chemotherapy may potentially enhance DFS and OS. However, large-scale randomized controlled trials with extended follow-up periods are warranted to obtain more robust evidence regarding survival outcomes, particularly long-term survival.
Regarding the safety-related endpoints, we comprehensively analyzed the incidence of TRAEs, SAEs, surgical complications, and perioperative mortality. Our findings revealed that the pooled incidence of TRAEs was slightly lower in the NICT group (64.4%) compared to the NCT group (73.8%). However, the difference did not reach statistical significance. Similarly, the incidence of SAEs was comparable between the NCT and NICT groups, with rates of 9.3% and 11.5%, respectively. Most grade 3 or higher TRAEs were haematologic, including leukopenia, neutropenia, anaemia, and decreased platelet count. These results indicate that adding immunotherapy to chemotherapy in the neoadjuvant setting does not significantly increase the risk of severe adverse events. Nevertheless, monitoring and managing haematological adverse events during neoadjuvant therapy is essential. Furthermore, we assessed the incidence of surgical complications, specifically anastomotic leakage, pneumonia, and postoperative hoarseness. Our analysis revealed no significant differences in the incidence of these complications between the NCT and NICT groups. This suggests that the incorporation of immunotherapy into chemotherapy does not have a substantial impact on the occurrence of postoperative complications. Additionally, fatal surgical complications were rare in our analysis. Specifically, two patients in the NCT group died due to pneumonia and sudden death, respectively27,34. In the NICT group, one patient died due to severe septic shock following anastomotic leakage33. These findings collectively indicate that the safety profile of NICT is acceptable.
Study type was identified as a significant source of heterogeneity in the analysis. The OR for achieving MPR with ICT was higher in retrospective studies compared to prospective studies (OR=4.16; 95% CI, 2.67–6.47 versus OR=2.17; 95% CI, 1.13–4.20), but the difference was not statistically significant (P=0.11). Similarly, in retrospective studies, the OR for achieving pCR with NICT was higher (OR=5.37; 95% CI, 3.32–8.69 versus OR=2.64; 95% CI, 1.28–5.45), and the difference was not statistically significant (P=0.11). One plausible explanation is selection bias in retrospective studies. Researchers conducting retrospective studies often have the advantage of selecting patients who had a favourable response to neoadjuvant immunotherapy. This selection bias may exclude patients with poor responses or experienced adverse events, resulting in an artificially higher OR for achieving MPR and pCR. On the other hand, prospective studies aim to enrol a more representative sample of patients, including those with varying responses to treatment, which may lead to a more balanced and accurate estimation of treatment effects. It is important to note that the sample sizes of the subgroup analyses are relatively small, and caution is required in interpreting these findings, with larger cohorts needed to verify the accuracy of these results. Despite the heterogeneity observed based on study type, it is crucial to note that these findings do not undermine the overall conclusions derived from the previous analyses. The results from both retrospective and prospective studies collectively support the efficacy of NICT in achieving favourable pathological responses. In the subgroup analysis of chemotherapy regimens, heterogeneity in the occurrence of pulmonary infection was observed (I2=68.4%). Within this subgroup, there appeared to be a trend suggesting a potential impact of the chemotherapy regimen on pulmonary infection. Specifically, in the TP subgroup, the OR for the incidence of pulmonary infection was relatively higher compared to the DP subgroup (OR=2.04; 95% CI, 0.76–5.49 versus OR=0.51; 95% CI, 0.16–1.63). However, it is essential to note that this difference did not reach statistical significance (P=0.08).
Subgroup analysis showed heterogeneity in TRAEs among the retrospective study subgroup (I2=72%). Systematically excluding studies, Qiao et al.32 or Zhang et al.34, reduced heterogeneity (I2=0%; I2=6%). Excluding Qiao et al.32 did not change the conclusion. Excluding Zhang et al.34 showed lower TRAEs in NICT versus NCT (OR=0.38, 95% CL, 0.18–0.80; P=0.01), impacting the conclusion. Qiao et al.32 study had significant differences in adverse reactions (37/48, 77.1% versus 189/206, 91.7%, P=0.003) leading to biased results. Consequently, Caution is advised in interpreting this conclusion, highlighting the need for further confirmatory studies.
