ABSTRACT
Objective
The aim of this systematic review was to update the current level of evidence for spinal manipulation in influencing various biochemical markers in healthy and/or symptomatic population.
Methods
This is a systematic review update. Various databases were searched (inception till May 2023) and fifteen trials (737 participants) that met the inclusion criteria were included in the review. Two authors independently screened, extracted and assessed the risk of bias in included studies. Outcome measure data were synthesized using standard mean differences and meta-analysis for the primary outcome (biochemical markers). The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) was used for assessing the quality of the body of evidence for each outcome of interest.
Results
There was low-quality evidence that spinal manipulation influenced various biochemical markers (not pooled). There was low-quality evidence of significant difference that spinal manipulation is better (SMD −0.42, 95% CI − 0.74 to −0.1) than control in eliciting changes in cortisol levels immediately after intervention. Low-quality evidence further indicated (not pooled) that spinal manipulation can influence inflammatory markers such as interleukins levels post-intervention. There was also very low-quality evidence that spinal manipulation does not influence substance-P, neurotensin, oxytocin, orexin-A, testosterone and epinephrine/nor-epinephrine.
Conclusion
Spinal manipulation may influence inflammatory and cortisol post-intervention. However, the wider prediction intervals in most outcome measures point to the need for future research to clarify and establish the clinical relevance of these changes.
KEYWORDS: Spinal Manipulation, Biochemical Markers, Pain Markers, Inflammatory Markers, Cortisol
Introduction
Spinal manipulation (SM) is a specific hands-on approach used by several different healthcare disciplines commonly for the intended purposes of reducing spinal pain and reducing disability [1–5]. Early theories on the mechanisms of therapeutic effects following SM centered within a biomechanical paradigm. According to the biomechanical model, an SM can cause changes in the biomechanics of the spine which allows it to function in a more optimal state [6,7]. However, accumulating evidence clearly demonstrates a shift toward a neurophysiological paradigm [8–25]. According to the neurophysiological paradigm, a mechanical input such as an SM may trigger a cascade of neurophysiological response at both spinal and supraspinal levels [7,10,14,24].
Pain modulation following SM is a net result of complex neural interactions between various physiological systems involving different biochemical mediators [26]. Several neuropeptides such as substance-P (SP), neurotensin, oxytocin and orexin-A influence pain modulation through widespread effects in the nervous system [27,28]. As these chemicals are primarily released at the injury site, they also influence the initiation of inflammatory process. This in turn results in the production of numerous pro-inflammatory and immuno-regulatory cytokines and neurotransmitters (e.g., tumor necrosis factor α (TNF-α); interleukins (IL)) [29,30]. Furthermore, endogenous opioids (ex: -endorphins); hormones (e.g. cortisol) and catecholamine’s (epinephrine and nor-epinephrine) modulate several immune parameters associated with the inflammatory process [31–33].
It has been hypothesized that SM activates the liberation of various biochemical markers such as SP, TNF-α from neural tissues resulting in its hypoalgesia and/or anti-inflammatory effects [34]. This is based on evidence that have demonstrated that SM can influence biochemical markers such as SP [35] neurotensin and oxytocin; -endorphins [10] and hormones such as cortisol [15,36]. A systematic review undertaken by our team previously established a ‘moderate’ level evidence that SM may influence various biochemical markers following SM [37]. Specifically, SM can increase substance-p, neurotensin, oxytocin and interleukin levels and may influence cortisol levels post-intervention [37].
Our previous systematic review [37] employed valid methods and has been widely cited suggesting that our review is current and topical. Further, since the publication of our review, there has been significant interest in this topic area with several new studies published. Taking into consideration these factors and a possibility that the level of evidence may change with the findings from new studies, we considered that it was timely to provide an update of our systematic review as recommended previously [38,39].
The aim of this systematic review update was to provide an update on:
The effects of SM on biochemical markers in humans.
Establish the level of evidence for changes in biochemical biomarkers following an SM.
