Abstract
Rationale:
Designer receptors exclusively activated by designer drugs (DREADDs) are a tool for “remote control” of defined neuronal populations during behavior. These receptors are inert unless bound by an experimenter-administered designer drug, commonly clozapine-n-oxide (CNO). However, questions have emerged about the suitability of CNO as a systemically administered DREADD agonist.
Objectives:
Second-generation agonists such as JHU37160 (J60) have been developed, which may have more favorable properties than CNO. Here we sought to directly compare effects of CNO (0, 1, 5, & 10 mg/kg, i.p.) and J60 (0, 0.03, 0.3, & 3 mg/kg, i.p.) on operant food pursuit.
Methods:
Male and female TH:Cre+ rats and their wildtype (WT) littermates received cre-dependent hM4Di-mCherry vector injections into ventral tegmental area (VTA), causing inhibitory DREADD expression in VTA dopamine neurons of TH:Cre+ rats. All rats were trained to stably lever press for palatable food on a fixed ratio 10 schedule, and doses of both agonists were tested on separate days in counterbalanced order.
Results:
All three CNO doses reduced operant rewards earned in rats with DREADDs, and no CNO dose had behavioral effects in WT controls. The highest J60 dose tested significantly reduced responding in DREADD rats, but this dose also increased responding in WTs, indicating non-specific effects. The magnitude of CNO and J60 effects in TH:Cre+ rats were correlated and were present in both sexes.
Conclusions:
Findings demonstrate the usefulness of directly comparing DREADD agonists when optimizing behavioral chemogenetics, and highlight the importance of proper controls, regardless of the DREADD agonist employed.
Keywords: chemogenetics, dopamine, ventral tegmental area, clozapine-n-oxide, JHU37160
Introduction
Designer receptors exclusively activated by designer drugs (DREADDs) are a useful method for attaining “remote control” of neuronal populations (Armbruster et al., 2007; Rogan and Roth, 2011). These mutated receptors are derived from human muscarinic acetylcholine receptors but are not affected by endogenous neurotransmitters, rendering them normally inert when expressed in targeted neural populations. Yet when a drug capable of agonist binding to DREADDs is administered experimentally, the receptors engage endogenous G protein-coupled signaling pathways, resulting in net excitation or inhibition of neuronal firing, and/or neurotransmitter release (Alexander et al., 2009; Atasoy and Sternson, 2018; Brodnik et al., 2020; Buchta et al., 2017; Mahler et al., 2019, 2014; Martinez et al., 2023; Song et al., 2022; Stachniak et al., 2014). Especially when coupled with genetic strategies such as cre-driver rodent lines, DREADDs are a powerful tool for manipulating G protein-coupled receptors in phenotypically-defined neural populations, elucidating the consequences of neural manipulations on behaviors and other outcomes (Burnett and Krashes, 2016; Ferguson et al., 2013; Fortress et al., 2015; Mazzone et al., 2018; O’Neal et al., 2020; Rinker et al., 2017; Rorabaugh et al., 2017; Zhu and Roth, 2015).
One of the most useful features of DREADDs is their “lock and key” nature—the premise that DREADDs (the “lock”) are inert in the absence of an exogenous ligand (the “key”), and that their ligand, when administered, acts “Exclusively” at DREADD receptors. Yet recent evidence suggests that CNO does not efficiently penetrate the blood brain barrier, and it binds relatively weakly at DREADDs. Instead, it is likely that metabolic conversion of CNO to clozapine, which enters the brain efficiently and binds DREADDs potently, is directly responsible for behavioral effects of systemically administered CNO (Gomez et al., 2017). In other words, CNO essentially acts as a pro-drug for clozapine, the direct DREADD agonist. Since at high enough concentrations clozapine can also bind endogenous receptors, it is therefore also possible for CNO to have off-target effects (Gomez et al., 2017; Ilg et al., 2018; MacLaren et al., 2016; Manvich et al., 2018; Porter et al., 2017). CNO is frequently found to have no measurable behavioral effects in the absence of DREADDs (Mahler and Aston-Jones, 2018; Smith et al., 2016; Urban and Roth, 2015; Whissell et al., 2016), but other experiments have found non-specific effects (Bonaventura et al., 2019; Gomez et al., 2017; MacLaren et al., 2016; Manvich et al., 2018; Martinez et al., 2019; Porter et al., 2017; Raper et al., 2017). The reasons for these varying results are unknown, but may involve species differences, the behavioral task tested, the presence of other experimentally-administered drugs, and CNO dose, route of administration, and dosing frequency (Campbell and Marchant, 2018; MacLaren et al., 2016). It is thus essential to compare CNO effects in subjects with and without DREADDs, to determine specificity of observed behavioral changes to manipulation of the targeted neural population.
Regardless, there is a clear need for a next generation of DREADD agonist that binds potently and selectively to DREADDs at doses in which it has no off-target behavioral effects. Several groups have proposed alternatives to CNO for behavioral chemogenetic experiments. One strategy is to administer low doses of clozapine, which binds at lower doses to DREADDs than at endogenous receptors (Desloovere et al., 2021; Jendryka et al., 2019). Other candidate drugs with varying advantages and disadvantages include deschloroclozapine (DCZ), C21, olanzapine, and JHU37160 (J60) (Bonaventura et al., 2019; Desloovere et al., 2021; Ferrari et al., 2022; Fleury Curado et al., 2021; Goutaudier et al., 2020; Kljakic et al., 2022; Nagai et al., 2020; Nentwig et al., 2022; Thompson et al., 2018).
One of these compounds, J60, was developed by an NIH team who showed it has acceptable behavioral and other effects in mice (Bonaventura et al., 2019), a finding that has been replicated by several other groups (Desloovere et al., 2021; Flerlage et al., 2022; Fleury Curado et al., 2021; Giannotti et al., 2021; Heinsbroek et al., 2021; Huang et al., 2021; Lewis et al., 2020; Li and Hollis, 2021; Salimi-Nezhad et al., 2023; Zhang et al., 2020). J60 efficiently crosses the blood-brain-barrier, it binds directly to central DREADD receptors after i.p. administration, in mice it is behaviorally-effective at both excitatory (hM3Dq) and inhibitory (hM4Di) DREADDs, and it also shows promise in primates (Bonaventura et al., 2019). We also showed in the same report that it is effective at stimulating locomotor activity at hM3Dq excitatory DREADDs in VTA dopamine neurons of TH:Cre+ rats, even at very low doses that had no clear off-target actions (Bonaventura et al., 2019). One group showed 0.1 mg/kg J60 in rats has specific behavioral effects at hM4Di DREADDs, but behavioral effects of other doses with inhibitory DREADDs have not yet been reported in rats (Giannotti et al., 2021; Heinsbroek et al., 2021).
