Abstract
Background
Technological advances have enabled continuous monitoring of vital signs (CMVS) by wearable, wireless devices on general hospital wards to facilitate early detection of clinical deterioration, which could potentially improve clinical outcomes. However, evidence on the impact of these CMVS systems on patient outcomes is limited. This research aimed to explore the effect of CMVS on the clinical outcomes in major abdominal surgery patients in a general surgery ward.
Methods
A single-centre before–after study was conducted from October 2019 to June 2022. Patients in the intervention group received CMVS in addition to conventional intermittent vital sign monitoring (standard care for control group). With CMVS, heart rate and respiratory rate were measured every 5 min by a patch sensor. Proactive vital signs trends assessments and, when necessary, subsequent nursing activities were performed every nursing shift. The primary outcome of interest was the length of hospital stay (LOS); also, 12 patient-related outcomes were analysed. In the CMVS group, follow-up nursing activities of deviating vital signs trends were described and patient acceptability was measured. Post-hoc subgroup analysis was performed for colorectal and hepatopancreatobiliary surgery.
Results
A total of 908 patients were included (colorectal: n = 650; hepatopancreatobiliary: n = 257). Overall, median LOS was lower in the CMVS group (5.0 versus 5.5 days; P = 0.012), respectively. Post-hoc subgroup analysis showed this reduction in LOS was mostly observed in the colorectal group and not in the hepatopancreatobiliary group. Apart from a decrease in nurse-to-house-officer calls (from 15.3% to 7.7%; P = 0.007), all secondary clinical outcomes were similar in CMVS and control groups. However, a non-significant trend towards less-severe complications and reduced ICU LOS was observed in the CMVS group. In CMVS patients, 109 additional nursing activities were performed and 83% of patients indicated CMVS was acceptable.
Conclusion
CMVS was associated with a significant reduction in LOS, while other clinical outcomes were unchanged. CMVS triggered additional nursing activities such as extra patient assessments and therapeutic interventions.
A before–after study including 908 abdominal surgery patients showed wearable continuous vital signs monitoring on the general ward was associated with a significant decrease in length of stay. Besides a decrease in nurse-to-house-officer calls, secondary outcomes were similar in intervention and control groups. Also, the intervention triggered additional interventions such as extra patient assessments and therapeutic interventions.
Introduction
Postoperative complications after major abdominal surgery may occur in up to 44% of all patients1,2, impact a broad range of patient outcomes and also considerably increase costs3–10. They not only increase mortality and prolong hospital stay, but also result in the need for an increased level of post-discharge care and a higher readmission rate. Furthermore, long-term outcomes such as quality of life and functional performance are negatively affected10,11.
Severe postoperative complications are commonly associated with clinical deterioration and, when detected early, timely intervention may reduce morbidity and mortality12. Vital sign deviations usually precede clinical deterioration. To promote identification of patients at risk, simple physiological parameter-based protocols are broadly implemented on general wards13,14. Generally, the five key vital signs15 (blood pressure, blood oxygen saturation, heart rate, respiratory rate and body temperature) are measured manually 1–3 times a day in general wards and aggregated into a single number using the Early Warning Scores (EWS) system. A critical limitation of these systems is that the physiological measurements are intermittent, there is poor protocol adherence and sometimes inaccurate vital sign recording16–18. Patients may unexpectedly deteriorate, which may go unnoticed in between routine vital signs measurements19.
Over the last decade, new technological advances facilitated the introduction of continuous monitoring of vital signs (CMVS) by wearable, wireless devices on general wards. These CMVS interventions allow earlier detection of clinical deterioration and may improve clinical outcomes, in particular reduced complication severity, reduction of failure to rescue events and fewer ICU admissions, all of which combined may decrease total length of stay12,18,20–22. However, evidence for a positive effect on clinical outcomes in general ward patients with wearable devices is scarce23,24. This may be explained by the challenging implementation of CMVS in clinical workflows25–27.
Successful implementation is essential before any potential effectiveness of continuous monitoring can be reliably demonstrated. Therefore, a CMVS intervention was developed and its feasibility evaluated in two previous studies28,29. Subsequently, an interventional study with a hybrid design focusing on both evaluation of the implementation and the effectiveness of the intervention was set up30. The success of this implementation strategy is described elsewhere31. Here, the findings regarding the impact of CMVS on the surgical ward on clinical outcomes in major abdominal surgery patients, consisting of elective colorectal and hepatopancreatobiliary (HPB) surgery, are described as compared to a historical control group. The primary aim was to explore the effect of CMVS on length of hospital stay (LOS). Secondary aims were to explore the effects of CMVS on a broad range of other clinical outcome measures.
Methods
Study design
A single-centre before–after study as part of a type 2 hybrid design30 was conducted from October 2019 to July 2022 in a 1250-bed teaching hospital in the Netherlands. This study is reported in concordance with the STROBE guidelines and was registered in the ISRCTN registry (ISRCTN37125996)32.
Participants and setting
Patients admitted to the surgical ward for elective major abdominal surgery, including both colorectal (average of 230 surgeries per year) and HPB (average of 115 surgeries per year) resections, were eligible to participate in the study. Inclusion criteria were: ≥ 18 years old and expected hospitalization of ≥2 days. Patients admitted between October 2019 and November 2021 were retrospectively included in the pre-implementation group as controls. From November 2021 to June 2022, patients were prospectively included in the intervention group (post-implementation group). No substantive changes were made to unit staffing, or to hospital protocols, departmental safety and quality policies during the 2.5-year study period. Patients were excluded when the primary indication for hospitalization was acute (not elective), had a palliative indication, a known allergy for any of the materials of the sensor or when they participated in another (potentially conflicting) study.
Continuous monitoring of vital signs intervention
Pre-implementation, the standard of care was intermittent manual monitoring using the Modified Early Warning Score (MEWS) every 24 h according to the local hospital protocol. For every MEWS, besides subjective measurements, five vital signs were recorded: respiratory rate (RR), heart rate (HR), blood pressure (BP), core temperature and oxygen saturation33 (Supplementary material). Vital signs were measured manually using a blood pressure measuring device with a pulse oximeter, an ear thermometer, and by visual inspection of RR.
Post-implementation, in addition to the standard MEWS protocol, patients were continuously monitored by the Conformité Européene marked Philips Healthdot and Intellivue Guardian Solution (IGS) software system (Philips, Eindhoven, The Netherlands). The wireless monitoring sensor is embedded in a patch worn on the patient’s chest (Supplementary Material, Fig. S1); it continuously records heart rate (HR) in beats per minute and respiratory rate (RR) in respirations per minute using a chest accelerometer. Every 5-min interval, the vital signs measurements were wirelessly transmitted to the IGS software. Within the IGS software, vital sign trends are visualized and, complementary to the hospital MEWS protocol, a partial MEWS-score (D-EWS) was aggregated every hour based on the thresholds for HR and RR. As the device measures only two vital signs, the intervention was used in addition to the standard-of-care intermittent manual measurements. Based on the feasibility study findings, instead of using an alarm strategy, nurses routinely assessed current vital signs and their trends every 4 h (that is, twice per 8-h shift) without alarms29. At the end of every shift, they reported the D-EWS score, possible abnormalities, deviations and subsequent nursing activities in the electronic health record (EHR). A full description of the intervention and implementation strategy is reported elsewhere34.
Outcomes of interest
The primary outcome for the study was the effect of CMVS in hospital LOS in days. Discharge time before 2p.m. was considered as a 0.5-day based on routine workflows for operating room and ward bed capacity. Secondary postoperative outcomes were divided into in-hospital and post-discharge outcomes. In-hospital outcomes were: proportion of long LOS (defined as +1 standard deviation or third quartile or higher of the control group); rapid response team (RRT) calls; nurse-to-house-officer (HO) calls (defined as junior resident calls between 6 p.m. and 8 a.m.) regarding deviating vital signs; unplanned ICU admissions, ICU LOS, reoperations; mortality <30 days after surgery; severe complications (severity IIIa to V according to the Clavien–Dindo classification38); and postoperative unplanned CT or MRI scans. Post-discharge outcomes were: readmissions <30 days after discharge; days alive at home39 (DAH30); discharge disposition; and type and amount of required post-discharge nursing care.
All nursing activities that were initiated and performed based on the CMVS trend assessments were documented by the nurses. These were divided into performing additional checks or interventions in consultation with a physician. In addition, patients completed a questionnaire consisting of the acceptability intervention measurement (AIM), a patient-reported experience measurement about comfort of the sensor and recommendation score on a scale of 1–5, an overall score on a scale of 1–10 and free space for remarks. The AIM questionnaire consisted of four statements about acceptance on a 5-point Likert scale (score 1–5). A median score of ≥ 3.5 was defined as sufficient acceptability40.
The following patient characteristics were collected: gender, age, length, weight, BMA, ASA classification, procedure (laparoscopic or open), malignancy (none, solid tumour or metastasis), nutritional status (the short nutritional assessment questionnaire score with score ≥3 or higher as malnutrition35), smoking status (yes, no or prior), alcohol use (yes, no), preoperative haemoglobin (Hb), co-morbidities (Charlson Comorbidity Index (CCI) score ranging 0–12) and MEWS measurement frequency36,37. For the CMVS group, the distribution of D-EWS scores was presented.
Study size
Estimation of the sample size was calculated with MedCalc (MedCalc Software Ltd, Ostend, Belgium). A two-tailed alpha of 5%, power of 0.80 and LOS (in hours) with 150.1 (intervention) versus 187.7 h (control) in ratio 1:4, resulted in at least 180 patients required for the intervention group and 720 for the control group. LOS in the CMVS group was prospectively recorded, and LOS in the control group was derived from the hospital EHR. An additional 10% for potentially non-parametric testing resulted in at least 198 patients in the intervention group and 792 patients in the control group.