Operational definitions
Systematic review update: The update of a systematic review is defined as ‘a new edition of a published systematic review with changes that can include new data, new methods, or new analysis to the previous version’ [38]. This may include the following: updating the search; updating risk of bias tools; synthesis of new papers; adjusting the conclusions of a review [39].
Biochemical Markers: For the purpose of this systematic review update, biochemical markers were classified into the following three categories: (1) neuropeptides (2) inflammatory and (3) endocrine biomarkers.
Methods
This review has been reported based on Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [40]. The review protocol was prospectively registered on the International Prospective Register of Systematic Reviews (PROSPERO: CRD42016049473).
Types of studies
Randomized controlled trials (RCT) or controlled clinical trials that involved humans (healthy or painful), measured biochemical markers were eligible for this review. Only articles published in English language were included. Further, published conference abstracts, pilot studies and dissertations were excluded.
Types of participants
Studies involving humans were eligible. There were no restrictions based on age, gender and severity of pain.
Types of intervention
The intervention of interest was SM provided either by a physiotherapist, osteopath, or chiropractor. SM is defined as a high-velocity, low-amplitude thrust technique that is often associated with a cavitation [41]. The comparator (control) group could be any of the following: no intervention, usual care group, GP care, sham therapy or any other therapy.
Types of outcome(s)
The outcome measures of interest included the following biochemical markers: (1) neuropeptides (e.g. neurotensin, oxytocin, SP) (2) inflammatory (e.g. TNF, IL) and (3) endocrine (e.g. cortisol, epinephrine, nor-epinephrine) biomarkers from any body fluids.
Search strategy
In consultation with a librarian, it was decided that the previous search strategy was relevant and no changes were required. A replacement approach as recommended by Cochrane was utilized where the previous review was used as one source of studies. A bibliographic search (Table 1) was performed through the following databases: Medline, AMED, EMBASE, CINAHL, SPORTSDiscus, PubMed, Cochrane Library, Web of Science, Physiotherapy Evidence Database, and SCOPUS (from inception till May 2023).
Table 1.
Search strategy.
| Phase 1 | Phase 2 | Phase 3 |
|---|---|---|
|
|
|
Data management
Articles obtained by the systematic search in the above-mentioned databases were exported to Covidence (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia; www.covidence.org) and managed in Covidence throughout the review process.
Study selection
Duplicates were automatically detected and removed by Covidence. However, one reviewer (KSK) went through the titles to ensure all duplicates were removed. Full texts of the remaining articles were then screened by two independent reviewers (KSK and LT). Any disagreements between reviewers at any stage of the selection process were resolved through consensus and discussion. A third reviewer was available if required.
Data extraction and management
Three reviewers (KSK, JDR and LT) collected data independently from included studies using a standardized data collection form in Covidence. The following were extracted: (1) study characteristics: funding, settings, design and country (2) patient characteristics: age, gender, severity of condition (if applicable) (3) intervention characteristics: number of intervention groups, content of each intervention (4) Outcome/data results: outcome measures (biomarkers) used, time points used and duration of follow-up (Table 2). Any disagreements were resolved by reaching a consensus.
Table 2.
Characteristics of included studies.