In general, there is a notable lack of head-to-head behavioral comparisons of DREADD agonists, delaying the field from advancing toward consensus on the best compound for use in behavioral neuroscience experiments. Toward this goal, we conducted a preliminary experiment comparing CNO to J60 in tyrosine hydroxylase (TH):Cre rats with inhibitory hM4Di DREADDs in ventral tegmental area (VTA) dopamine neurons. We tested multiple doses of each compound in the same animals, examining effects on performance of an instrumental task—pressing a lever on a fixed-ratio (FR)10 schedule for palatable food pellets. Operant responding for reward is a readily replicable task that is usually stable once trained and thus optimal for within-subject manipulations, and which is sensitive to dopamine neuron manipulations (Mahler et al., 2019). We employed palatable food as the reinforcer here for simplicity, and to avoid potential issues with intravenous catheter patency needed for an equivalent experiment with self-administered drugs. Our results indicate that high doses of both CNO and J60 similarly suppressed instrumental responding in rats with inhibitory DREADDs, though the highest dose of J60 tested had the notable paradoxical effect of enhancing lever pressing in control rats without DREADDs. These data support the idea that either of these compounds can be used in such behavioral neuroscience experiments, though careful dosing optimization, and direct comparison of effects in animals with and without DREADDs is required. We also hope this report will inspire further direct comparisons of DREADD agonists to one another across different classes of behaviors and ranges of doses.
Methods
All procedures were approved by the Institutional Animal Care and Use Committee at UC Irvine and are in accordance with the NIH Guide for the Care and Use of Animals.
Subjects:
Long Evans transgenic TH:Cre+ (N=13) and wildtype TH:Cre− (WT; N=9) rats (N=9 males, N=13 females) were bred in-house, and housed in pairs as adults in ventilated tub cages with corncob bedding and ad libitum chow and water. Rats were at least 75 days old at the start of experiments. Rats were housed in reverse 12:12 hr lighting, and behavior experiments took place during the dark cycle.
Drugs:
CNO was provided by the NIDA Drug Supply Program, stored in desiccated, opaque powder aliquots at 4 °C, and prepared daily, mixed in 5% dimethyl sulfoxide (DMSO) in saline solution. J60 was provided by the NIMH Drug Supply Program, stored in desiccated, opaque powder aliquots at 4 °C, and prepared weekly, mixed in 5% DMSO saline solution and also stored at 4°C.
Viral Vector and Surgery:
A validated (Mahler et al., 2019, 2014) AAV2-hSyn-DIO-hM4D(Gi)-mCherry vector (titer ≥ 5×1012 vg/mL) was attained from AddGene (catalog number 44362-AAV2). Rats were anesthetized with 2.5% isoflurane, with meloxicam analgesic (1.0 mg/kg), then stereotaxically injected via glass pipette and Picospritzer with 0.75 μl of the vector bilaterally (Martinez et al., 2023), aimed at the VTA (coordinates relative to bregma (mm): −5.5 AP, +/− 0.8 ML, −8.1 DV). Pipettes were left in place for 5 min to reduce spread prior to removal. Rats were allowed at least 10 days to recover following surgery before beginning training. At least 28 days elapsed between virus injection and the first administration of CNO or J60, allowing sufficient time for robust, persistent DREADD/reporter expression in VTA dopamine neurons (Brodnik et al., 2020; Mahler et al., 2019, 2014).
Behavioral Training and Testing:
After recovering from surgery, animals were trained to lever press for highly palatable, banana-flavored, sucrose-, fat-, and protein-containing pellets (Bio-Serv, catalog #F0059) in a Med Associates rat operant conditioning box, enclosed in a sound-proof chamber. In daily 1 hr sessions, rats began on a fixed ratio (FR) 1 schedule and moved up to FR3, FR5 then FR10 when their responding was consistent for 3 consecutive days. No genotype or sex difference was seen in the number of days to progress to FR10, or to stabilize on FR10 responding (ps>0.05). After at least 8 days at FR10, and when stability criterion was achieved (less than 33% change in responding for 2 consecutive days), testing with DREADD agonists commenced. Animals were tested in a counterbalanced, pseudo-random order, with tests of all CNO doses (vehicle, 1, 5, 10 mg/kg) conducted prior to beginning a counterbalanced series of J60 tests (vehicle, 0.03, 0.3, 3 mg/kg). We first tested all CNO doses prior to moving on to J60 doses, opening the possibility of impacts of prior CNO on J60 efficacy. However, behavior on the two vehicle (VEH) day tests were equivalent (p=0.577; VEH tests are hereafter averaged for analyses and figures), arguing against persistent effects of CNO. Furthermore, there were no changes in responding between the day prior to, and the day after any dose of CNO or J60 (p > 0.35), also suggesting that both agonists had effects only on the day they were administered and did not cause persistent action and/or agonist-induced plasticity. After each test, stable responding was re-established prior to re-testing, and 48 h elapsed between any two tests. Experimental timeline is shown in Fig. 1.
Fig. 1. Schematic of experimental timeline.

Following hM4Di DREADD injection into the VTA, rats underwent fixed ratio (FR) training for palatable food and were stably responding at FR10 before testing with DREADD agonists began. All CNO doses and a VEH test were counterbalanced, followed by counterbalanced J60 doses and another VEH test. Animals were sacrificed after all tests were completed to confirm DREADD expression.
Confirmation of DREADD Expression:
After the final test was completed, animals were transcardially perfused with ice cold 0.9% saline followed by 4% paraformaldehyde. Extracted brains were cryoprotected in 20% sucrose, sectioned at 40 μm in a cryostat, and blocked in 3% normal donkey serum PBST. Tissue was incubated for 16 hr in rabbit anti-DsRed (Clontech; 1:5000) and mouse anti-TH antibodies (Immunostar; 1:1000) in PBST-azide with 2% normal donkey serum. After washing, slices were incubated in the dark for 4 hr in AlexaFluor-donkey anti-rabbit 594 and donkey anti-mouse 488 (Thermo Fisher Scientific), washed, then incubated with 4′,6-diamidino-2-phenylindole (DAPI; 1:1000) in PB for 5 mins, washed, mounted, and coverslipped with Fluoromount (Thermo Fisher Scientific). mCherry, TH, and DAPI expression was imaged at 10x, and the zone of expression in each hemisphere of each rat was verified relative to VTA borders using a rat brain atlas (Paxinos and Watson, 2006) (Fig. 2). Colocalization of TH and mCherry was also visualized at 63x magnification, and showed specific expression of mCherry in VTA dopamine neurons, as previously reported (Mahler et al., 2019, 2014).