Statistical analysis
LOS was compared between the CMVS and control groups. Multiple imputation was performed to handle missing data when present. Normally distributed continuous data were presented as means and s.d. and tested with unpaired t-tests. Likewise, non-normally distributed data are presented as median and i.q.r. and were tested with Mann–Whitney U-tests. Normality was checked by the Kolmogorov–Smirnov test and visually by a Q-Q plot and histogram. Nominal data were presented with frequencies and percentages (n, %) and tested with χ² test or Fisher exact tests based on assumptions. Post-hoc analysis of the subgroups colorectal and HPB surgery were performed to compare outcomes between CMVS and control groups.
A multivariable analysis to determine impact of CMVS on log-transformed LOS was performed while controlling for gender, type of surgery (colorectal or HPB), procedure, Charlson Comorbidity Index, significant different co-morbidities, complications and group. Multicollinearity was present if the variance inflation factor was ≥5. Over time, effects were analysed by comparison of median LOS over years. All data were analysed with IBM SPSS Statistics 26 for Windows (IBM Armork, New York, USA) and P < 0.05 was considered significant.
Ethics
The Daily Board of the Medical Ethics Committee Isala reviewed the protocol (protocol 20211114) and declared the Medical Research Involving Human Subjects Act (also known by its Dutch abbreviation ‘WMO’) did not apply for the study. The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from patients participating in the post-implementation group. A waiver was provided for patients in the pre-implementation group.
Results
Study characteristics
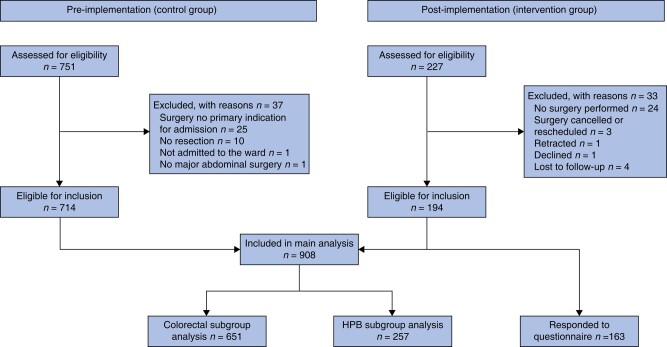
A total of 978 patients were screened and after exclusion, 908 were eligible for analysis: 714 controls and 194 intervention patients (Fig. 1). Proportion of ASA class 3–4 was higher in the CMVS group (35.1% versus 25.9%; P = 0.012) although the CCI score was lower for the CMVS group (5.2 versus 5.8; P = 0.004; Table 1). Table 1 presents the characteristics of all patients.
Fig. 1.
Flowchart of the study
HPB, hepatopancreatobiliary.
Table 1.
Patient characteristics
| Control group (n = 714) | CMVS group (n = 194) | P | |
|---|---|---|---|
| Sex | 0.313 | ||
| Male | 361 (50.6) | 106 (54.6) | |
| Female | 353 (49.4) | 88 (45.4) | |
| Age, mean (s.d.) | 66.8 (13.1) | 68.1 (11.9) | 0.236 |
| Length, mean (s.d.) | 172.8 (11.7) | 174.1 (10.2) | 0.163 |
| Weight, mean (s.d.) | 79.0 (15.9) | 80.5 (16.8) | 0.258 |
| BMI, mean (s.d.) | 26.3 (4.7) | 26.5 (4.7) | 0.607 |
| ASA classification | 0.012* | ||
| 1–2 | 529 (74.1) | 126 (64.9) | |
| 3–4 | 185 (25.9) | 68 (35.1) | |
| Type | 0.399 | ||
| Colorectal | 507 (71.0) | 143 (74.1) | |
| HPB | 207 (29.0) | 50 (25.9) | |
| Procedure | 0.299 | ||
| Laparoscopic | 552 (73.1) | 149 (76.8) | |
| Open | 192 (26.9) | 45 (23.3) | |
| Malignancy | 0.310 | ||
| No tumour | 126 (17.6) | 35 (18.0) | |
| Solid tumour | 480 (67.2) | 138 (71.1) | |
| Metastasis | 108 (15.1) | 21 (10.9) | |
| CCI, mean (s.d.) | 5.8 (2.8) | 5.2 (2.5) | 0.004* |
| Myocardial infarction | 67 (9.4) | 18 | 1.000 |
| COPD | 83 (11.6) | 10 (5.2) | 0.011* |
| Hypertension | 360 (50.4) | 46 (23.7) | 0.000* |
| Cerebral vascular accident | 88 (12.3) | 16 (8.2) | 0.128 |
| Chronic heart failure | 39 | 4 | 0.055 |
| Chronic kidney disease | 11 | 0 | 0.211 |
| Dementia | 4 | 2 | 0.614 |
| Connective tissue disease | 17 | 3 | 0.592 |
| Peptic ulcer disease | 9 | 2 | 1.000 |
| Liver disease | 6 | 0 | 0.351 |
| Hemiplegia | 3 | 1 | 1.000 |
| Leukaemia | 5 | 1 | 1.000 |
| Lymphoma | 23 | 1 | 0.074 |
| AIDS | 0 | 0 | n/a |
| Preoperative Hb, mean (s.d.) | 7.9 (1.1) | 7.7 (1.2) | 0.061 |
| Smoking status | 0.963 | ||
| No | 281 (39.4) | 75 (38.7) | |
| Prior | 353 (49.4) | 96 (49.5) | |
| Yes | 80 (11.2) | 23 (11.9) | |
| Alcohol use | 364 (51.0) | 88 (45.6) | 0.184 |
| Nutritional status | 0.161 | ||
| No malnourishment | 584 (81.8) | 167 (86.1) | |
| Malnourishment | 130 (18.2) | 27 (13.9) | |
| MEWS measurement frequency, median (i.q.r.) | 1.7 (1.3–4.1) | 1.6 (1.2–4.3) | 0.217 |
| Hours of CMVS, mean (s.d.) | n/a | 118 (105) | n/a |
| D-EWS | n/a | 17 176 | n/a |
| Score 0 | 3853 (22.4) | ||
| Score 1–2 | 12 670 (73.8) | ||
| Score 3 or higher | 653 (3.8) |
Values are n (%) unless otherwise stated. HPB, hepatopancreatobiliary; CCI, Charlson Comorbidity Index; CMVS, continuous vital sign monitoring; D-EWS, partial MEWS; Hb, haemoglobin; MEWS, Modified Early Warning Score; n/a, not applicable. *Statistically significant.
In the post-hoc subgroup analysis, several statistically significant baseline differences were present (Table 2). In the colorectal CMVS group, less rectal resections were performed and perioperative Hb and ASA classification were higher. In the HPB CMVS-group, more pancreas resections were performed, CCI score was lower, and there were more active smokers in comparison with the control group.
Table 2.
Patient characteristics of subgroups
| Colorectal surgery (n = 651) | HPB surgery (n = 257) | |||||
|---|---|---|---|---|---|---|
| Controls (n = 507) | CMVS (n = 144) | P | Control (n = 207) | CMVS (n = 50) | P | |
| Sex | 0.502 | 0.343 | ||||
| Male | 248 (48.9) | 75 (52.1) | 113 (54.6) | 31 (62.0) | ||
| Female | 259 (51.1) | 69 (47.9) | 94 (45.4) | 29 (58.0) | ||
| Age, mean (s.d.) | 67.0 (13.5) | 68.6 (12.2) | 0.201 | 66.3 (12.0) | 66.4 (11.0) | 0.962 |
| Length, mean (s.d.) | 172.7 (9.8) | 174.0 (10.3) | 0.188 | 173.0 (15.3) | 174.5 (9.8) | 0.522 |
| Weight, mean (s.d.) | 79.1 (16.1) | 80.9 (16.0) | 0.238 | 78.6 (15.6) | 79.4 (19.5) | 0.828 |
| BMI, mean (s.d.) | 26.4 (4.8) | 26.6 (4.4) | 0.553 | 26.0 (4.4) | 26.0 (5.6) | 0.948 |
| ASA classification | 0.008* | 0.449 | ||||
| 1–2 | 393 (77.5) | 96 (66.7) | 136 (65.7) | 30 (60.0) | ||
| 3–4 | 114 (22.5) | 48 (33.3) | 71 (34.4) | 20 (40.0) | ||
| Type | 0.011* | 0.019* | ||||
| Colon/liver | 366 (72.2) | 119 (82.6) | 100 (51.7) | 15 (70.0) | ||
| Rectal/pancreas | 141 (27.8) | 25 (17.4) | 107 (48.3) | 35 (30.0) | ||
| Procedure | 0.288 | 0.883 | ||||
| Laparoscopic | 441 (87.0) | 130 (90.3) | 81 (39.1) | 19 (38.0) | ||
| Open | 66 (13.0) | 14 (9.7) | 126 (60.9) | 31 (62.0) | ||
| Malignancy | 0.563 | 0.058 | ||||
| No tumour | 103 (20.3) | 26 (18.1) | 23 (11.1) | 9 (18.0) | ||
| Solid tumour | 382 (75.3) | 109 (75.7) | 98 (47.3) | 29 (58.0) | ||
| Metastasis | 22 (4.3) | 9 (6.9) | 86 (41.5) | 12 (24.0) | ||
| CCI, mean (s.d.) | 5.4 (2.6) | 5.1 (2.3) | 0.225 | 6.8 (3.0) | 5.5 (2.9) | 0.005* |
| Preoperative Hb, mean (s.d.) | 7.9 (1.1) | 7.7 (1.2) | 0.038* | 8.0 (1.1) | 8.0 (1.1) | 0.967 |
| Smoking status | 0.197 | 0.002* | ||||
| No | 202 (39.8) | 64 (44.4) | 79 (38.2) | 11 (22.0) | ||
| Prior | 244 (48.1) | 70 (48.6) | 109 (52.7) | 26 (52.0) | ||
| Yes | 61 (12.0) | 10 (7.0) | 19 (9.2) | 13 (26.0) | ||
| Alcohol use | 276 (54.4) | 70 (486) | 0.216 | 88 (42.5) | 19 (38.0) | 0.561 |
| Nutritional status | 0.239 | 0.581 | ||||
| No malnourishment | 439 (86.6) | 130 (90.3) | 145 (70.0) | 37 (74.0) | ||
| Malnourishment | 68 (13.4) | 14 (9.7) | 62 (30.0) | 13 (26.0) | ||
Values are n (%) unless otherwise stated. HPB, hepatopancreatobiliary; CMVS, continuous vital signs monitoring intervention; CCI, Charlson Comorbidity Index; Hb, haemoglobin. *Statistically significant.