| Author, year | Methods/participant characteristics | Intervention | Outcome Measure(s)/ time points | Findings | Notes |
|---|---|---|---|---|---|
| Achalandabaso2014 | 3 groups RCT | Placebo SM vs SM (cervical-Thoracic) | Blood samples (plasma and serum) | No changes in any of the studied damage markers | |
| Randomized: 30 healthy subjects volunteers | Placebo SM Control group: n=10 received following the cervical manipulation protocol with regard to hand contact, without intention of mobilization, nor application of tissue tension by the operator | CPK, LDH, CRP, Troponin-I, Myoglobin, NSE, aldolaseBefore and right after intervention and 2 hours after | |||
| Gender: 16 male – 14 female subjects | Cervical group: n=10 received HVLA thrust at C4 and C5 cervical spine in supine, with left rotation and right-side bending | ||||
| Age: 27.6 - 29.8 - 28.6 (y, mean 3 groups Ctrl, Cerv, Th) | Thoracic Manipulation: n=10 received HVLA thrust at levels T3-T4 and T4-T5 | ||||
| Settings: healthy students from the University of Jaen | |||||
| Brennan 1991 | 3 groups RCT. | SM (vs) sham (vs) soft tissue | plasma concentration | ↑SP in SM group | Funded by a grant from the Foundation for Chiropractic Education and Research. |
| Randomised: 99 healthy volunteers. | SMT group: 42 participants received a thoracic SMT (T1 to aT6). | CBC | |||
| Gender: 67 males, 32 females | Sham group: 38 participants received sham manipulation (low velocity, low amplitude thrust). | SP | |||
| Age: 26.2 (mean) | Soft tissue group: 19 participants received soft tissue manipulation to either the left or right gluteal area. | 15 minutes pre and 15 minutes post-intervention | |||
| Setting: Research department, Chiropractic college. | |||||
| Christian 1987 | 4 groups RCT. | Pain-free SM group (vs.) pain SM group (vs.) pain-free sham group (vs.) pain sham group. | Plasma samples | No changes in any outcome measures | Supported by a grant-in aid from NHMRC, Australia |
| Randomised: 40. 20 with pain and 20 pain-free. | Pain-free SM group: 10 asymptomatic participants received chiropractic SMT. | Cortisol | |||
| Gender: only male participants | Pain SM group: 10 participants with pain received chiropractic SMT. | ACTH | |||
| Age: 18 to 30 (range) | Pain-free sham group: 10 asymptomatic participants received sham intervention where a very slight pressure was exerted on the neck. | β-endorphin | |||
| Setting: chiropractic teaching clinic | Pain sham group: 10 participants with pain received sham intervention. | Pre-intervention, 5 and 30 minutes post-intervention. | |||
| Duarte2022 | 3 groups RCT99 healthy young adults mostly chiropractic studentsGender: 10 female – 89 maleAge: 25.6 years (mean)Setting: Canadian Memorial Chiropractic College Simulation Laboratory and Life Science Laboratory | Spinal manipulation therapy vs ControlSingle interventionControl (preload only): n= 33Single thoracic SMT with a total peak force of 400N: n=33Single thoracic SMT with a total peak force of 800N: n=33 | 14 different inflammatory biomarkers (pro, anti, dual role, chemokine, and growth factor) was assessed by multiplex arrayGM-CSFIFN-γIL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-13, IL-17A, IL-23TNF-α | Select plasma pro-inflammatory and dual-role cytokines were elevated by higher compared to lower SMT force btw-group (800N vs 400N) difference was observed on interferon-gamma, IL-5, IL-6, while a within-group difference (800N: immediately vs 20 minutes post-intervention) was observed on IL-6 | This research project was funded by the Internal Research Support Fund at Canadian Memorial Chiropractic College |
| Kovanur Sampath2017 | RCT:2 Groups | Thoracic SM vs Sham | Salivary Cortisol | Thoracic SM resulted in an immediate decrease in salivary cortisol concentration and reduced T/C ratio 6h after intervention. | Funded by a grant from the New Zealand Manipulative Physiotherapists Association |
| 24 healthy men | SM: n=12 received HVLA thrust at T5 vertebra | Salivary Testosterone | SM did not differentially alter oxyhemoglobin, testosterone, or HRV vs responses in the sham group | ||
| Age: 18-45 y | upon expiration (single thrust) | T/C Ratio | |||