Fig. 2. Bilateral VTA Dopamine Neuron DREADD Expression:
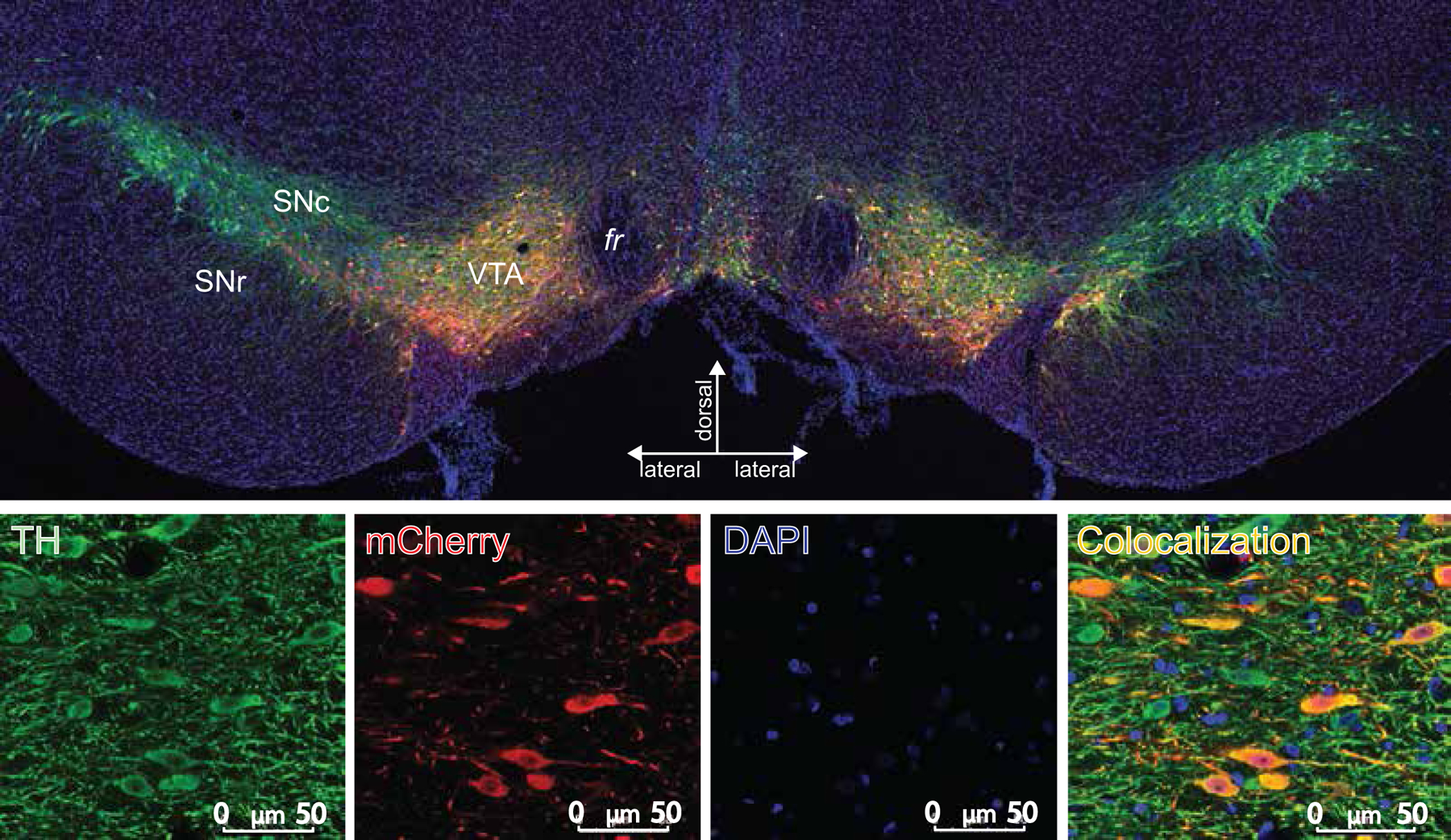
(Top) Typical expression of AAV2-hSyn-DIO-hM4D(Gi)-mCherry vector in a TH:Cre+ rat is depicted in a coronal view. mCherry (the cre-dependent DREADD reporter; red stain) is expressed nearly exclusively in TH+ neurons (green stain) of VTA; DAPI counterstain (blue). (Bottom) Each stain is shown separately at higher magnification (Scale bar=50 μm)
Statistical Analyses:
Analysis of drug effects was primarily conducted on change from baseline data, since rats’ baselines could drift somewhat over the course of training. We averaged active lever presses, inactive lever presses, and rewards earned on the 2 days prior to a test day, and subtracted that average from test day values. We first performed three-way ANOVAs for each drug, with dose (within subjects, 4 doses of CNO or J60), genotype (between subjects, TH:Cre+ and WT), and sex (F/M) factors. When sex was added to 3-way ANOVAs, significant interactions with sex did not emerge—though our sample was likely under-powered to detect sex differences, so future work should explore whether subtle sex differences in specific or non-specific effects of DREADD agonists exist. Separate ANOVAs were used to analyze drug and genotype effects on active and inactive lever presses, and significant findings were followed up with more targeted one-way ANOVAs and Sidak correction for multiple comparisons. Pearson’s correlation was used to determine whether behaviorally inhibitory effects of CNO and J60 were correlated. In all cases, two-tailed tests with significance thresholds of p>0.05 were used. Statistical analyses were conducted in R.
Results
Viral Expression:
TH:Cre+ rats exhibited hM4Di-mCherry expression that was localized within VTA borders, and expression was observed to be highly selective to dopamine neurons, as previously described (Fig. 2) (Brodnik et al., 2020; Mahler et al., 2019, 2014). Two TH:Cre+ animals were excluded from behavioral analyses because they lacked mCherry expression in the VTA in one hemisphere, and one animal was excluded due to a broken operant lever during testing, for a total of 11 TH:Cre+ and 8 WT animals included in analyses.
No Sex or Genotype Effects on Responding:
In the absence of hM4Di DREADD manipulation, there were no sex differences in active lever responding on VEH day (p=0.130; mean(±SEM) responding in males = 897(±97.6), females = 650(±125)), nor were there differences between WT and TH:Cre+ animals (p=0.574; WT = 693(±141), TH:Cre+ = 798(±96.5)).
Effects of CNO on FR10 Responding:
CNO inhibited active lever pressing relative to baseline in TH:Cre+ rats but not WTs (CNO dose X genotype interaction on change from pre-test baseline responding: F3,45=4.50, p=0.00760; Fig.3A). In TH:Cre+ animals, there was a main effect of dose (F3,30=9.64, p=0.000129), which was driven by suppression of responding, relative to vehicle, at 5 and 10 mg/kg, and a strong trend at the lowest dose (1 mg/kg: p=0.0758; 5 mg/kg: p=0.0013; 10 mg/kg: p=0.0015). There was no main effect of CNO doses in WT animals (F3,18=0.189, p=0.902; Fig.3C. To confirm these results, we also analyzed raw presses on each test day, in addition to change from baseline in pressing. TH:Cre+ rats responded less than their WT littermates on CNO days (CNO dose X genotype interaction for raw active lever pressing: F3, 45=4.48, p=0.00779). There was a drop in active lever responding in TH:Cre+ animals relative to VEH (main effect of dose: (F3,30=7.27, p=0.000829) driven by the 5 and 10 mg/kg doses (5 mg/kg: p=0.00230, mean(±SEM) responding in TH:Cre+=424(±81.9), WT=763(±114); 10 mg/kg p=0.0126, TH:Cre+=430(±92.5), WT=762(±153)), and a trend for suppression in active lever pressing with the 1 mg/kg dose (1 mg/kg p=0.195, TH:Cre+=530(±99.2), WT=709(±126)).