Length of stay
Median (i.q.r.) LOS for the total CMVS group was 5.0 (3.5–8.6) days versus 5.5 (4.0–10.0) days in the control group (P = 0.012; Table 3). After controlling for patient and surgical characteristics with multivariate analysis, this difference was maintained with an unstandardized coefficient of −0.043 (95% c.i. −0.077 to −0.009). Except for gender and CCI score, all other variables in the model added statistically significantly to the prediction of LOS. Significant different co-morbidities such as hypertension and chronic obstructive pulmonary disease were added to the model in a separate analysis but did not change the outcome (Table S1).
Table 3.
Clinical outcomes of major abdominal surgery
| Control group (n = 714) | CMVS group (n = 194) | P | |
|---|---|---|---|
| Length of stay, median (i.q.r.) | 5.5 (4.0–10.0) | 5.0 (3.5–8.6) | 0.012‡* |
| In-hospital outcomes | |||
| Long LOS | 179 (25.1) | 40 (20.6) | 0.199 |
| Rapid response team calls | 2 | 3 | 0.068† |
| House Officer calls | 109 (15.3) | 15 (7.7) | 0.007‡ |
| ICU admissions | 17 | 3 | 0.592† |
| ICU LOS, median (i.q.r.) | 7.0 (3.0–18.5) | 3.0 (2.25–3.00) | 0.132a |
| Mortality | 5 | 1 | 1.000† |
| Reoperations | 53 (7.4) | 14 (7.2) | 0.922 |
| Unplanned diagnostics | |||
| CT | 96 (13.4) | 28 (14.4) | 0.722 |
| MRI | 5 | 0 | 0.590† |
| Complication rate | 70 (9.8) | 19 (9.8) | 0.997 |
| Complication severity | 83 (100.0) | 23 (100.0) | 0.808† |
| IIIa | 25 | 7 | 1.000† |
| IIIb | 41 | 13 | 0.545 |
| IVa | 8 | 2 | 1.000† |
| IVb | 6 | 0 | 0.336 |
| V | 3 | 1 | 1.000† |
| Post-discharge outcomes | |||
| Readmissions | 77 (10.8) | 25 (12.9) | 0.411 |
| Readmissions’ LOS, median (i.q.r.) | 6.5 (4.0–9.5) | 4.0 (2.0–7.0) | 0.014‡* |
| DAH30, median (i.q.r.) | 24.0 (18.9–26.0) | 24.5 (20.5–26.5) | 0.005‡* |
| Discharge disposition | |||
| Independent | 467 (65.4) | 116 (59.8) | 0.148 |
| Home care | 211 (29.6) | 75 (38.9) | 0.015‡ |
| Rehabilitation centre | 24 | 2 | 0.093 |
| Nursing home | 3 | 0 | 1.000† |
| Other ward | 5 | 0 | 0.590† |
| Deceased | 3 | 1 | 1.000† |
| Hospice | 1 | 0 | 1.000† |
| Frequency of post-hospital care | 0.123 | ||
| 1 | 152 (61.8) | 44 (61.1) | |
| 2 | 66 (26.8) | 25 (34.7) | |
| ≥ 3 | 28 (11.4) | 3 (4.2) | |
| Type of post-hospital care | |||
| ADL | 116 (16.2) | 33 (17.1) | 0.827 |
| Stoma | 114 (16.0) | 24 (12.4) | 0.259 |
| Medication | 77 (10.8) | 26 (13.5) | 0.307 |
| Wound care | 57 (8.0) | 19 (9.8) | 0.384 |
| Tube feeding | 17 | 6 | 0.605† |
| Drain care | 14 | 12 | 0.005‡ |
| Catheter | 13 | 6 | 0.263† |
| Other | 6 | 0 | 0.351 |
Values are n (%) unless otherwise stated. ADL, activities of daily living; LOS, length of stay; DAH30, days alive at home 30 post-surgery. *Mann–Whitney U-test. †Fisher Exact test. ‡Statistically significant.
Post-hoc subgroup analysis showed that in patients undergoing colorectal procedures, LOS in the CMVS group was lower than in the control group (median LOS 4.0 versus 4.5 days; P = 0.001). In the patients undergoing HPB surgery, median LOS was similar between the CMVS and control groups (9.0 versus 9.0 days; P = 0.754; Table 4). After multivariate analysis, this difference was maintained for the colorectal patients, whereas LOS remained similar in HPB patients (Table S1). No time effect was present (Supplementary Material, Table S2).
Table 4.
Clinical outcomes of subgroups
| Colorectal surgery (n = 651) | HPB surgery (n = 257) | |||||
|---|---|---|---|---|---|---|
| Control (n = 507) | CMVS (n = 144) | P | Control (n = 217) | CMVS (n = 50) | P | |
| Length of stay, median (i.q.r.) | 4.5 (3.5–7.5) | 4.0 (3.0–6.0) | 0.001‡* | 9.0 (6.0–15.0) | 9.0 (7.0–13.6) | 0.754* |
| In-hospital outcomes | ||||||
| Long LOS | 127 (25.0) | 25 (16.4) | 0.054* | 53 (25.6) | 11 (22.0) | 0.597 |
| Rapid response team | 2 | 2 | 0.214† | 0 | 1 | 0.195† |
| House Officer calls | 74 (14.6) | 12 (8.3) | 0.050 | 35 (16.9) | 3 (6.0) | 0.051 |
| ICU admissions | 9 | 2 | 0.1.000† | 8 | 1 | 1.000† |
| ICU LOS, median (i.q.r.) | 8.0 (3.0–15.0) | 3.0 (3.0–3.0) | 0.150* | 4.0 (2.3–18.8) | n/a | n/a |
| Mortality | 4 | 1 | 1.000† | 1 | 0 | 1.000† |
| Reoperations | 42 | 12 | 0.967 | 11 | 2 | 1.000† |
| Unplanned diagnostics | ||||||
| CT | 60 (11.8) | 17 (11.8) | 0.992 | 36 (17.4) | 11 (22.0) | 0.449 |
| MRI | 2 | 0 | 1.000† | 3 | 0 | 1.000† |
| Complication rate | 44 | 14 | 0.698 | 26 | 5 | 0.618 |
| Complication severity | 51 | 18 | 0.916† | 32 | 5 | 0.790† |
| IIIa | 9 | 4 | 0.730† | 16 | 3 | 1.000† |
| IIIb | 34 | 12 | 1.000† | 7 | 1 | 1.000† |
| IVa | 3 | 1 | 1.000† | 5 | 1 | 1.000† |
| IVb | 3 | 0 | 0.562† | 3 | 0 | 1.000† |
| V | 2 | 1 | 1.000† | 1 | 0 | 1.000† |
| Post-hospital outcomes | ||||||
| Readmissions | 55 (10.8) | 19 (13.2) | 0.434 | 22 | 6 | 0.780 |
| Readmissions, LOS, median (i.q.r.) | 6.3 (4.0–9.4) | 3.0 (2.0–7.5) | 0.014‡* | 6.5 (3.1–12.5) | 5.5 (2.8–8.5) | 0.397* |
| DAH30, median (i.q.r.) | 25.0 (21.0–26.5) | 25.5 (23.0–27.0) | 0.001‡* | 20.5 (14.0–24.0) | 20.8 (16.4–23.0) | 0.874* |
| Discharge disposition | ||||||
| Independent | 344 (67.9) | 94 (65.7) | 0.634 | 123 (59.4) | 21 (42.0) | 0.026‡ |
| Home care | 137 (27.0) | 46 (32.2) | 0.227 | 74 (35.7) | 29 (58.0) | 0.004‡ |
| Rehabilitation centre | 18 | 2 | 0.274 | 6 | 0 | 0.600† |
| Nursing home | 2 | 0 | 1.000† | 1 | 0 | 1.000† |
| Other ward | 3 | 0 | 1.000† | 2 | 0 | 1.000† |
| Deceased | 2 | 1 | 0.526† | 2 | 0 | 1.000† |
| Hospice | 1 | 0 | 1.000† | 0 | 0 | n/a |
| Frequency of post-hospital care | 0.223 | 0.635 | ||||
| 1 | 113 (66.9) | 26 (60.5) | 39 | 18 | ||
| 2 | 44 | 16 | 25 | 9 | ||
| ≥ 3 | 12 | 1 | 9 | 2 | ||
| Type of post-hospital care | ||||||
| ADL | 65 (12.8) | 19 (13.3) | 0.883 | 51 (24.6) | 14 (28.0) | 0.624 |
| Stoma | 107 (21.1) | 22 (15.4) | 0.130 | 7 | 2 | 0.688† |
| Medication | 33 | 15 | 0.108 | 44 | 11 | 0.908 |
| Tube feeding | 2 | 1 | 0.526† | 15 | 5 | 0.566† |
| Wound care | 25 | 13 | 0.061 | 32 | 6 | 0.536 |
| Drain care | 2 | 0 | 1.000† | 12 | 12 | 0.000‡† |
| Catheter care | 11 | 5 | 0.366† | 2 | 1 | 0.479† |
| Other | 3 | 0 | 1.000† | 3 | 0 | 1.000† |
Values are n (%) unless otherwise stated. ADL, activities of daily living; HPB, hepatopancreatobiliary; CMVS, continuous vital signs monitoring intervention; LOS, length of stay; DAH30, days alive at home 30 post-surgery. *Mann–Whitney U-test. †Fisher Exact test. ‡Statistically significant.