| Setting: Controlled laboratory study | Sham: n=12 same setup without HVLA thrust | HRV | |||
| Oxyhemoglobin concentration (right calf muscle) | |||||
| Before, at 5 minutes, 30 minutes and approximately 6 hours after intervention | |||||
| Kovanur Sampath2021 | Randomized 2-sequence, 2-period crossover trial | SM vs sham 2 session in cross-over | Salivary samples | Statistically significant condition by time interaction was found for the T/C ratio (mean difference: −0.16;CI:−0.33 to 0.006: P < .05) and TOI (mean difference: 1.35; CI:−1.3 to 4.1: P < .05) of calf muscle but not for Achilles tendon (P = .6); No difference was found for heart rate variability (P = .5) | Funded by a grant from the New Zealand Manipulative Physiotherapists Association |
| 24 participants with Achilles tendinopathy >3mo | Sequence 1 (sham intervention and then thoracic spinal manipulation) or sequence 2 (thoracic spinal manipulation and then sham intervention) | T/C Ratio (Salivary samples) | |||
| Age: 48 ±7 y | Session duration : 10 seconds | HRV (/ECG) | |||
| Gender: Male: 10 ; Female: 14 | SM: n=24 received thoracic spinal manipulation HVLA on T5 vertebra upon expiration | Total oxygenation index calf muscle and Achille tendon (/near-infrared spectroscopy) | |||
| Setting: University Laboratory with washout period of 1week | Sham intervention: n=24 received same setup, not place a fixating hand against thoracic spine and without HVLA thrust | TC Ratio: Pre-intervention, at 5 minutes, 30 minutes, and 6 hours post-intervention | |||
| Lohman2019 | RCT 2 groups | Cervical SM vs sham CSM | Serum concentration using the Milliplex Map Magnetic Bead Panel Immunoassay on the Luminex 200 Platform | CSM group, significant increases in pre vs post-manipulation mean oxytocin (154.5±60.1 vs 185.1±75.6, p= .012); neurotensin (116.0±26.5 vs.136.4±34.1, p< . 001); orexin A (52.2±31.1 vs 73.8±38.8, p< .01) but no significant differences in mean cortisol (p= .052) (Serum concentration) | Supported by Loma Linda University. |
| Randomized 28 female subjects with non-specific mechanical neck pain | One session | Oxytocin | |||
| Age: 37.1 – 30.1 (CSM – Sham) | CSM: n=13 received a cervical spine manipulation HVLA thrust in rotation | Neurotensin | |||
| Setting: Loma Linda University | Sham CSM: n=15 received sham CSM without moving the individual or carrying out the final thrust procedure | Orexin A | |||
| Cortisol | |||||
| Molina-Ortega 2014 | 3 groups RCT | Control (vs) Cervical SM (vs) Thoracic SM | Serum samples | ↑SP, ↑PPT in CSM group | |
| Randomised: 30 healthy volunteers | Control group: 10 participants received no intervention. | NO2 | No effects on NO2 | ||
| Gender: 16 male, 14 female | Cervical manipulation group: 10 participants received cervical manipulation. | SP | |||
| Age: 27.8 (mean) | Thoracic manipulation group: 10 participants received thoracic manipulation. | PPT (Algometer) | |||
| Setting: University Research Department | Pre-intervention, immediately after and 2 hours post-intervention. | ||||
| Pascual-Vaca2017 | Randomized controlled blinded clinical study | The experimental group (EG, n=23) received a spinal manipulation of the thoracolumbar junction, and the control group (CG, n=23) received a sham procedure | PPT algometer (spinous process T10 to L1 and quadratus Lumbarum)Urinary pH | significant changes in PPT in both quadratus lumborum (P<0.001) as well as in the spinous processes of all of the evaluated levels (P<0.05). No changes in urinary pH were observed (P=0.419) | |
| 46 patients suffering from renal lithiasis ; 27 men (59%) and 19 women (41%) with an average age of 38.5 (SD=6.80) and a Body Mass Index (BMI) of 25.07 (SD=3.12) | EG: High speed movement with low amplitude, bilaterally on T12-L1 at the end of ROM rotating patient | Pre-Post (immediately after intervention) | |||
| Settings/location: Nephrology Departments of 2 hospitals and one private consultancy of physiotherapy in Valencia (Spain) | CG: The therapist placed one hand on the sacrum and the other hand on the middle thoracic region, without performing any action for 90 seconds. A rest time of 10 minutes was also taken before taking the post intervention measurements. | ||||