Fig. 3. CNO and J60 Effects on Operant FR10 Responding.
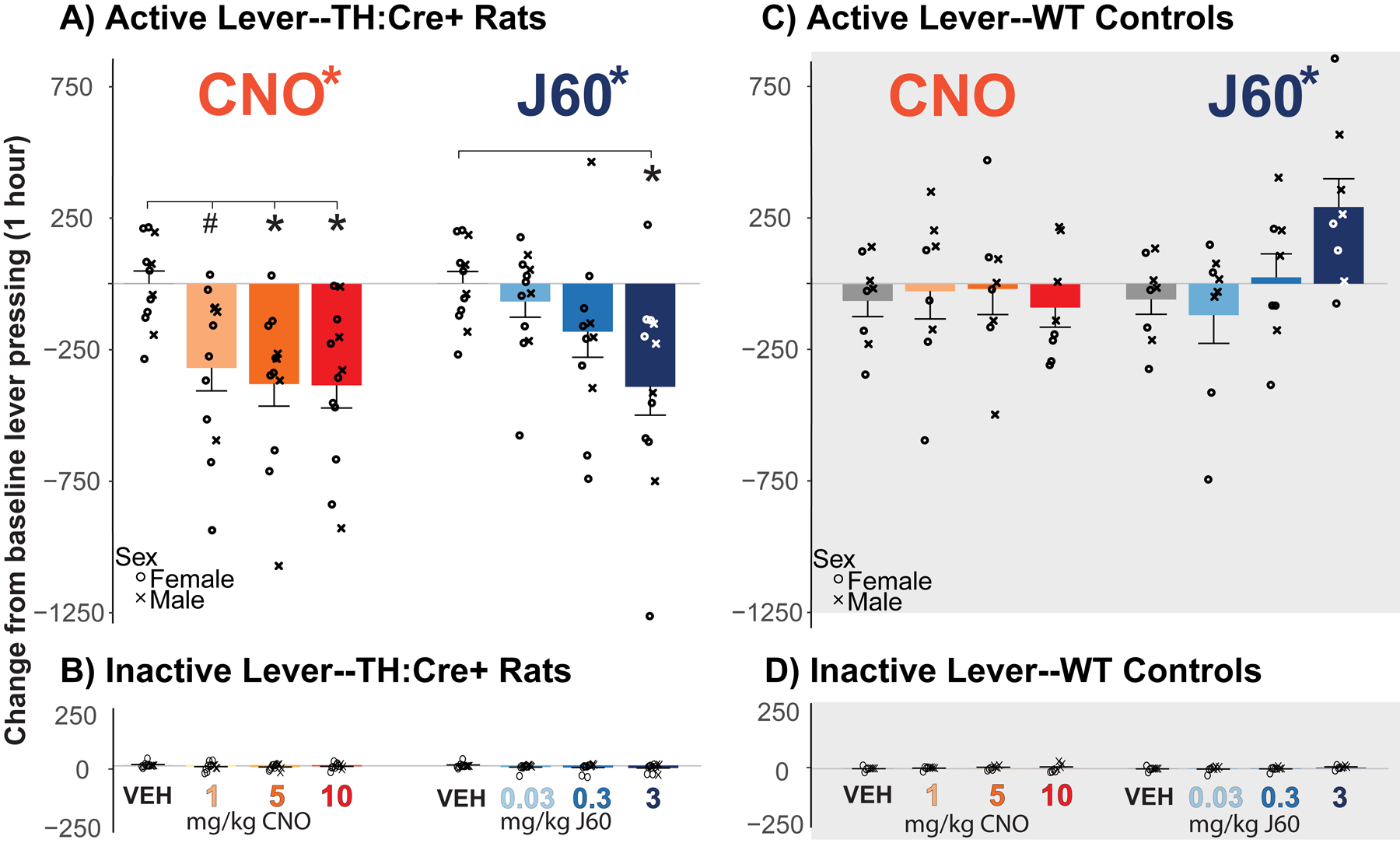
A) Change in active lever presses from 2 day prior average baseline with administration of CNO and J60 in TH:Cre+ animals is shown. There was a main effect of both CNO and J60 on active lever presses in TH:Cre+ animals, with posthoc test results comparing each dose to vehicle indicated with *; p<0.05). B) Change in inactive lever presses from baseline in TH:Cre+ animals. C&D) Change in (C) active and (D) inactive lever pressing is shown for WT animals. There was a main effect of J60 on active lever pressing in WT animals. Crosses and circles depict data from individual male and female animals, respectively.
CNO similarly suppressed the baseline-relative number of rewards earned in TH:Cre+, but not WT rats (dose X genotype interaction: F3,45= 4.50, p= 0.00761). In TH:Cre+ rats, there was a main effect CNO dose (F3,30=9.95, p=0.000103), driven by a drop in rewards earned between VEH and all CNO doses (1 mg/kg: p=0.0479; 5 mg/kg: p=0.000190; 10 mg/kg: p=0.00150).There was no effect of CNO on rewards earned in WT animals (F3,21=0.232, p=0.872).
Effects of J60 on FR10 Responding:
J60 also had distinct, dose-dependent effects on change from baseline active lever pressing in TH:Cre+ and WT rats (genotype X dose interaction: F3,45=8.41, p=0.000150). In TH:Cre+ rats, there was a main effect of dose (Fig. 3A; F3,30=5.67, p=0.00337), driven by a drop in active lever pressing between VEH and high-dose J60 (3 mg/kg; p=0.019). However there was also an effect of J60 dose in WT animals (Fig. 3C; F3,21=4.20, p=0.0177), and though there were no significant changes from VEH at any dose in posthoc analyses, the high dose trended toward increasing responding (3 mg/kg: p=0.138). Looking at raw pressing on test days, TH:Cre+ animals responded less than their WT littermates after J60 (dose X genotype interaction: F3, 45=9.21, p=0.0000727). In TH:Cre+ animals there was a drop in active lever responding after J60 (main effect of dose: F3,30=4.21, p=0.0133) with suppression at the 3 mg/kg dose (p=0.017, mean(±SEM) responding in TH:Cre+=479(±75.8), WT=986(±176)), but no suppression of active lever pressing with the 0.3 or 0.03 mg/kg doses compared to VEH (ps >0.5; 0.3 mg/kg TH:Cre+=633(±81.4), WT=724(±166); 0.03 mg/kg TH:Cre+=703(±87.6), WT=608(±146)).