Currently, there is a lack of predictive biomarkers for neoadjuvant immunotherapy in oesophageal cancer. Multiple studies show no significant correlation between PD-L1 and pathological response in ESCC45–47. However, Yang et al. 48 found higher PD-L1 levels and tumour mutation burden (TMB) in the pCR group. Additionally, high mismatch repair deficiency/microsatellite instability (dMMR/MSI-H) may also serve as a potential biomarker49. TMB correlates with efficacy in advanced oesophageal adenocarcinoma50. More research is needed for meta-regression analysis and optimal biomarker identification.
Despite its valuable findings, this systematic review and meta-analysis are not without limitations. Firstly, it should be noted that the majority of the included studies were retrospective clinical trials, with only three RCTs being part of this review. The limited number of RCTs introduces the potential for bias and may impact the overall quality of evidence. Additionally, the reliance on a clinical trial derived from conference abstracts without access to official publications poses limitations on bias assessment and may introduce publication bias. Secondly, it is essential to acknowledge that all the included studies focused on the Asian population with ESCC, limiting the generalizability of the conclusions to patients with ESCC from other ethnic backgrounds. Thirdly, slight variations exist in the clinical staging among the included articles. Patients at different disease stages may have varying responses to neoadjuvant treatments and differences in tolerance to treatment-related adverse events. These subtleties could impact the conclusions of this analysis. Fourthly, the lack of available data hindered the evaluation of effective biomarkers, such as the combined positive score and tumour proportion score, in neoadjuvant immunotherapy. Further investigation into the role of these biomarkers is warranted to better understand their impact on treatment outcomes. Furthermore, the number of studies and patients included in the subgroup analyses was relatively small, necessitating a cautious interpretation of the heterogeneity observed. Lastly, the lack of long-term survival outcome data was a common issue among the included studies. This limitation is understandable, given that obtaining final results for long-term survival requires considerable time. However, as the number of active trials on neoadjuvant immunotherapy or chemoimmunotherapy continues to grow, future studies with larger sample sizes and more RCTs are expected to provide further validation.
In conclusion, our meta-analysis confirms the efficacy and safety of NICT in locally advanced oesophageal squamous cell carcinoma. NICT exhibits superior outcomes in terms of pCR and MPR rates compared to conventional NCT without increased toxicity or postoperative complications. These findings lay a foundation for future research. However, larger multicenter RCTs and longer-term follow-ups are required to validate and refine these results.
Ethical approval
Not applicable.
Source of funding
This work was supported by the projects of Xiamen Science and Technology Commission(3502Z20209028).
Author contribution
J.X., and Y.C.: conception or design of the work; J.X., Y.C. and Z.H.: data collection and quality assessment; J.X., Y.C. and Z.H.: analysis and interpretation of data; J.X., and Y.C.: drafting the work; J.X., H.D. and S.K.: critical review and final approval.
Conflicts of interest disclosure
No conflict of interest.
Research registration unique identifying number (UIN)
PROSPERO database (CRD42023395193).
Guarantor
Jinxin Xu.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Footnotes
J.X., Y.C. and Z.H. contribute equally to the article.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/international-journal-of-surgery.
Published online 5 October 2023
Contributor Information
Jinxin Xu, Email: 2297203944@qq.com.
Yingjie Cai, Email: xmzscyj2023@163.com.
Zhinuan Hong, Email: hongzhinuan@163.com.
Hongbing Duan, Email: dhb3100@Sina.com.