| Plaza-Manzano 2014 | 3 groups RCT. | Control (vs.) cervical manipulation (vs.) thoracic manipulation | Serum samples | ↑neurotensin, ↑oxytocin in CSM and TSM groups immediately. | |
| Randomised: 30 healthy participants. | Control group: 10 participants received no intervention. | neurotensin | ↑cortisol in CSM group immediately. | ||
| Gender: 16 males, 14 females | Cervical manipulation group: 10 participants received cervical manipulation. | oxytocin | No changes in orexin-A. | ||
| Age: 27.8 (mean) | Thoracic manipulation group: 10 participants received thoracic manipulation. | orexin A | |||
| Setting: University Research setting. | cortisol. | ||||
| Samples were collected before, immediately after and 2 hours after manipulation. | |||||
| Puhl 2012 | 2 group RCT. | SMT (vs.) Sham. | Plasma samples | No changes in E or NE levels. | Only 36 included in final analysis. |
| Randomised: 56 healthy participants. | SMT group: 18 participants received a thoracic SMT. | NE | 2 subjects developed adverse reaction (vertigo) post-randomisation during catheter insertion. | ||
| Gender: 19 males, 17 females. | Sham group: 18 participants received sham manipulation (identical setup like SMT but without the thrust). | E | No adverse events after intervention | ||
| Age: 26.1(mean). | Pre-intervention, immediately after and 15 minutes post-intervention. | Funded by Research division, Canadian Memorial Chiropractic College | |||
| Setting: Chiropractic teaching clinic. | |||||
| Teodorczyk-Injeyan 2006 | 3 groups RCT. | SMT (vs.) Sham (vs.) control. | Serum samples | ↓ IL-1ß No effects on TNF-α or SP | Funded by Public Health Services Grant, Canada |
| Randomised: 64, healthy participants | SMT group: 24 participants received a thoracic SMT. | TNF-α | |||
| Gender: 28 males, 36 females | Sham group: 20 participants received sham manipulation (identical setup like SMT but without the thrust). | SP | |||
| Age: 24.7 (mean) | Control group: participants (n=20) did not receive any treatment. | IL-1 | |||
| Setting: Chiropractic College | Pre-intervention, 20 minutes and 2 hours post-intervention. | ||||
| Teodorczyk-Injeyan 2010 | 3 groups RCT. | SMT-C (vs.) SMT-NC (vs.) control. | Serum samples | ↑IgG, ↑IgM in SM-C group at 20-minutes and 2 hours post-intervention. | Funded by Public Health Services Grant, Canada |
| Randomised: 74 healthy participants | SM with cavitation group: 27 participants received a thoracic SMT with an audible cavitation. | PBMC | |||
| Gender: 31 males, 43 females | SM without cavitation: 25 participants received sham manipulation (identical setup like SMT but without cavitation). | IgG | |||
| Age: 24.7 (mean) | Control group: participants (n=22) in this group did not receive any treatment. | IgM | |||
| Setting: Chiropractic College | Pre-intervention, 20 minutes and 2 hours post-intervention. | ||||
| Valera-Calero2019 | 3 groups RCT | Cervical manipulation vs cervical mobilization vs sham manipulation in patients with chronic mechanical neck pain. | Salivary cortisol levelsPre-Post intervention | A significant and comparable increase in cortisol levels was observed immediately after cervical manipulation and mobilization (both P<0.001) | |
| 83 patients with chronic mechanical neck pain | Cervical spine manipulation (n=28) velocity, mid-range, left rotational force to C5-C6, with right side bending and left rotation | Reduced neck pain and decreased disability immediately after manipulation. | |||
| Age: Mean±SD | Cervical mobilization (n=28) grade III postero-anterior joint oscillatory mobilization technique applied to the articular pillar of C5/6 on the subject’s symptomatic side | ||||
| cMAN 35.64±8.11 | Sham manipulation (n=27) eliminated the joint preload and thrust component | ||||
| cMOB 37.25±10.54 | |||||
| Sham 36.96±8.89 | |||||
| Gender : 51 women, 32 men | |||||
| Setting: University of Alcala de Henares: outpatient (referrals from office workers) | |||||
| Whelan 2002 | 3 groups RCT. | Control Group: 10 participants were just supine lying. No manipulation or vertebral positioning done. | Salivary samples | No effects on basal cortisol levels. | Supported by New York Research Committee |