J60 had similar effects on number of rewards earned as it did on active lever responding (genotype X dose interaction (F3,45=8.56, p=0.000131). In TH:Cre+ rats, there was a main effect of dose (F3,30=6.60, p=0.00147), driven by a drop in rewards earned between VEH and J60 3 mg/kg (p=0.00301). There was also an effect of dose in WT animals (F3,21=3.79, p=0.0257), driven by a strong trend in food pellets earned at the high dose (3 mg/kg: p=0.078).
Inactive Lever Responding:
There were no significant effects of either CNO or J60 on inactive lever pressing relative to baseline in either genotype (Fig. 3B, 3D; dose x genotype interaction; CNO: F3,45=1.09, p=0.360; J60: F3,45=1.59, p=0.203). Accordingly, in TH:Cre+ rats there was no effect of CNO (F3,30=1.56, p=0.218) or J60 (F3,30=1.32, p=0.284). Likewise, in WT rats there was no effect of either CNO (F3,21=0.178, p=0.910) or J60 (F3,21=1.16, p=0.349).
Comparison of CNO to J60-Inhibited Responding:
We next sought to determine whether the ability of CNO and J60 to suppress responding were of similar magnitude in individual animals. No statistical difference between the DREADD agonist drugs was observed in a dose (Low; Mid; High) X drug (CNO; J60) X sex repeated measures ANOVA in TH:Cre+ rats (no main effect of dose: F2,18= 2.67, p=0.0962, or drug: F1,9=2.72, p=0.133, and no dose x drug interaction (F2,18=2.38, p=0.121).
To further query whether behavioral effects of chemogenetic VTA dopamine neuron inhibition with CNO versus J60 were related in individual rats, we next examined correlations between baseline-relative pressing after moderate (CNO: 5 mg/kg; J60: 0.3 mg/kg) or high doses of each drug (CNO: 10 mg/kg; J60: 3 mg/kg), in TH:Cre+ or WT rats. For the moderate doses, pressing suppression by both drugs was not correlated in TH:Cre+ rats (p=0.298, r=0.345) but was in WT littermates (p=0.0260, r=−0.768). For the high doses, CNO- and J60-suppression of pressing was highly correlated in TH:Cre+ rats (Fig. 4A; p=0.0179, r=0.694), but no such effects were seen in WT littermates (Fig. 4B; p=0.458, r=−0.308).
Fig. 4. Suppression of Pressing by High Dose CNO and J60 is Correlated in TH:Cre+, but not WT rats.

A&B) Correlation between effect of high dose CNO and J60 is shown in A) TH:Cre+ and B) WT animals. Data is baseline-relative. Crosses and circles depict data from individual male and female rats, respectively.
Discussion
DREADDs are a common approach for manipulating neural populations and circuits of behaving animals in neuroscience experiments. However, there remains controversy over which is the best agonist drug for engaging DREADDs. Therefore, we elected to test two prominent DREADD agonists (clozapine-n-oxide; CNO, and JHU37160; J60) head-to-head, using behaving TH:Cre+ rats expressing hM4Di inhibitory DREADDs in VTA dopamine neurons, or WT littermates without DREADDs. Using an operant reward seeking task (FR10 lever pressing for palatable food), we found that both agonists inhibited reward seeking and rewards obtained in hM4Di DREADD-expressing animals, and CNO did so at doses that did not affect behavior in WT controls. We also found that J60 enhanced reward attainment in WT rats at the highest dose (3 mg/kg), despite strongly suppressing seeking in TH:Cre+ rats at the same dose. The magnitude of high dose CNO- and J60-suppression of reward pursuit across rats was also correlated in rats with VTA dopamine neuron hM4Di DREADDs, but not in WT controls. Taken together, these results suggest that both CNO and J60 can activate inhibitory DREADDs in VTA dopamine neurons to suppress operant food seeking. An important implication of these studies is that whatever agonist drug is used in a DREADD experiment, it is essential to compare its effects in experimental animals to effects in control animals without DREADD expression.
CNO significantly reduced palatable food rewards earned in hM4Di DREADD rats at all tested doses (1, 5, & 10 mg/kg) without having any behavioral effect in non-DREADD WT rats. J60 significantly reduced food pursuit in TH:Cre+ rats at the highest tested dose (3 mg/kg), but this dose also showed signs of increasing responding in WT animals, suggesting nonspecific effects. Interestingly, J60 did not increase responding on the inactive lever, which may indicate that the off-target effects involve increased instrumental seeking of palatable food, rather than non-specific arousal or locomotor activation. Supporting the qualitatively similar efficacy of CNO and J60, we found that the highest tested doses of both drugs elicited statistically equivalent behavioral effects in the same animals, and that the magnitude of effects elicited by these drug doses in individual animals was correlated.
We picked these specific doses of CNO based on precedent within our own lab and in the field more broadly. We’ve seen specific behavioral effects of CNO in rats at doses of 1, 5, 10, and even up to 20 mg/kg (Farrell et al., 2021, 2019; Lawson et al., 2021; Mahler et al., 2014). J60 has previously been tested at doses from 0.01 to 0.3 mg/kg with specific behavioral effects (Bonaventura et al., 2019; Desloovere et al., 2021; Giannotti et al., 2021; Heinsbroek et al., 2021). The high dose of J60 used here, 3 mg/kg, is likely the highest ever tested. At this dose, J60 effectively inhibits active lever pressing in animals with DREADDs, but also showed nonspecific effects of increasing reward obtainment in WT rats. It is not presently clear which receptor this high dose of J60, or its potential metabolites, might act at to produce these non-specific response-facilitating effects.
In contrast to J60, we did not observe any off-target effects of CNO at any tested dose. Some prior studies have found non-selective effects of CNO in DREADD-free rats or mice (Bonaventura et al., 2019; Desloovere et al., 2021; Gomez et al., 2017; Jendryka et al., 2019; MacLaren et al., 2016; Manvich et al., 2018; Porter et al., 2017; Raper et al., 2017), though we and many others have failed to find CNO-only effects on behavior in operant responding for food and drugs in our prior work (Farrell et al., 2021, 2019; Mahler et al., 2014). It is thus possible that the non-specific behavioral effects of CNO are dependent upon the behavior being tested. It is also possible that CNO effects are exacerbated by the presence of other drugs, potentially due to competitive metabolism of drugs that could enhance overall exposure to the agonist or its metabolites, such as clozapine (Mahler et al., 2019). Such metabolic competition may vary between species, strains, and sexes, and can also depend on the animals’ health—it is clearly a topic that requires further, dedicated study. Regardless, we strongly recommend that all DREADD studies using CNO or other agonists employ proper control groups to account for the potentially task-specific effects of CNO (or any DREADD agonist) in the absence of DREADDs.