Secondary outcomes
In-hospital outcomes
The number/percentage of nurse-to-HO calls was significantly lower in the intervention group, 7.5% versus 15.3% in the control group (P = 0.007; Table 3) and in the subgroup analysis for both groups (8.3% versus 14.6%; P = 0.05 in colorectal patients and 16.9% versus 6.0%; P = 0.051 in HPB patients; Table 4). None of the other outcomes differed statistically significantly between the CMVS and control groups including complication rates, the number of RRT calls and unplanned ICU admissions. This was also true for the post-hoc subgroup analysis (Table 4). Although overall complication rates did not differ between groups, a non-significant increase in complication severity IIIb (49.4% to 56.5%) and decrease in severity IVb (from 7.2% to 0%) was observed. A trend towards a reduced median ICU LOS in the CMVS group (3.0 versus 8.0 days) was observed.
Post-discharge outcomes
DAH30 was higher in the CMVS group (median 24.5 versus 24.0 days; P = 0.005) and more patients were discharged with a need for home care (38.9% versus 29.6%; P = 0.015). Although the readmission rate did not differ between groups, LOS of readmissions was lower in the control group (median 6.5 versus 4.0 days; P = 0.014). None of the other outcomes were different between the intervention and control groups (Table 3). In the post-hoc subgroup analysis, DAH30 and LOS of readmissions were different only in colorectal patients (Table 4). In HPB patients, more patients in the CMVS group were discharged with a need for care (59.4% versus 42.0%; P = 0.026), resulting in more patients who were discharged with a need for home care (58.0% versus 35.7%; P = 0.004).
Performed nursing activities
Based on trends assessments, 109 nursing activities were performed in 68 patients (35.1%) of which 70 (64.2%) were performed independently by the nurse and 39 (35.8%) in consultation with a physician (Table 5). Nurses independently performed nine (8.3%) additional measurements and 61 additional patient assessments resulting in wait-and-see (56.0%). In consultation with a physician, 10 (9.2%) diagnostic and 28 (25.7%) therapeutic interventions were performed.
Table 5.
Performed nursing activities based on trend assessments
| Nursing activity | n (%) |
|---|---|
| Activity performed by nurse | 70 (100) |
| Patient assessment (wait-and-see) | 61 (87.1) |
| Addition manual check measurement with MEWS | 9 (12.9) |
| Interventions performed in consultation with a physician | 39 |
| Consulted physician but wait-and-see | 1 |
| Diagnostics | 10 |
| Blood test: blood culture | 2 |
| Chest X-ray | 2 |
| Electrocardiogram | 1 |
| CT scan | 3 |
| Blood test: arterial blood gas | 1 |
| Therapy | 28 |
| Analgesics | 11 |
| Oxygen supplementation | 4 |
| Bronchodilators | 4 |
| Fluid challenge | 3 |
| Beta-blockers | 1 |
| Diuretics | 2 |
| Breathing exercise | 2 |
| Digoxin | 1 |
MEWS, Modified Early Warning Score; X-ray, energetic high-frequency electromagnetic radiation.
Patient experiences
A total of 163 questionnaires were completed (84%). Of patients, 76.7% (n = 125) rated the intervention 8 out of 10 or higher resulting in a median satisfaction of 8.0 out of 10 (i.q.r. 8–9; Table 6). Of patients, 83.4% (n = 136) found the intervention acceptable, resulting in a median (i.q.r.) acceptability of 4 out of 5 (3.6–5.0). The majority of patients found the patch easy to wear (88.6%), felt safer (71.2%) and would wear the patch again (92.6%). There were no significant differences between colorectal and HPB groups.
Table 6.
Patient experience based on the questionnaire
| n = 163 | |||
|---|---|---|---|
| Acceptability score, range 0–5, median (i.q.r.) | 4.0 (3.75–5.0) | ||
| Satisfaction rating, range 0–10, median (i.q.r.) | 8.0 (8.0–9.0) | ||
| Disagree (1–2) | Neutral (3) | Agree (4–5) | |
| Comfort, range 0–5, median (i.q.r.) | 6 (3.7) | 11 (6.8) | 145 (88.9) |
| Feeling safer, range 0–5, median (i.q.r.) | 9 (5.5) | 38 (23.3) | 116 (71.2) |
| Wear again, range 0–5, median (i.q.r.) | 2 (1.2) | 10 (6.1) | 151 (92.6) |
In addition, patients made 47 remarks (Table S3). There were statements about the desire to have more insight into their own vital signs measurements and the results and impact of the CMVS intervention. Furthermore, most patients mentioned they were not bothered at all by wearing the sensor. In contrast, several patients mentioned negative aspects of the wearability of the sensor about it being too hard, especially when laying on their side in bed, and the need for replacement when diagnostic tests had to be done. Also, patients mentioned an increased feeling of safety by wearing the sensor.
Discussion
In this study the effects of CMVS on the general ward on LOS and a broad range of other clinical outcomes in major abdominal surgery patients were explored. Adequate implementation of the CMVS intervention on the surgical ward was previously demonstrated and reported34. The results of the current study show that the addition of CMVS to the standard care was associated with a small, but statistically significant reduction in LOS. Besides, in the CMVS group, the number of nurse-to-HO calls was significantly reduced (15% to 8%). Based on trends assessments, 35% of patients received additional nursing activities. Patients highly accepted the CMVS intervention.
In the post-hoc subgroup analysis, the association of CMVS with reduced LOS was maintained in the colorectal group but not in the HPB group. This may be explained by a difference in the post-operative complication profile. Both surgery types may be complicated by anastomotic leaks, intra-abdominal abscess, or bleeding, all of which are accompanied by deviating vital signs41. In the HPB group, however, pancreatic resections result in delayed gastric emptying in 10–30% of patients, which delays normal oral intake and significantly prolongs LOS, but is not associated with deviating vital signs42,43.
Importantly, LOS in colorectal surgery has been significantly reduced since the introduction of Enhanced Recovery After Surgery (ERAS) protocols over a decade ago44. In this study the ERAS protocols were unchanged and strictly applied throughout the entire study period (including historical controls). The study period coincided in part with the coronavirus disease 2019 (COVID-19) pandemic, but this did not affect outcomes, because the care for elective major abdominal (mostly oncological) surgery patients was not affected in the hospital, as they were given priority over all other usual care.
Even though the observed reduction in LOS may suggest that CMVS has enabled more rapid detection and intervention in case of clinical deterioration, no significant differences were found in complication rate, complication severity, RRT calls, ICU admissions and ICU LOS. This study may not have had sufficient statistical power to determine differences in these rare outcomes. Nonetheless, a non-significant trend towards less-severe complications was noted in the CMVS group, which could have been the result of additional interventions triggered by early detection. Also, in the CMVS group we observed a trend towards a reduced median ICU LOS (3.0 versus 8.0 days), which is closely associated with the severity of complications45.
LOS is considered an important indicator for assessing the efficiency of hospital management, quality of patient care and functional evaluation46. From the point of view of the healthcare provider, a shorter LOS results in lower medical costs and increases bed capacity, which is especially important in times of scarcity as during the COVID-19 pandemic and ongoing nursing shortages47–49. However, early discharge may increase the need for home care and other resource utilization, which must be accounted for in total healthcare cost estimations48. This is supported by the finding showing increased use of home care in the CMVS group.
Given the successful implementation of CMVS in this study, nurses may have been more attentive to vital signs monitoring, resulting in proactive assessment of the patient condition allowing for accurate and thorough nursing care. In fact, 35% of patients received additional nursing activities based on trends assessments, including interventions such as optimizing analgesia, which may have contributed to timely patient discharge and reduced LOS. Also, fewer nurse-to-HO calls for both groups during evening and night shifts after implementation of CMVS were observed, which is important because it may reduce the burden on the on-call physicians50,51. Although this decrease is difficult to explain based on this study, it is possible that abnormalities were noticed earlier and were adequately dealt with by nurses, obviating the need for care escalation to physician on-call.
The present study used a proactive method of trend assessment every 4 h as opposed to a reactive method of threshold-based alarms for trend monitoring. In a previous feasibility study in the same hospital, an active alarm strategy impaired nurse acceptance and compliance, possibly due to alarm fatigue25,28,29. The optimal frequency of vital sign measurements on general wards is unknown, but should be high enough to detect early changes in vital signs well before the onset of life-threatening events52. Routine monitoring vital sign trends every 4 h without alarms may be considered adequate to detect vital sign trend deviations indicating an imminent systemic inflammatory response syndrome caused by postoperative complications53–55. More frequent monitoring assessments may not be needed, as vital signs monitoring in the general ward setting is not aimed at detecting severe acute events such as cardiac arrest.
Besides clinical outcome measures, other positive effects of CMVS on patient-centred outcomes are important to consider when assessing the utility of CMVS (or considering the pros and cons of implementing CMVS as standard care). For instance, this study shows that patient satisfaction and acceptance of the CMVS was very high. The perceived feeling of safety and high comfort of the sensor should be considered an important patient-reported outcome for the implementation of CMVS, especially considering these outcome measures scored much higher than other wearable devices in previous studies in which significant proportions prematurely discontinued the CMVS56,57. Comparison of results with prior studies is complicated given the heterogeneity in study designs, patient populations and outcomes and, more importantly, because of the wide range of different CMVS interventions, with regard to sensors, alarm strategy and follow-up of deviating vital signs.
In comparison with other literature, the results from previous studies on the impact of CMVS on LOS are diverse. In this study, the reduction in hospital LOS was modest but statistically significant. Two other before–after studies, in relatively large cohorts of medical and surgical patients with comparable LOS, did not show a significant reduction by CMVS56,58. Interestingly, one of these studies reported beneficial results for patients on unplanned ICU admissions and RRT calls56. One meta-analysis covering five studies showed a non-significant weighted mean reduction in LOS of 0.09 days59. One possible explanation for these results is that the incidence of major adverse events was rare, and therefore had no impact on median LOS. Another meta-analysis, covering three studies comparable to this study, did show a trend towards a reduction of LOS by a weighted mean reduction of 3.3 days21. However, confidence intervals were wide (−8.8 to 2.2 days) and therefore this meta-analysis failed to demonstrate a significant association between CMVS and LOS. A recent before–after study with a comparable intervention also showed a significant LOS reduction of 0.7 days60. However, the mean LOS in that study was twice as long as our colorectal group (8.0 days versus 4.0 days), which is not considered state-of-the-art when using ERAS protocols. Another study with a bed-based continuous monitoring device, measuring the same two vital signs, was consistent with the results of this study, showing a significant reduction in LOS of 0.4 days58. However, these patients could not be monitored during mobilization on the ward (only when supine).