| Randomised: 30 healthy student volunteers | Sham group: 10 participants were lying supine with their cervical spine positioned but without any manipulation. | Cortisol | |||
| Gender: only male participants | CM Group: An upper cervical manipulation was performed on 10 participants. | 5 consecutive weeks. | |||
| Age: unavailable | Week-1: 5 consecutive days. | ||||
| Setting: Research Department, Chiropractic college. | Week 2-5: pre-intervention, 5 and 60 minutes after intervention |
Note: ACTH – Adreno-Corticotropic Hormone, C – Control, CSM – Cervical Spinal Manipulation, I – Intervention, Ig – Immunoglobulin, IL – Interleukin, NO2 – Nitric Oxide, PBMC – Peripheral Blood Mononuclear Cells, PPT – Pressure Pain Threshold, SM – Spinal Manipulation, SM-C – Spinal Manipulation with Cavitation, SM-NC – Spinal Manipulation with No Cavitation, SP – Substance-P, ST – Soft Tissue, TSM – Thoracic Spinal Manipulation, TNF – Tumour Necrosis Factor, VC – Venipuncture Control.
Risk of bias
The Cochrane Collaboration’s tool for assessing risk of bias [42] available as part of Covidence was used by two reviewers (KSK, LT, JDR and OT) independently to assess the risk of bias in the included studies. Any disagreements were resolved through consensus. If consensus could not be obtained a third reviewer was available to enable a final decision. A study was considered to have low risk of bias if the random sequence generation, allocation concealment and incomplete outcome data domains were adequately met. While the use of the recent Cochrane’s risk of bias (RoB 2) tool [43] has been encouraged, it was not mandatory to use RoB-2 for a review update.
Summary measures
Meta-analyses were performed where it was appropriate to pool data from multiple studies at two time points (1) immediate and (2) short-term. For the purpose of this review, immediate was defined as the measurement point immediately (up to 30 minutes) after intervention and short-term was defined as the measurement point up to 24 hours after intervention. Mean and standard deviations for outcome measures were extracted into Cochrane’s online Review Manager (RevMan Web, version 1.22.0) [44] software to analyze the comparative data between each intervention effect.
Measures of treatment effects
All outcomes of interest were examined as a standardized mean difference (SMD) and a random effects model was used whereby the overall effects are adjusted to include an estimate of the degree of variation or heterogeneity across studies. An effect size (Cohen’s d; small − 0.2; medium − 0.5 and large − 0.8) [45] and a 95% confidence interval were calculated for each treatment comparison.
Dealing with missing data
The authors were contacted in cases of missing data. For data that were graphically displayed, a software tool (https://automeris.io/WebPlotDigitizer/) was used, which is consistent with the original review.
Assessment of heterogeneity
Clinical heterogeneity was evaluated by determining if different clinical factors (characteristics of participants, interventions, outcome measure) varied between trials and could potentially influence the treatment effect. Statistical heterogeneity was determined using Chi-square and I [2] statistics (25%, 50% and 75% representing low, moderate and high heterogeneity respectively). If the heterogeneity was more than 50% (representing moderate heterogeneity), a sensitivity analysis was conducted to identify the cause of statistical heterogeneity.
Prediction interval
We calculated prediction interval (PI) as I2 statistics may not point to the clinical implications of the observed heterogeneity. The PI represents interval within which the effect size of a new study would fall if the new study was randomly selected from the same population of studies that are included in the meta-analysis [46]. Reporting a prediction interval in addition to the summary estimate, CI and I2 statistics have been recommended to capture the range of true effects that can be expected in future settings [47,48]. The formula to calculate PI is available [49] however, a pre-set template that is available from www.meta-analysis.com was used for calculating PIs in this review.