Limitations and Future Directions:
These studies, testing in the same rats the relative efficacy of two common DREADD agonist drugs in eliciting hM4Di-dependent behavioral effects, leave several important questions unanswered. Instead of fully counterbalancing drug dosing, J60 doses were always given after CNO doses. The effects of J60 on behavior could therefore be impacted by prior CNO administrations, or repeated engagement hM4Di receptors by CNO. That being said, we have previously found that repeated CNO administrations in TH:Cre rats with hM4Di DREADDs in dopamine neurons did not have lingering effects on operant reward seeking (Farrell et al., 2019; Mahler et al., 2019, 2014), nor did we find lingering effects of either agonist here. Yet we acknowledge that it is still possible that CNO-induced plasticity could have had subtle effects here that impacted subsequent efficacy of J60.
We tested 3 doses of each agonist drug, but these are not necessarily the optimal doses for controlling behavior via selective actions at hM4Di DREADDs. For example, while we observed a non-specific effect of high-dose J60, it is possible that a dose between 0.3 and 3 mg/kg would have had strong behavioral effects on this task that were highly specific to DREADDs. Previously, 0.1 mg/kg J60 (i.p.) in rats was reported to hM4Di DREADD-specifically suppress neural activity and alter drug seeking behavior (Giannotti et al., 2021; Heinsbroek et al., 2021), and both 0.1 and 1 mg/kg doses have been reported to effectively alter behavior and suppress neuronal activity at hM4Di DREADDs in mice (Bonaventura et al., 2019; Li and Hollis, 2021; Zhang et al., 2020). A group using both CNO and J60 found no differences between behavioral changes elicited by 0.1 mg/kg J60 and 3 mg/kg CNO at hM4Di DREADDs in mice (Lewis et al., 2020), and J60 is also effective at 0.1 and 1 mg/kg doses in rats and mice at hM3Dq DREADDs (Huang et al., 2021; Salimi-Nezhad et al., 2023; Zhang et al., 2020). These doses of J60 may therefore have selective behavioral effects depending on the task and neural substrate targeted, and potentially FR10 responding for palatable food is relatively insensitive to J60-induced dopamine neuron inhibition. The current study only assessed palatable food self-administration, and since non-specific effects of DREADD agonists can vary by behavioral task employed (Mahler and Aston-Jones, 2018; Smith et al., 2016), additional behavioral assays should be examined in future studies to determine their sensitivity to J60 and CNO off-target effects. Further work should also examine the neural substates responsible for VTA dopamine neuron inhibition behavioral effects. Further dose characterization with J60 remains to be thoroughly characterized in both rats and mice, and future work should verify selectivity and efficacy of dosing in the behavioral model of interest.
In addition, other DREADD agonists are also promising, and should be similarly tested empirically. For example, compound 21 (Ferrari et al., 2022; Jendryka et al., 2019; Kljakic et al., 2022; Thompson et al., 2018), perlapine (Chen et al., 2015; Kljakic et al., 2022; Thompson et al., 2018), DCZ (Nagai et al., 2020; Nentwig et al., 2022; Raper and Galvan, 2022; Upright and Baxter, 2020), and olanzapine (Goossens et al., 2021; Upright and Baxter, 2020; Weston et al., 2019) have been reported to have strong and selective effects at DREADDs, without pronounced off-target actions. We hope that the field will soon converge upon the “best” DREADD agonist for most behavioral experiments.
Though preliminary, these data are also valuable as proof of concept for testing DREADD agonists against one another in the same animals, in a direct and empirical manner. We hope this report will inspire others to similarly test other promising DREADD agonist drugs for their selective efficacy in head-to-head comparisons in other species and strains of model organisms and in other task conditions.
Acknowledgements:
We thank NIDA Drug Supply Program for supplying CNO, and NIMH Drug Supply Program for supplying JHU37160.
Funding:
Funding was provided by NIH grants P50 DA044118, T32 MH119049, R01 DA055849, and U01 DA053826.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
Data Availability Statement:
Data will be made available upon reasonable request.
References
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL, 2009. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63, 27–39. 10.1016/j.neuron.2009.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL, 2007. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci 104, 5163–5168. 10.1073/pnas.0700293104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Sternson SM, 2018. Chemogenetic Tools for Causal Cellular and Neuronal Biology. Physiol. Rev 98, 391–418. 10.1152/physrev.00009.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura J, Eldridge MAG, Hu F, Gomez JL, Sanchez-Soto M, Abramyan AM, Lam S, Boehm MA, Ruiz C, Farrell MR, Moreno A, Galal Faress IM, Andersen N, Lin JY, Moaddel R, Morris PJ, Shi L, Sibley DR, Mahler SV, Nabavi S, Pomper MG, Bonci A, Horti AG, Richmond BJ, Michaelides M, 2019. High-potency ligands for DREADD imaging and activation in rodents and monkeys. Nat. Commun 10, 4627. 10.1038/s41467-019-12236-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodnik ZD, Xu W, Batra A, Lewandowski SI, Ruiz CM, Mortensen OV, Kortagere S, Mahler SV, España RA, 2020. Chemogenetic Manipulation of Dopamine Neurons Dictates Cocaine Potency at Distal Dopamine Transporters. J. Neurosci 40, 8767–8779. 10.1523/JNEUROSCI.0894-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchta WC, Mahler SV, Harlan B, Aston-Jones GS, Riegel AC, 2017. Dopamine terminals from the ventral tegmental area gate intrinsic inhibition in the prefrontal cortex. Physiol. Rep 5, e13198. 10.14814/phy2.13198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett CJ, Krashes MJ, 2016. Resolving Behavioral Output via Chemogenetic Designer Receptors Exclusively Activated by Designer Drugs. J. Neurosci 36, 9268–9282. 10.1523/JNEUROSCI.1333-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EJ, Marchant NJ, 2018. The use of chemogenetics in behavioural neuroscience: receptor variants, targeting approaches and caveats. Br. J. Pharmacol 175, 994–1003. 10.1111/bph.14146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Choo H, Huang X-P, Yang X, Stone O, Roth BL, Jin J, 2015. The First Structure–Activity Relationship Studies for Designer Receptors Exclusively Activated by Designer Drugs. ACS Chem. Neurosci. 6, 476–484. 