Finally, the CMVS patch device used in this study was found to be highly acceptable to patients. This outcome was in line with previous studies using disposable finger probes or patches as devices23,58. In contrast, in another study, in 21% of patients a wrist-worn device was prematurely removed, indicating patient acceptability was relatively low compared to the patch device worn in our study56.
Important strengths of this study are the selective inclusion of highly complex abdominal surgery (colorectal and HPB), robust characterization of patient characteristics (including CCI and ASA scores) and an array of clinical outcome data, as well as the significant length of the study period. However, several limitations should be considered when interpreting the results. First, due to the before–after design, time trends and unobserved confounding factors may have affected changes in the outcomes and it precludes strong inferences regarding causal effects. An RCT therefore may be more suitable. On the other hand, the chosen design enabled assessment of the impact under ‘real-world conditions’, ensuring results may be better translatable to clinical practice than a RCT30,61. In fact, the complex nature of the CMVS intervention and implementation also impedes randomization of two different vital signs monitoring protocols in parallel on the same ward but could also lower the consent rate62,63. Furthermore, RCTs are costly and time-consuming, so not ideal for rapidly developing eHealth technologies such as CMVS systems24,64. Importantly, during the entire study period no changes were made in patient management or policies in, for example, EWS system and early discharge (ERAS) protocols. Although compliance with the ERAS items was not systematically measured, the hypothesis is that the ERAS items are routinely completed for all patients and that significant changes in compliance are unlikely. Additionally, no significant changes over time in median LOS data of historical controls during the study period were found. Furthermore, despite the COVID-19 pandemic, the clinical care and workflow for major abdominal surgery patients was unaffected and continued in a similar fashion in the hospital. Also, besides patient-, diagnosis- and intervention-related factors, LOS is determined by multiple factors unrelated to clinical outcomes such as discharge delay due to rehabilitation or home care capacity shortages66–72. Given the sizable control group and prolonged study period, it is assumed any variations in these factors were adequately controlled for in this study.
Furthermore, the study was limited to elective surgery and not emergency surgery. It is conceivable that the effects of the intervention are larger in emergency surgery patients given that postoperative complications occur more frequently in this group65. The study was conducted in a single hospital setting, and the results might not be generalizable to other institutions or types of hospitals.
There are also several statistical limitations. Given the exploratory nature of the study, many outcome measures were measured and compared. The multiple outcomes might have caused statistical multiplicity. For this reason, they should be interpreted as exploratory and Ps are presented to three decimal places. In addition, the calculated sample size was not completely reached and this study may not have had sufficient statistical power to detect differences in rare outcomes (such as unplanned ICU admissions, RRT calls and ICU LOS). This is especially true for subgroup analysis.
Despite the promising findings, more robust prospective multicentre studies are needed to establish the true added value of CMVS for clinical care and analyse its causal effects on general wards. Such prospective trials should include a simultaneous evaluation of the quality and success of CMVS implementation, which is essential before any clinical value can be established. Analysis of the follow-up nursing activities on deviating trends and its consequences should also be included in such studies rather than just focusing on major patient outcomes such as complication severity, RRT calls, unplanned ICU admissions or mortality. All the proactive nursing activities collectively may contribute to the prevention of more serious complications and prolonged hospitalization times.
As an alternative to proactive trend assessment, machine-learning algorithms may be developed that provide reliable personalized clinical decision support tools to facilitate correct and timely interpretation of vital signs trends. This may contribute to the development of highly efficient alarm strategies. This will prevent unnecessary diagnostic procedures and overtreatment by reducing the number of irrelevant and false-positive alarms and may improve workflow efficiency on the ward52,53,73,74.
In addition, future availability of advanced and validated multiparameter CMVS wireless sensors, which are sufficiently accurate, patient-friendly and comprehensive, may allow the discontinuation of standard manual vital sign measurements by nurses, especially because measuring other vital signs (for example, body temperature and blood oxygen saturation) are still relevant for adequate detection of surgical complications. This may not only improve clinical outcomes to a greater extent56 but also reduce nursing workload and increase efficiency of inpatient care, which seems important for successful implementation of wearable CMVS systems on the ward31.
Considering that inpatient hospital stays are becoming increasingly shorter, postoperative complications and clinical deterioration will inevitably occur more frequently at home75. Therefore, continuing CMVS after discharge—which is possible with the sensor used in the study—may be considered to allow monitoring and timely detection at home76. This may further lower barriers for safe early discharge. In addition, functionality of providing patients with insight into their own vital signs via an app may generate more patient involvement in their own health and benefit recovery.
In conclusion, CMVS using wearable wireless sensors and proactive trend assessments was associated with a significant decrease in length of stay for colorectal surgery patients but not for HPB surgery patients. Although all other clinical outcomes were similar in both groups, a non-significant trend towards less-severe complications and reduced ICU LOS was noted in the CMVS group. CMVS with the sensor used in this study was highly accepted by patients. It is important to note that CMVS triggered additional nursing activities such as patient assessments and therapeutic interventions, which may eventually result in attenuation of the severity of postoperative complications. Future studies should focus on additional interventions prompted by CMVS and its consequences in carefully selected patient groups with a relatively high risk of deterioration to establish the causal effects of CMVS and enhance the quality and safety of postoperative care.
Supplementary Material
Acknowledgements
The authors would like to thank all patients and nurses for participation in the study, Dr Mireille Edens for auditing on the data analysis, Research Department Athena Care for their support in the data collection and Philips Healthcare and Isala I&I for technical preparations.
Contributor Information
Jobbe P L Leenen, Department of Surgery, Isala, Zwolle, The Netherlands; Connected Care Centre, Isala, Zwolle, The Netherlands; Research Group IT Innovations in Healthcare, Windesheim University of Applied Sciences, Zwolle, The Netherlands.
Vera Ardesch, Flex pool department, Isala, Zwolle, The Netherlands.
Cor J Kalkman, Department of Anaesthesiology, University Medical Centre Utrecht, Utrecht University, Utrecht, The Netherlands.
Lisette Schoonhoven, Julius Centre for Health Sciences and Primary Care, University Medical Centre Utrecht, Utrecht University, Utrecht, The Netherlands; School of Health Sciences, Faculty of Environmental and Life Sciences, University of Southampton, Southampton, UK.
Gijs A Patijn, Department of Surgery, Isala, Zwolle, The Netherlands; Connected Care Centre, Isala, Zwolle, The Netherlands.
Author contributions
Jobbe Leenen (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing—original draft, Writing—review & editing), Vera Ardesch (Data curation, Formal analysis, Validation, Writing—review & editing), Cor Kalkman (Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Writing—original draft, Writing—review & editing), Lisette Schoonhoven (Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Writing—original draft, Writing—review & editing), and Gijs Patijn (Conceptualization, Data curation, Funding acquisition, Methodology, Supervision, Validation, Writing—original draft, Writing—review & editing).
Funding
The Isala Innovation & Science Funds (grant number INNO2016) funded the study and Transitiegelden (Project ID2021-0012) financially supported procurement of the wearable sensors.
Disclosure
None of the authors has conflicting interests. The manufacturer of the CMVS system (Philips Healthcare, Eindhoven, The Netherlands) did not play a role in the design, implementation, interpretation or reporting of the study. Sensors were provided at a reduced price for the study.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The data set of this study is available upon request by contacting the corresponding author.