Sunkui Ke, Email: xmzsksk2021@163.com.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. Zheng RS, Zhang SW, Zeng HM, et al. Cancer incidence and mortality in China, 2016[J]. J Natl Cancer Cent 2022;2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381–387. [DOI] [PubMed] [Google Scholar]
- 4. Baba NYY, Kinoshita K, Iwatsuki M, et al. And prognostic features of patients with esophageal cancer and multiple primary cancers: a retrospective single-institution study. Ann Surg 2018;267:478–83. [DOI] [PubMed] [Google Scholar]
- 5. Huang TX, Fu L. The immune landscape of esophageal cancer. Cancer Commun (Lond) 2019; 39:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pasquali S, Yim G, Vohra RS, et al. Survival after neoadjuvant and adjuvant treatments compared to surgery alone for resectable esophageal carcinoma: a network meta-analysis. Ann Surg 2017;265:481–91. [DOI] [PubMed] [Google Scholar]
- 7. Bushan K, Sharma S. Neoadjuvant chemotherapy and surgery versus surgery alone in resectable esophageal cancer. Indian J Cancer 2015;52:413–416. [DOI] [PubMed] [Google Scholar]
- 8. van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 9. Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase Ⅲ multicenter, randomized, open-label clinical trial. Jo Clin Oncol 2018;36:2796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68–74. [DOI] [PubMed] [Google Scholar]
- 11. Qin H, Liu F, Zhang Y, et al. Comparison of neoadjuvant immunotherapy versus routine neoadjuvant therapy for patients with locally advanced esophageal cancer: a systematic review and meta-analysis. Front Immunol 2023;14:1108213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Janjigian JBYY, Calvo E, Kim JW, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol 2018;36:2836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kojima MAST, Muro K, Francois E, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol 2020;38:4138–48. [DOI] [PubMed] [Google Scholar]
- 14. Sun J-M, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 2021;398:759–771. [DOI] [PubMed] [Google Scholar]
- 15. National Comprehensive Cancer Network . Esophageal and esophagogastric junction cancers. version 2 2021. [DOI] [PubMed]
- 16. Shen D, Chen Q, Wu J, et al. The safety and efficacy of neoadjuvant PD-1 inhibitor with chemotherapy for locally advanced esophageal squamous cell carcinoma. J Gastrointest Oncol 2021;12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu J, Yang Y, Liu Z, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer 2022;10:e004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shang X, Zhao G, Liang F, et al. Safety and effectiveness of pembrolizumab combined with paclitaxel and cisplatin as neoadjuvant therapy followed by surgery for locally advanced resectable (stage Ⅲ) esophageal squamous cell carcinoma: a study protocol for a prospective, single-arm, single-center, open-label, phase-II trial (Keystone-001). Ann Transl Med 2022;10:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu J, Yan C, Li Z, et al. Efficacy and safety of neoadjuvant chemoimmunotherapy in resectable esophageal squamous cell carcinoma: a meta-analysis. Ann Surg Oncol 2023;30:1597–1613. [DOI] [PubMed] [Google Scholar]
- 20. Wang Z, Shao C, Wang Y, et al. Efficacy and safety of neoadjuvant immunotherapy in surgically resectable esophageal cancer: a systematic review and meta-analysis. Int J Surg 2022;104:106767. [DOI] [PubMed] [Google Scholar]
- 21. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 22. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol 2015;1:1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 26. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions (Version 510). The Cochrane Collaboration; 2011. [Google Scholar]
- 27. Li Y, Zhou A, Liu S, et al. Comparing a PD-L1 inhibitor plus chemotherapy to chemotherapy alone in neoadjuvant therapy for locally advanced ESCC: a randomized Phase II clinical trial: a randomized clinical trial of neoadjuvant therapy for ESCC. BMC Med 2023;21:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang R, DS, Song, et al. Efficacy and safety of camrelizumab combined with chemotherapy versus chemotherapy alone as preoperative neoadjuvant therapy for resectable locally advanced esophageal squamous cell carcinoma: Preliminary results from a multicenter, prospective, randomized controlled study. J Clin Oncol 2023;41(suppl 16; abstr 4064). [Google Scholar]
- 29. Xiao Y, Huoqian L, Yangyi M, et al. Clinical trial of carrelizumab injection combined with operation in the treatment of patients with stageⅡ/Ⅲ esophageal squamous cell carcinoma. Chin J Clin Pharmacol 2021;37:3323–3325. [Google Scholar]