10.1021/cn500325v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desloovere J, Boon P, Larsen LE, Goossens M-G, Delbeke J, Carrette E, Wadman W, Vonck K, Raedt R, 2021. Chemogenetic Seizure Control with Clozapine and the Novel Ligand JHU37160 Outperforms the Effects of Levetiracetam in the Intrahippocampal Kainic Acid Mouse Model. Neurotherapeutics. 10.1007/s13311-021-01160-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MR, Esteban JSD, Faget L, Floresco SB, Hnasko TS, Mahler SV, 2021. Ventral Pallidum GABA Neurons Mediate Motivation Underlying Risky Choice. J. Neurosci 41, 4500–4513. 10.1523/JNEUROSCI.2039-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MR, Ruiz CM, Castillo E, Faget L, Khanbijian C, Liu S, Schoch H, Rojas G, Huerta MY, Hnasko TS, Mahler SV, 2019. Ventral pallidum is essential for cocaine relapse after voluntary abstinence in rats. Neuropsychopharmacology 44, 2174–2185. 10.1038/s41386-019-0507-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Phillips PEM, Roth BL, Wess J, Neumaier JF, 2013. Direct-Pathway Striatal Neurons Regulate the Retention of Decision-Making Strategies. J. Neurosci 33, 11668–11676. 10.1523/JNEUROSCI.4783-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LL, Ogbeide-Latario OE, Gompf HS, Anaclet C, 2022. Validation of DREADD Agonists and Administration Route in a Murine Model of Sleep Enhancement. J. Neurosci. Methods 109679. 10.1016/j.jneumeth.2022.109679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flerlage WJ, Langlois LD, Rusnak M, Simmons SC, Gouty S, Armstrong RC, Cox BM, Symes AJ, Tsuda MC, Nugent FS, 2022. Involvement of Lateral Habenula Dysfunction in Repetitive Mild Traumatic Brain Injury–Induced Motivational Deficits. J. Neurotrauma neu.2022.0224. 10.1089/neu.2022.0224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury Curado T, Pho H, Freire C, Amorim MR, Bonaventura J, Kim LJ, Lee R, Cabassa ME, Streeter SR, Branco LG, Sennes LU, Fishbein K, Spencer RG, Schwartz AR, Brennick MJ, Michaelides M, Fuller DD, Polotsky VY, 2021. Designer Receptors Exclusively Activated by Designer Drugs Approach to Treatment of Sleep-disordered Breathing. Am. J. Respir. Crit. Care Med 203, 102–110. 10.1164/rccm.202002-0321OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Hamlett ED, Vazey EM, Aston-Jones G, Cass WA, Boger HA, Granholm A-CE, 2015. Designer Receptors Enhance Memory in a Mouse Model of Down Syndrome. J. Neurosci 35, 1343–1353. 10.1523/JNEUROSCI.2658-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotti G, Gong S, Fayette N, Heinsbroek JA, Orfila JE, Herson PS, Ford CP, Peters J, 2021. Extinction blunts paraventricular thalamic contributions to heroin relapse. Cell Rep. 36, 109605. 10.1016/j.celrep.2021.109605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M, 2017. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507. 10.1126/science.aan2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens M-G, Boon P, Wadman W, Van den Haute C, Baekelandt V, Verstraete AG, Vonck K, Larsen LE, Sprengers M, Carrette E, Desloovere J, Meurs A, Delbeke J, Vanhove C, Raedt R, 2021. Long-term chemogenetic suppression of seizures in a multifocal rat model of temporal lobe epilepsy. Epilepsia 62, 659–670. 10.1111/epi.16840 [DOI] [PubMed] [Google Scholar]
- Goutaudier R, Coizet V, Carcenac C, Carnicella S, 2020. Compound 21, a two-edged sword with both DREADD-selective and off-target outcomes in rats. PLOS ONE 15, e0238156. 10.1371/journal.pone.0238156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek JA, Giannotti G, Mandel MR, Josey M, Aston-Jones G, James MH, Peters J, 2021. A common limiter circuit for opioid choice and relapse identified in a rodent addiction model. Nat. Commun 12, 4788. 10.1038/s41467-021-25080-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Guan F, Licinio J, Wong M-L, Yang Y, 2021. Activation of septal OXTr neurons induces anxiety- but not depressive-like behaviors. Mol. Psychiatry 26, 7270–7279. 10.1038/s41380-021-01283-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg A-K, Enkel T, Bartsch D, Bähner F, 2018. Behavioral Effects of Acute Systemic Low-Dose Clozapine in Wild-Type Rats: Implications for the Use of DREADDs in Behavioral Neuroscience. Front. Behav. Neurosci 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendryka M, Palchaudhuri M, Ursu D, van der Veen B, Liss B, Kätzel D, Nissen W, Pekcec A, 2019. Pharmacokinetic and pharmacodynamic actions of clozapine-N-oxide, clozapine, and compound 21 in DREADD-based chemogenetics in mice. Sci. Rep 9, 4522. 10.1038/s41598-019-41088-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kljakic O, Hogan-Cann AE, Yang H, Dover B, Al-Onaizi M, Prado MAM, Prado VF, 2022. Chemogenetic activation of VGLUT3-expressing neurons decreases movement. Eur. J. Pharmacol 935, 175298. 10.1016/j.ejphar.2022.175298 [DOI] [PubMed] [Google Scholar]
- Lawson KA, Flores AY, Hokenson RE, Ruiz CM, Mahler SV, 2021. Nucleus Accumbens Chemogenetic Inhibition Suppresses Amphetamine-Induced Ultrasonic Vocalizations in Male and Female Rats. Brain Sci. 11, 1255. 10.3390/brainsci11101255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RG, Serra M, Radl D, Gori M, Tran C, Michalak SE, Vanderwal CD, Borrelli E, 2020. Dopaminergic Control of Striatal Cholinergic Interneurons Underlies Cocaine-Induced Psychostimulation. Cell Rep. 31, 107527. 10.1016/j.celrep.2020.107527 [DOI] [PubMed] [Google Scholar]
- Li Y, Hollis E, 2021. Basal Forebrain Cholinergic Neurons Selectively Drive Coordinated Motor Learning in Mice. J. Neurosci 41, 10148–10160. 10.1523/JNEUROSCI.1152-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DAA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, España RA, Clark SD, 2016. Clozapine N-Oxide Administration Produces Behavioral Effects in Long-Evans Rats: Implications for Designing DREADD Experiments. eNeuro 3, ENEURO.0219–16.2016. 10.1523/ENEURO.0219-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones G, 2018. CNO Evil? Considerations for the Use of DREADDs in Behavioral Neuroscience. Neuropsychopharmacology 43, 934–936. 10.1038/npp.2017.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Brodnik ZD, Cox BM, Buchta WC, Bentzley BS, Quintanilla J, Cope ZA, Lin EC, Riedy MD, Scofield MD, Messinger J, Ruiz CM, Riegel AC, España RA, Aston-Jones G, 2019. Chemogenetic Manipulations of Ventral Tegmental Area Dopamine Neurons Reveal Multifaceted Roles in Cocaine Abuse. J. Neurosci 39, 503–518. 10.1523/JNEUROSCI.0537-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G, 2014. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat. Neurosci 17, 577–585. 10.1038/nn.3664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, Webster KA, Foster SL, Farrell MS, Ritchie JC, Porter JH, Weinshenker D, 2018. The DREADD agonist clozapine N-oxide (CNO) is reverse-metabolized to clozapine and produces clozapine-like interoceptive stimulus effects in rats and mice. Sci. Rep 8, 3840. 10.1038/s41598-018-22116-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez MX, Farrell MR, Mahler SV, 2023. Pathway-specific chemogenetic manipulation by applying ligand to axonally-expressed DREADDs, in: Vectorology for Optogenetics and Chemogenetics. [Google Scholar]