References
- 1. Jakobson T, Karjagin J, Vipp L, Padar M, Parik A-H, Starkopf L et al. Postoperative complications and mortality after major gastrointestinal surgery. Medicina (Kaunas) 2014;50:111–117 [DOI] [PubMed] [Google Scholar]
- 2. Simões CM, Carmona MJC, Hajjar LA, Vincent J-L, Landoni G, Belletti A et al. Predictors of major complications after elective abdominal surgery in cancer patients. BMC Anesthesiol 2018;18:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Čečka F, Jon B, Čermáková E, Šubrt Z, Ferko A. Impact of postoperative complications on clinical and economic consequences in pancreatic surgery. Ann Surg Treat Res 2016;90:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Law WL, Choi HK, Lee YM, Ho JWC. The impact of postoperative complications on long-term outcomes following curative resection for colorectal cancer. Ann Surg Oncol 2007;14:2559–2566 [DOI] [PubMed] [Google Scholar]
- 5. Straatman J, Cuesta MA, de Lange-de Klerk ESM, van der Peet DL. Hospital cost-analysis of complications after major abdominal surgery. Dig Surg 2015;32:150–156 [DOI] [PubMed] [Google Scholar]
- 6. Healy MA, Mullard AJ, Campbell DAJ, Dimick JB. Hospital and payer costs associated with surgical complications. JAMA Surg 2016;151:823–830 [DOI] [PubMed] [Google Scholar]
- 7. Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DAJ. Hospital costs associated with surgical complications: a report from the private-sector national surgical quality improvement program. J Am Coll Surg 2004;199:531–537 [DOI] [PubMed] [Google Scholar]
- 8. Vonlanthen R, Slankamenac K, Breitenstein S, Puhan MA, Muller MK, Hahnloser D et al. The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg 2011;254:907–913 [DOI] [PubMed] [Google Scholar]
- 9. Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet (London, England) 2012;380:1059–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tevis SE, Kennedy GD. Postoperative complications and implications on patient-centered outcomes. J Surg Res 2013;181:106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown SR, Mathew R, Keding A, Marshall HC, Brown JM, Jayne DG. The impact of postoperative complications on long-term quality of life after curative colorectal cancer surgery. Ann Surg 2014;259:916–923 [DOI] [PubMed] [Google Scholar]
- 12. Ludikhuize J, Smorenburg SM, de Rooij SE, de Jonge E. Identification of deteriorating patients on general wards; measurement of vital parameters and potential effectiveness of the Modified Early Warning Score. J Crit Care 2012;27:424.e7–424.e13 [DOI] [PubMed] [Google Scholar]
- 13. Sessler DI, Saugel B. Beyond “failure to rescue”: the time has come for continuous ward monitoring. Br J Anaesth 2019;122:304–306 [DOI] [PubMed] [Google Scholar]
- 14. Churpek MM, Yuen TC, Edelson DP. Predicting clinical deterioration in the hospital: the impact of outcome selection. Resuscitation 2013;84:564–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahrens T. The most important vital signs are not being measured. Aust Crit Care 2008;21:3–5 [DOI] [PubMed] [Google Scholar]
- 16. Eddahchouri Y, Koeneman M, Plokker M, Brouwer E, van de Belt TH, van Goor H et al. Low compliance to a vital sign safety protocol on general hospital wards: a retrospective cohort study. Int J Nurs Stud 2021;115:103849. [DOI] [PubMed] [Google Scholar]
- 17. Hands C, Reid E, Meredith P, Smith GB, Prytherch DR, Schmidt PE et al. Patterns in the recording of vital signs and early warning scores: compliance with a clinical escalation protocol. BMJ Qual Saf 2013;22:719–726 [DOI] [PubMed] [Google Scholar]
- 18. Downey CL, Tahir W, Randell R, Brown JM, Jayne DG. Strengths and limitations of early warning scores: a systematic review and narrative synthesis. Int J Nurs Stud 2017;76:106–119 [DOI] [PubMed] [Google Scholar]
- 19. Brown H, Terrence J, Vasquez P, Bates DW, Zimlichman E. Continuous monitoring in an inpatient medical-surgical unit: a controlled clinical trial. Am J Med 2014;127:226–232 [DOI] [PubMed] [Google Scholar]
- 20. Weenk M, van Goor H, Frietman B, Engelen LJ, van Laarhoven CJ, Smit J et al. Continuous monitoring of vital signs using wearable devices on the general ward: pilot study. JMIR mHealth uHealth 2017;5:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun L, Joshi M, Khan SN, Ashrafian H, Darzi A. Clinical impact of multi-parameter continuous non-invasive monitoring in hospital wards: a systematic review and meta-analysis. J R Soc Med 2020;113:217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watkinson PJ, Barber VS, Price JD, Hann A, Tarassenko L, Young JD. A randomised controlled trial of the effect of continuous electronic physiological monitoring on the adverse event rate in high risk medical and surgical patients. Anaesthesia 2006;61:1031–1039 [DOI] [PubMed] [Google Scholar]
- 23. Leenen JPL, Leerentveld C, van Dijk JD, van Westreenen HDL, Schoonhoven L, Patijn GA. Current evidence for continuous vital signs monitoring by wearable wireless devices in hospitalized adults: systematic review. J Med Internet Res 2020;22:e18636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Michard F, Sessler DI. Ward Monitoring 3.0. Br J Anaesth 2018;121:999–1001 [DOI] [PubMed] [Google Scholar]
- 25. Leenen JPL, Dijkman EM, van Hout A, Kalkman CJ, Schoonhoven L, Patijn GA. Nurses’ experiences with continuous vital sign monitoring on the general surgical ward: a qualitative study based on the behaviour change wheel. BMC Nurs 2022;21:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Areia C, King E, Ede J, Young L, Tarassenko L, Watkinson P et al. Experiences of current vital signs monitoring practices and views of wearable monitoring: a qualitative study in patients and nurses. J Adv Nurs 2022;78:810–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Rossum M, Leenen J, Kingma F, Breteler M, van Hillegersberg R, Ruurda J et al. Expectations of continuous vital signs monitoring for recognizing complications after esophagectomy: interview study among nurses and surgeons. JMIR Perioper Med 2021;4:e22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leenen JPL, Dijkman EM, van Dijk JD, van Westreenen HL, Kalkman C, Schoonhoven L et al. Feasibility of continuous monitoring of vital signs in surgical patients on a general ward: an observational cohort study. BMJ Open 2021;11:e042735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leenen JPL, Rasing HJM, van Dijk JD, Kalkman CJ, Schoonhoven L, Patijn GA. Feasibility of wireless continuous monitoring of vital signs without using alarms on a general surgical ward: a mixed methods study. PLoS One 2022;17:e0265435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50:217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leenen JPL, Rasing HJM, Kalkman CJ, Schoonhoven L, Patijn GA. Process evaluation of a wireless wearable continuous vital signs monitoring intervention in 2 general hospital wards: mixed methods study. JMIR Nurs 2023;6:e44061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. von Elm E, Altman D, Egger M, Pocock S, Gøtzsche P, Vandenbroucke J et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duus CL, Aasvang EK, Olsen RM, Sørensen HBD, Jørgensen LN, Achiam MP et al. Continuous vital sign monitoring after major abdominal surgery—quantification of micro events. Acta Anaesthesiol Scand 2018;62:1200–1208 [DOI] [PubMed] [Google Scholar]
- 34. Leenen JPL, Rasing H, Kalkman CJ, Schoonhoven L, Patijn GA. Process evaluation of a wireless wearable continuous vital signs monitoring intervention in 2 general hospital wards: mixed methods study. JMIR Nurs 2023;6:e44061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kruizenga HM, Seidell JC, de Vet HCW, Wierdsma NJ, van Bokhorst-de van der Schueren MAE. Development and validation of a hospital screening tool for malnutrition: the Short Nutritional Assessment Questionnaire (SNAQ). Clin Nutr 2005;24:75–82 [DOI] [PubMed] [Google Scholar]
- 36. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 37. de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. A critical review of available methods. J Clin Epidemiol 2003;56:221–229 [DOI] [PubMed] [Google Scholar]
- 38. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–196 [DOI] [PubMed] [Google Scholar]
- 39. Bell M, Eriksson LI, Svensson T, Hallqvist L, Granath F, Reilly J et al. Days at home after surgery: an integrated and efficient outcome measure for clinical trials and quality assurance. EClinicalMedicine 2019;11:18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weiner BJ, Lewis CC, Stanick C, Powell BJ, Dorsey CN, Clary AS et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci 2017;12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kirchhoff P, Clavien P-A, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg 2010;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ho C-K, Kleeff J, Friess H, Büchler MW. Complications of pancreatic surgery. HPB Off J Int Hepato Pancreato Biliary Assoc 2005;7:99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mohammed S, Van Buren Ii G, McElhany A, Silberfein EJ, Fisher WE. Delayed gastric emptying following pancreaticoduodenectomy: incidence, risk factors, and healthcare utilization. World J Gastrointest Surg 2017;9:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dutch Institute for Clinical Auditing (DICA) . DICA Jaarrapport 2021 [Internet]. Leiden; 2022. Available from: https://dica.nl/media/2970/DICAjaarrapportage2021.pdf
- 45. Vlayen A, Verelst S, Bekkering GE, Schrooten W, Hellings J, Claes N. Incidence and preventability of adverse events requiring intensive care admission: a systematic review. J Eval Clin Pract 2012;18:485–497 [DOI] [PubMed] [Google Scholar]
- 46. Collins TC, Daley J, Henderson WH, Khuri SF. Risk factors for prolonged length of stay after major elective surgery. Ann Surg 1999;230:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Berger E, Winkelmann J, Eckhardt H, Nimptsch U, Panteli D, Reichebner C et al. A country-level analysis comparing hospital capacity and utilisation during the first COVID-19 wave across Europe. Health Policy 2022;126:373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rotter T, Kinsman L, James E, Machotta A, Gothe H, Willis J et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev 2010;3:CD006632. [DOI] [PubMed] [Google Scholar]
- 49. Bueno H, Ross JS, Wang Y, Chen J, Vidán MT, Normand S-LT et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA 2010;303:2141–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hassan M, Hussain T, Ahmed SM, Fraz TR, Rehmat Z. Perceived stress and stressors among house officers. Indian J Occup Environ Med 2014;18:145–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hayes CW, Rhee A, Detsky ME, Leblanc VR, Wax RS. Residents feel unprepared and unsupervised as leaders of cardiac arrest teams in teaching hospitals: a survey of internal medicine residents. Crit Care Med 2007;35:1668–1672 [DOI] [PubMed] [Google Scholar]