- 30. Huang B, Shi H, Gong X, et al. Comparison of efficacy and safety between pembrolizumab combined with chemotherapy and simple chemotherapy in neoadjuvant therapy for esophageal squamous cell carcinoma. J Gastrointest Oncol 2021;12:2013–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jing S-W, Zhai C, Zhang W, et al. Comparison of neoadjuvant immunotherapy plus chemotherapy versus chemotherapy alone for patients with locally advanced esophageal squamous cell carcinoma: a propensity score matching. Front Immunol 2022;13:970534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qiao Y, Zhao C, Li X, et al. Efficacy and safety of camrelizumab in combination with neoadjuvant chemotherapy for ESCC and its impact on esophagectomy. Front Immunol 2022;13:953229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou R-Q, Luo J, Li L-J, et al. Neoadjuvant plus chemotherapy in locally advanced oesophageal squamous cell carcinoma: a retrospective cohort study. BMC Surg 2023;23:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang B, Zhao H, Wu X, et al. Perioperative outcomes of neoadjuvant chemotherapy plus camrelizumab compared with chemotherapy alone and chemoradiotherapy for locally advanced esophageal squamous cell cancer. Front Immunol 2023;14:1066527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hong Z, Xu J, Chen Z, et al. Additional neoadjuvant immunotherapy does not increase the risk of anastomotic leakage after esophagectomy for esophageal squamous cell carcinoma: a multicenter retrospective cohort study. Int J Surg 2023;109:2168–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ge F, Huo Z, Cai X, et al. Evaluation of clinical and safety outcomes of neoadjuvant immunotherapy combined with chemotherapy for patients with resectable esophageal cancer: a systematic review and meta-analysis. JAMA network Open 2022;5:e2239778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taylor LJ, Greenberg CC, Lidor AO, et al. Utilization of surgical treatment for local and locoregional esophageal cancer: analysis of the National Cancer Data Base. Cancer 2017;123:410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Z, Hong Z-N, Xie S, et al. Neoadjuvant sintilimab plus chemotherapy for locally advanced esophageal squamous cell carcinoma: a single-arm, single-center, phase 2 trial (ESONICT-1). Ann Transl Med 2021;9:1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kato K, Ito Y, Daiko H, et al. A randomized controlled phase Ⅲ trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study[J]. J Clin Oncol 2022;40(4_suppl):238. [Google Scholar]
- 40. Zhao Q, Yu J, Meng X. A good start of immunotherapy in esophageal cancer. Cancer medicine 2019;8:4519–4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. The Lancet 2021;398:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Luo H, Lu J, Bai Y, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA 2021;326:916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med 2021;384:1191–1203. [DOI] [PubMed] [Google Scholar]
- 44. WM, Whited JR, et al. Therapy in locally advanced esophageal cancer: a national cancer database analysis. J Gastrointest Surg 2018;22:187–19. [DOI] [PubMed] [Google Scholar]
- 45. Duan H, Wang T, Luo Z, et al. A multicenter single-arm trial of sintilimab in combination with chemotherapy for neoadjuvant treatment of resectable esophageal cancer (SIN-ICE study). Ann Transl Med 2021;9:1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. He W, Leng X, Mao T, et al. Toripalimab plus paclitaxel and carboplatin as neoadjuvant therapy in locally advanced resectable esophageal squamous cell carcinoma. Oncologist 2022;3:e18–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li C, Zhao S, Zheng Y, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer 2021;144:232–241. [DOI] [PubMed] [Google Scholar]
- 48. Yang W, Xing X, Yeung S-CJ, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer 2022;10:e003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dhakras P, Uboha N, Horner V, et al. Gastrointestinal cancers: Current biomarkers in esophageal and gastric adenocarcinoma. Trans Gastroenterol Hepatol 2020;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee KW, Van Cutsem E, Bang YJ, et al. Association of tumor mutational burden with effificacy of Pembrolizumab ±Chemotherapy as fifirst-line therapy for gastric cancer in the phase iii keynote-062 study. Clin Cancer Res an Off J Am Assoc Cancer Res 2022;28:3489–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