- Martinez VK, Saldana-Morales F, Sun JJ, Zhu PJ, Costa-Mattioli M, Ray RS, 2019. Off-Target Effects of Clozapine-N-Oxide on the Chemosensory Reflex Are Masked by High Stress Levels. Front. Physiol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone CM, Pati D, Michaelides M, DiBerto J, Fox JH, Tipton G, Anderson C, Duffy K, McKlveen JM, Hardaway JA, Magness ST, Falls WA, Hammack SE, McElligott ZA, Hurd YL, Kash TL, 2018. Acute engagement of Gq-mediated signaling in the bed nucleus of the stria terminalis induces anxiety-like behavior. Mol. Psychiatry 23, 143–153. 10.1038/mp.2016.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Miyakawa N, Takuwa H, Hori Y, Oyama K, Ji B, Takahashi M, Huang X-P, Slocum ST, DiBerto JF, Xiong Y, Urushihata T, Hirabayashi T, Fujimoto A, Mimura K, English JG, Liu J, Inoue K, Kumata K, Seki C, Ono M, Shimojo M, Zhang M-R, Tomita Y, Nakahara J, Suhara T, Takada M, Higuchi M, Jin J, Roth BL, Minamimoto T, 2020. Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat. Neurosci 23, 1157–1167. 10.1038/s41593-020-0661-3 [DOI] [PubMed] [Google Scholar]
- Nentwig TB, Obray JD, Vaughan DT, Chandler LJ, 2022. Behavioral and slice electrophysiological assessment of DREADD ligand, deschloroclozapine (DCZ) in rats. Sci. Rep 12, 6595. 10.1038/s41598-022-10668-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal TJ, Nooney MN, Thien K, Ferguson SM, 2020. Chemogenetic modulation of accumbens direct or indirect pathways bidirectionally alters reinstatement of heroin-seeking in high- but not low-risk rats. Neuropsychopharmacology 45, 1251–1262. 10.1038/s41386-019-0571-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2006. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition. Elsevier. [Google Scholar]
- Porter JH, Manvich DF, Webster KA, Foster SL, Farrell MS, Weinshenker D, 2017. Behavioral and Pharmacokinetic Properties of the Putatively-Inert DREADD Ligand Clozapine-N-Oxide (CNO) in Rats and Mice. FASEB J. 31, lb594–lb594. [Google Scholar]
- Raper J, Galvan A, 2022. Applications of chemogenetics in non-human primates. Curr. Opin. Pharmacol 102204. 10.1016/j.coph.2022.102204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Morrison RD, Daniels JS, Howell L, Bachevalier J, Wichmann T, Galvan A, 2017. Metabolism and Distribution of Clozapine-N-oxide: Implications for Nonhuman Primate Chemogenetics. ACS Chem. Neurosci 8, 1570–1576. 10.1021/acschemneuro.7b00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, Kash TL, Navarro M, Thiele TE, 2017. Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge Ethanol Intake. Biol. Psychiatry, The Extended Amygdala and Addiction 81, 930–940. 10.1016/j.biopsych.2016.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan SC, Roth BL, 2011. Remote Control of Neuronal Signaling. Pharmacol. Rev 63, 291–315. 10.1124/pr.110.003020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorabaugh JM, Chalermpalanupap T, Botz-Zapp CA, Fu VM, Lembeck NA, Cohen RM, Weinshenker D, 2017. Chemogenetic locus coeruleus activation restores reversal learning in a rat model of Alzheimer’s disease. Brain 140, 3023–3038. 10.1093/brain/awx232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Nezhad N, Missault S, Reinoso AN, Hassani A, Amiri M, Keliris GA, 2023. The impact of selective and non-selective medial septum stimulation on hippocampal neuronal oscillations: A study based on modeling and experiments. Neurobiol. Dis 106052. 10.1016/j.nbd.2023.106052 [DOI] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW, Mahler SV, 2016. DREADDs: Use and Application in Behavioral Neuroscience. Behav. Neurosci 130, 137–155. 10.1037/bne0000135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Patel RV, Sharif M, Ashokan A, Michaelides M, 2022. Chemogenetics as a neuromodulatory approach to treating neuropsychiatric diseases and disorders. Mol. Ther 30, 990–1005. 10.1016/j.ymthe.2021.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachniak TJ, Ghosh A, Sternson SM, 2014. Chemogenetic Synaptic Silencing of Neural Circuits Localizes a Hypothalamus→Midbrain Pathway for Feeding Behavior. Neuron 82, 797–808. 10.1016/j.neuron.2014.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KJ, Khajehali E, Bradley SJ, Navarrete JS, Huang XP, Slocum S, Jin J, Liu J, Xiong Y, Olsen RHJ, Diberto JF, Boyt KM, Pina MM, Pati D, Molloy C, Bundgaard C, Sexton PM, Kash TL, Krashes MJ, Christopoulos A, Roth BL, Tobin AB, 2018. DREADD Agonist 21 Is an Effective Agonist for Muscarinic-Based DREADDs in Vitro and in Vivo. ACS Pharmacol. Transl. Sci 1, 61–72. 10.1021/acsptsci.8b00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upright NA, Baxter MG, 2020. Effect of chemogenetic actuator drugs on prefrontal cortex-dependent working memory in nonhuman primates. Neuropsychopharmacology 45, 1793–1798. 10.1038/s41386-020-0660-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban DJ, Roth BL, 2015. DREADDs (Designer Receptors Exclusively Activated by Designer Drugs): Chemogenetic Tools with Therapeutic Utility. Annu. Rev. Pharmacol. Toxicol 55, 399–417. 10.1146/annurev-pharmtox-010814-124803 [DOI] [PubMed] [Google Scholar]
- Weston M, Kaserer T, Wu A, Mouravlev A, Carpenter JC, Snowball A, Knauss S, von Schimmelmann M, During MJ, Lignani G, Schorge S, Young D, Kullmann DM, Lieb A, 2019. Olanzapine: A potent agonist at the hM4D(Gi) DREADD amenable to clinical translation of chemogenetics. Sci. Adv 5, eaaw1567. 10.1126/sciadv.aaw1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whissell PD, Tohyama S, Martin LJ, 2016. The Use of DREADDs to Deconstruct Behavior. Front. Genet 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen D, Sweeney P, Yang Y, 2020. An excitatory ventromedial hypothalamus to paraventricular thalamus circuit that suppresses food intake. Nat. Commun 11, 6326. 10.1038/s41467-020-20093-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Roth BL, 2015. DREADD: A Chemogenetic GPCR Signaling Platform. Int. J. Neuropsychopharmacol 18, pyu007. 10.1093/ijnp/pyu007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.