- 52. Weenk M, Bredie S, Koeneman M, Hesselink G, van Goor H, van de Belt T. Continuous monitoring of vital signs in the general ward using wearable devices: randomized controlled trial. J Med Internet Res 2020;22:e15471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van Rossum MC, Vlaskamp LB, Posthuma LM, Visscher MJ, Breteler MJM, Hermens HJ et al. Adaptive threshold-based alarm strategies for continuous vital signs monitoring. J Clin Monit Comput 2021;36:407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brekke IJ, Puntervoll LH, Pedersen PB, Kellett J, Brabrand M. The value of vital sign trends in predicting and monitoring clinical deterioration: a systematic review. PLoS One 2019;14:e0210875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Churpek MM, Adhikari R, Edelson DP. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation 2016;102:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Eddahchouri Y, Peelen R V, Koeneman M, Touw HRW, van Goor H, Bredie SJH. Effect of continuous wireless vital sign monitoring on unplanned ICU admissions and rapid response team calls: a before-and-after study. Br J Anaesth 2022;128:857–863 [DOI] [PubMed] [Google Scholar]
- 57. Downey C, Randell R, Brown J, Jayne D. Continuous versus intermittent vital signs monitoring using a wearable, wireless patch in patients admitted to surgical wards: pilot cluster randomized controlled trial. J Med Internet Res 2018;20:e10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Taenzer AH, Pyke JB, McGrath SP, Blike GT. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology 2010;112:282–287 [DOI] [PubMed] [Google Scholar]
- 59. Areia C, Biggs C, Santos M, Thurley N, Gerry S, Tarassenko L et al. The impact of wearable continuous vital sign monitoring on deterioration detection and clinical outcomes in hospitalised patients: a systematic review and meta-analysis. Crit Care 2021;25:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vroman H, Mosch D, Eijkenaar F, Naujokat E, Mohr B, Medic G et al. Continuous vital sign monitoring in patients after elective abdominal surgery: a retrospective study on clinical outcomes and costs. J Comp Eff Res 2023;2:e220176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Saugel B, Joosten A, Scheeren TWL. Perioperative goal-directed therapy: what’s the best study design to investigate its impact on patient outcome? J Clin Monit Comput 2019;33:361–363 [DOI] [PubMed] [Google Scholar]
- 62. Minary L, Trompette J, Kivits J, Cambon L, Tarquinio C, Alla F. Which design to evaluate complex interventions? Toward a methodological framework through a systematic review. BMC Med Res Methodol 2019;19:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jacques RM, Ahmed R, Harper J, Ranjan A, Saeed I, Simpson RM et al. Recruitment, consent and retention of participants in randomised controlled trials: a review of trials published in the National Institute for Health Research (NIHR) journals library (1997–2020). BMJ Open 2022;12:e059230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mohr DC, Schueller SM, Riley WT, Brown CH, Cuijpers P, Duan N et al. Trials of intervention principles: evaluation methods for evolving behavioral intervention technologies. J Med Internet Res 2015;17:e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mullen MG, Michaels AD, Mehaffey JH, Guidry CA, Turrentine FE, Hedrick TL et al. Risk associated with complications and mortality after urgent surgery vs elective and emergency surgery: implications for defining “quality” and reporting outcomes for urgent surgery. JAMA Surg 2017;152:768–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kulinskaya E, Kornbrot D, Gao H. Length of stay as a performance indicator: robust statistical methodology. IMA J Manag Math 2005;16:369–381 [Google Scholar]
- 67. Brown EG, Yang A, Canter RJ, Bold RJ. Outcomes of pancreaticoduodenectomy: where should we focus our efforts on improving outcomes? JAMA Surg 2014;149:694–699 [DOI] [PubMed] [Google Scholar]
- 68. Mazmudar A, Castle J, Yang AD, Bentrem DJ. The association of length of hospital stay with readmission after elective pancreatic resection. J Surg Oncol 2018;118:7–14 [DOI] [PubMed] [Google Scholar]
- 69. Lingsma HF, Bottle A, Middleton S, Kievit J, Steyerberg EW, Marang-van de Mheen PJ. Evaluation of hospital outcomes: the relation between length-of-stay, readmission, and mortality in a large international administrative database. BMC Health Serv Res 2018;18:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Han TS, Murray P, Robin J, Wilkinson P, Fluck D, Fry CH. Evaluation of the association of length of stay in hospital and outcomes. Int J Qual Heal Care 2021;34:mzab160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shea S, Sideli R V, DuMouchel W, Pulver G, Arons RR, Clayton PD. Computer-generated informational messages directed to physicians: effect on length of hospital stay. J Am Med Inform Assoc 1995;2:58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stone K, Zwiggelaar R, Jones P, Mac Parthaláin N. A systematic review of the prediction of hospital length of stay: towards a unified framework. PLOS Digit Heal 2022;1:1–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Antink CH, Leonhardt S, Walter M. Reducing false alarms in the ICU by quantifying self-similarity of multimodal biosignals. Physiol Meas 2016;37:1233–1252 [DOI] [PubMed] [Google Scholar]
- 74. Eerikainen LM, Vanschoren J, Rooijakkers MJ, Vullings R, Aarts RM. Reduction of false arrhythmia alarms using signal selection and machine learning. Physiol Meas 2016;37:1204–1216 [DOI] [PubMed] [Google Scholar]
- 75. Zhuang C-L, Ye X-Z, Zhang X-D, Chen B-C, Yu Z. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum 2013;56:667–678 [DOI] [PubMed] [Google Scholar]
- 76. Leenen JPL, Ardesch V, Patijn GA. Remote home monitoring of continuous vital sign measurements by wearables in patients discharged after colorectal surgery: observational feasibility study. JMIR Perioper Med 2023;6:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data set of this study is available upon request by contacting the corresponding author.
References
- 1. Jakobson T, Karjagin J, Vipp L, Padar M, Parik A-H, Starkopf L et al. Postoperative complications and mortality after major gastrointestinal surgery. Medicina (Kaunas) 2014;50:111–117 [DOI] [PubMed] [Google Scholar]
- 2. Simões CM, Carmona MJC, Hajjar LA, Vincent J-L, Landoni G, Belletti A et al. Predictors of major complications after elective abdominal surgery in cancer patients. BMC Anesthesiol 2018;18:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Čečka F, Jon B, Čermáková E, Šubrt Z, Ferko A. Impact of postoperative complications on clinical and economic consequences in pancreatic surgery. Ann Surg Treat Res 2016;90:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Law WL, Choi HK, Lee YM, Ho JWC. The impact of postoperative complications on long-term outcomes following curative resection for colorectal cancer. Ann Surg Oncol 2007;14:2559–2566 [DOI] [PubMed] [Google Scholar]
- 5. Straatman J, Cuesta MA, de Lange-de Klerk ESM, van der Peet DL. Hospital cost-analysis of complications after major abdominal surgery. Dig Surg 2015;32:150–156 [DOI] [PubMed] [Google Scholar]
- 6. Healy MA, Mullard AJ, Campbell DAJ, Dimick JB. Hospital and payer costs associated with surgical complications. JAMA Surg 2016;151:823–830 [DOI] [PubMed] [Google Scholar]
- 7. Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DAJ. Hospital costs associated with surgical complications: a report from the private-sector national surgical quality improvement program. J Am Coll Surg 2004;199:531–537 [DOI] [PubMed] [Google Scholar]
- 8. Vonlanthen R, Slankamenac K, Breitenstein S, Puhan MA, Muller MK, Hahnloser D et al. The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg 2011;254:907–913 [DOI] [PubMed] [Google Scholar]
- 9. Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet (London, England) 2012;380:1059–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tevis SE, Kennedy GD. Postoperative complications and implications on patient-centered outcomes. J Surg Res 2013;181:106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown SR, Mathew R, Keding A, Marshall HC, Brown JM, Jayne DG. The impact of postoperative complications on long-term quality of life after curative colorectal cancer surgery. Ann Surg 2014;259:916–923 [DOI] [PubMed] [Google Scholar]
- 12. Ludikhuize J, Smorenburg SM, de Rooij SE, de Jonge E. Identification of deteriorating patients on general wards; measurement of vital parameters and potential effectiveness of the Modified Early Warning Score. J Crit Care 2012;27:424.e7–424.e13 [DOI] [PubMed] [Google Scholar]
- 13. Sessler DI, Saugel B. Beyond “failure to rescue”: the time has come for continuous ward monitoring. Br J Anaesth 2019;122:304–306 [DOI] [PubMed] [Google Scholar]
- 14. Churpek MM, Yuen TC, Edelson DP. Predicting clinical deterioration in the hospital: the impact of outcome selection. Resuscitation 2013;84:564–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahrens T. The most important vital signs are not being measured. Aust Crit Care 2008;21:3–5 [DOI] [PubMed] [Google Scholar]
- 16. Eddahchouri Y, Koeneman M, Plokker M, Brouwer E, van de Belt TH, van Goor H et al. Low compliance to a vital sign safety protocol on general hospital wards: a retrospective cohort study. Int J Nurs Stud 2021;115:103849. [DOI] [PubMed] [Google Scholar]
- 17. Hands C, Reid E, Meredith P, Smith GB, Prytherch DR, Schmidt PE et al. Patterns in the recording of vital signs and early warning scores: compliance with a clinical escalation protocol. BMJ Qual Saf 2013;22:719–726 [DOI] [PubMed] [Google Scholar]
- 18. Downey CL, Tahir W, Randell R, Brown JM, Jayne DG. Strengths and limitations of early warning scores: a systematic review and narrative synthesis. Int J Nurs Stud 2017;76:106–119 [DOI] [PubMed] [Google Scholar]
- 19. Brown H, Terrence J, Vasquez P, Bates DW, Zimlichman E. Continuous monitoring in an inpatient medical-surgical unit: a controlled clinical trial. Am J Med 2014;127:226–232 [DOI] [PubMed] [Google Scholar]
- 20. Weenk M, van Goor H, Frietman B, Engelen LJ, van Laarhoven CJ, Smit J et al. Continuous monitoring of vital signs using wearable devices on the general ward: pilot study. JMIR mHealth uHealth 2017;5:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun L, Joshi M, Khan SN, Ashrafian H, Darzi A. Clinical impact of multi-parameter continuous non-invasive monitoring in hospital wards: a systematic review and meta-analysis. J R Soc Med 2020;113:217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watkinson PJ, Barber VS, Price JD, Hann A, Tarassenko L, Young JD. A randomised controlled trial of the effect of continuous electronic physiological monitoring on the adverse event rate in high risk medical and surgical patients. Anaesthesia 2006;61:1031–1039 [DOI] [PubMed] [Google Scholar]
- 23. Leenen JPL, Leerentveld C, van Dijk JD, van Westreenen HDL, Schoonhoven L, Patijn GA. Current evidence for continuous vital signs monitoring by wearable wireless devices in hospitalized adults: systematic review. J Med Internet Res 2020;22:e18636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Michard F, Sessler DI. Ward Monitoring 3.0. Br J Anaesth 2018;121:999–1001 [DOI] [PubMed] [Google Scholar]
- 25. Leenen JPL, Dijkman EM, van Hout A, Kalkman CJ, Schoonhoven L, Patijn GA. Nurses’ experiences with continuous vital sign monitoring on the general surgical ward: a qualitative study based on the behaviour change wheel. BMC Nurs 2022;21:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Areia C, King E, Ede J, Young L, Tarassenko L, Watkinson P et al. Experiences of current vital signs monitoring practices and views of wearable monitoring: a qualitative study in patients and nurses. J Adv Nurs 2022;78:810–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Rossum M, Leenen J, Kingma F, Breteler M, van Hillegersberg R, Ruurda J et al. Expectations of continuous vital signs monitoring for recognizing complications after esophagectomy: interview study among nurses and surgeons. JMIR Perioper Med 2021;4:e22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leenen JPL, Dijkman EM, van Dijk JD, van Westreenen HL, Kalkman C, Schoonhoven L et al. Feasibility of continuous monitoring of vital signs in surgical patients on a general ward: an observational cohort study. BMJ Open 2021;11:e042735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leenen JPL, Rasing HJM, van Dijk JD, Kalkman CJ, Schoonhoven L, Patijn GA. Feasibility of wireless continuous monitoring of vital signs without using alarms on a general surgical ward: a mixed methods study. PLoS One 2022;17:e0265435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50:217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leenen JPL, Rasing HJM, Kalkman CJ, Schoonhoven L, Patijn GA. Process evaluation of a wireless wearable continuous vital signs monitoring intervention in 2 general hospital wards: mixed methods study. JMIR Nurs 2023;6:e44061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. von Elm E, Altman D, Egger M, Pocock S, Gøtzsche P, Vandenbroucke J et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duus CL, Aasvang EK, Olsen RM, Sørensen HBD, Jørgensen LN, Achiam MP et al. Continuous vital sign monitoring after major abdominal surgery—quantification of micro events. Acta Anaesthesiol Scand 2018;62:1200–1208 [DOI] [PubMed] [Google Scholar]
- 34. Leenen JPL, Rasing H, Kalkman CJ, Schoonhoven L, Patijn GA. Process evaluation of a wireless wearable continuous vital signs monitoring intervention in 2 general hospital wards: mixed methods study. JMIR Nurs 2023;6:e44061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kruizenga HM, Seidell JC, de Vet HCW, Wierdsma NJ, van Bokhorst-de van der Schueren MAE. Development and validation of a hospital screening tool for malnutrition: the Short Nutritional Assessment Questionnaire (SNAQ). Clin Nutr 2005;24:75–82 [DOI] [PubMed] [Google Scholar]
- 36. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 37. de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. A critical review of available methods. J Clin Epidemiol 2003;56:221–229 [DOI] [PubMed] [Google Scholar]
- 38. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–196 [DOI] [PubMed] [Google Scholar]
- 39. Bell M, Eriksson LI, Svensson T, Hallqvist L, Granath F, Reilly J et al. Days at home after surgery: an integrated and efficient outcome measure for clinical trials and quality assurance. EClinicalMedicine 2019;11:18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weiner BJ, Lewis CC, Stanick C, Powell BJ, Dorsey CN, Clary AS et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci 2017;12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kirchhoff P, Clavien P-A, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg 2010;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ho C-K, Kleeff J, Friess H, Büchler MW. Complications of pancreatic surgery. HPB Off J Int Hepato Pancreato Biliary Assoc 2005;7:99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mohammed S, Van Buren Ii G, McElhany A, Silberfein EJ, Fisher WE. Delayed gastric emptying following pancreaticoduodenectomy: incidence, risk factors, and healthcare utilization. World J Gastrointest Surg 2017;9:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dutch Institute for Clinical Auditing (DICA) . DICA Jaarrapport 2021 [Internet]. Leiden; 2022. Available from: https://dica.nl/media/2970/DICAjaarrapportage2021.pdf
- 45. Vlayen A, Verelst S, Bekkering GE, Schrooten W, Hellings J, Claes N. Incidence and preventability of adverse events requiring intensive care admission: a systematic review. J Eval Clin Pract 2012;18:485–497 [DOI] [PubMed] [Google Scholar]
- 46. Collins TC, Daley J, Henderson WH, Khuri SF. Risk factors for prolonged length of stay after major elective surgery. Ann Surg 1999;230:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Berger E, Winkelmann J, Eckhardt H, Nimptsch U, Panteli D, Reichebner C et al. A country-level analysis comparing hospital capacity and utilisation during the first COVID-19 wave across Europe. Health Policy 2022;126:373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rotter T, Kinsman L, James E, Machotta A, Gothe H, Willis J et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev 2010;3:CD006632. [DOI] [PubMed] [Google Scholar]
- 49. Bueno H, Ross JS, Wang Y, Chen J, Vidán MT, Normand S-LT et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA 2010;303:2141–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hassan M, Hussain T, Ahmed SM, Fraz TR, Rehmat Z. Perceived stress and stressors among house officers. Indian J Occup Environ Med 2014;18:145–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hayes CW, Rhee A, Detsky ME, Leblanc VR, Wax RS. Residents feel unprepared and unsupervised as leaders of cardiac arrest teams in teaching hospitals: a survey of internal medicine residents. Crit Care Med 2007;35:1668–1672 [DOI] [PubMed] [Google Scholar]
- 52. Weenk M, Bredie S, Koeneman M, Hesselink G, van Goor H, van de Belt T. Continuous monitoring of vital signs in the general ward using wearable devices: randomized controlled trial. J Med Internet Res 2020;22:e15471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van Rossum MC, Vlaskamp LB, Posthuma LM, Visscher MJ, Breteler MJM, Hermens HJ et al. Adaptive threshold-based alarm strategies for continuous vital signs monitoring. J Clin Monit Comput 2021;36:407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brekke IJ, Puntervoll LH, Pedersen PB, Kellett J, Brabrand M. The value of vital sign trends in predicting and monitoring clinical deterioration: a systematic review. PLoS One 2019;14:e0210875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Churpek MM, Adhikari R, Edelson DP. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation 2016;102:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Eddahchouri Y, Peelen R V, Koeneman M, Touw HRW, van Goor H, Bredie SJH. Effect of continuous wireless vital sign monitoring on unplanned ICU admissions and rapid response team calls: a before-and-after study. Br J Anaesth 2022;128:857–863 [DOI] [PubMed] [Google Scholar]
- 57. Downey C, Randell R, Brown J, Jayne D. Continuous versus intermittent vital signs monitoring using a wearable, wireless patch in patients admitted to surgical wards: pilot cluster randomized controlled trial. J Med Internet Res 2018;20:e10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Taenzer AH, Pyke JB, McGrath SP, Blike GT. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology 2010;112:282–287 [DOI] [PubMed] [Google Scholar]
- 59. Areia C, Biggs C, Santos M, Thurley N, Gerry S, Tarassenko L et al. The impact of wearable continuous vital sign monitoring on deterioration detection and clinical outcomes in hospitalised patients: a systematic review and meta-analysis. Crit Care 2021;25:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vroman H, Mosch D, Eijkenaar F, Naujokat E, Mohr B, Medic G et al. Continuous vital sign monitoring in patients after elective abdominal surgery: a retrospective study on clinical outcomes and costs. J Comp Eff Res 2023;2:e220176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Saugel B, Joosten A, Scheeren TWL. Perioperative goal-directed therapy: what’s the best study design to investigate its impact on patient outcome? J Clin Monit Comput 2019;33:361–363 [DOI] [PubMed] [Google Scholar]
- 62. Minary L, Trompette J, Kivits J, Cambon L, Tarquinio C, Alla F. Which design to evaluate complex interventions? Toward a methodological framework through a systematic review. BMC Med Res Methodol 2019;19:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jacques RM, Ahmed R, Harper J, Ranjan A, Saeed I, Simpson RM et al. Recruitment, consent and retention of participants in randomised controlled trials: a review of trials published in the National Institute for Health Research (NIHR) journals library (1997–2020). BMJ Open 2022;12:e059230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mohr DC, Schueller SM, Riley WT, Brown CH, Cuijpers P, Duan N et al. Trials of intervention principles: evaluation methods for evolving behavioral intervention technologies. J Med Internet Res 2015;17:e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mullen MG, Michaels AD, Mehaffey JH, Guidry CA, Turrentine FE, Hedrick TL et al. Risk associated with complications and mortality after urgent surgery vs elective and emergency surgery: implications for defining “quality” and reporting outcomes for urgent surgery. JAMA Surg 2017;152:768–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kulinskaya E, Kornbrot D, Gao H. Length of stay as a performance indicator: robust statistical methodology. IMA J Manag Math 2005;16:369–381 [Google Scholar]
- 67. Brown EG, Yang A, Canter RJ, Bold RJ. Outcomes of pancreaticoduodenectomy: where should we focus our efforts on improving outcomes? JAMA Surg 2014;149:694–699 [DOI] [PubMed] [Google Scholar]
- 68. Mazmudar A, Castle J, Yang AD, Bentrem DJ. The association of length of hospital stay with readmission after elective pancreatic resection. J Surg Oncol 2018;118:7–14 [DOI] [PubMed] [Google Scholar]
- 69. Lingsma HF, Bottle A, Middleton S, Kievit J, Steyerberg EW, Marang-van de Mheen PJ. Evaluation of hospital outcomes: the relation between length-of-stay, readmission, and mortality in a large international administrative database. BMC Health Serv Res 2018;18:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Han TS, Murray P, Robin J, Wilkinson P, Fluck D, Fry CH. Evaluation of the association of length of stay in hospital and outcomes. Int J Qual Heal Care 2021;34:mzab160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shea S, Sideli R V, DuMouchel W, Pulver G, Arons RR, Clayton PD. Computer-generated informational messages directed to physicians: effect on length of hospital stay. J Am Med Inform Assoc 1995;2:58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stone K, Zwiggelaar R, Jones P, Mac Parthaláin N. A systematic review of the prediction of hospital length of stay: towards a unified framework. PLOS Digit Heal 2022;1:1–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Antink CH, Leonhardt S, Walter M. Reducing false alarms in the ICU by quantifying self-similarity of multimodal biosignals. Physiol Meas 2016;37:1233–1252 [DOI] [PubMed] [Google Scholar]
- 74. Eerikainen LM, Vanschoren J, Rooijakkers MJ, Vullings R, Aarts RM. Reduction of false arrhythmia alarms using signal selection and machine learning. Physiol Meas 2016;37:1204–1216 [DOI] [PubMed] [Google Scholar]
- 75. Zhuang C-L, Ye X-Z, Zhang X-D, Chen B-C, Yu Z. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum 2013;56:667–678 [DOI] [PubMed] [Google Scholar]
- 76. Leenen JPL, Ardesch V, Patijn GA. Remote home monitoring of continuous vital sign measurements by wearables in patients discharged after colorectal surgery: observational feasibility study. JMIR Perioper Med 2023;6:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]