Assessment of reporting biases
Funnel plot has been recommended to assess publication bias in included studies. However, the funnel plot was not performed as the required statistical conditions were not met (10 or more studies).
Data synthesis
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system [50] was used to determine the overall quality of the evidence (high, moderate, low and very low).
Results
An updated search retrieved a total of 1466 records. After removal of duplicates, 1043 records were screened. Of the 12 full-text records that were assessed for eligibility, a total of seven studies that met the inclusion criteria were included in review. Together with the eight studies from the original review, a total of 15 studies were part of this systematic review update (refer Figure 1)
Figure 1.

PRISMA diagram of included studies.
Summary of included studies
A full description of included studies has been provided in the ‘characteristics of included studies’ (refer Table 1).
Methods
Out of 15 studies [15,18–21,35,36,51–58], nine studies were RCTs with three groups [21,35,36,51,52,54,56–58] five studies were RCTs with two groups [15,18–20,55] and one study had four groups [53].
Sample size
A total of 737 participants were examined in the studies. The sample size in the included studies ranged from 30 to 99 with only five studies recruiting more than 50 participants. All studies recruited participants in a single center.
Participants
The mean age of participants across all studies was 29.7 years. While 11 studies [15,20,21,35,51,53–58] included both male and female participants; three studies [19,36,52] included only male participants; and one study [18] included only female participants. Of the 15 studies, ten included healthy volunteers [15,19,21,35,36,51,52,54,56,57], four [18,20,53,58] included participants with pain (3 with neck pain and 1 with Achilles tendinopathy) and one study incuded participants with renal lithiasis.
Interventions
Two interventions were used by the researchers (1) cervical spine manipulation (either directed to atlanto-axial joint or cervical spine) (2) thoracic spine manipulation (either directed to T1 to T6, T12 or at the therapist’s discretion). In eight out of 15 studies (53%), thoracic spinal manipulation was the intervention used [15,19–21,52,54,55,57]. Four out of 15 studies (27%) used cervical manipulation [18,36,53,58] as the intervention and three out of 15 studies (20%) made use of both cervical and thoracic spinal manipulation interventions. While low velocity low amplitude thrust (mobilization) or setup for a thrust without manipulation was the commonly used sham procedure (n = 8), touch with no pressure was used as control (n = 7).
Outcome measures
A diverse range of outcome measures were reported in the studies including SP, neurotensin, cortisol, epinephrine/nor-epinephrine, interleukins, TNF, oxytocin and orexin-A. Most studies provided follow-up assessments at two time points: immediately (up to 30 minutes) and short-term (hours) after intervention.
Safety
Only one study [15] reported about withdrawal/adverse events. Another study [51]investigated changes in tissue damage markers after a spinal manipulation, which can be considered as an investigation about safety of spinal manipulation. Other studies did not report the presence/absence of adverse events and/or safety of spinal manipulation.
Risk of bias in included studies
The risk of bias was analyzed for all individual studies. Figure 2 provides a summary of the judgments of each methodological quality item for each study except for one study [53], random sequence generation was adequate in all other studies. Allocation concealment was considered ‘unclear’ in four studies [21,36,51,52] ‘inadequate’ in two studies [18,53] and ‘adequate’ in nine studies [15,19,20,35,54–58]. In manual therapy studies, blinding of participants and practitioners may not be possible. Hence all studies were rated as either ‘high’ risk or ‘unclear’ risk for this domain. Blinding of outcome assessors was explicit and considered ‘low’ risk in four studies [19,20,55,58], ‘unclear’ risk in eight studies [15,21,35,36,52,53,56,57] and ‘high’ risk in three studies [18,51,54]. Except for one study [15] in which participants withdrew post randomization, attrition bias was not detected in other studies. One study [58] was rated ‘high risk’ for other bias as there was considerable deviation from the study protocol. Of the 15 studies, 10 studies [15,18–21,36,52–54,57] received either full or partial funding. Five studies [35,51,55,56,58] did not report source of funding. One study [53] was rated ‘high risk’ overall as it did not meet random sequence generation and allocation concealment criteria.
Figure 2.
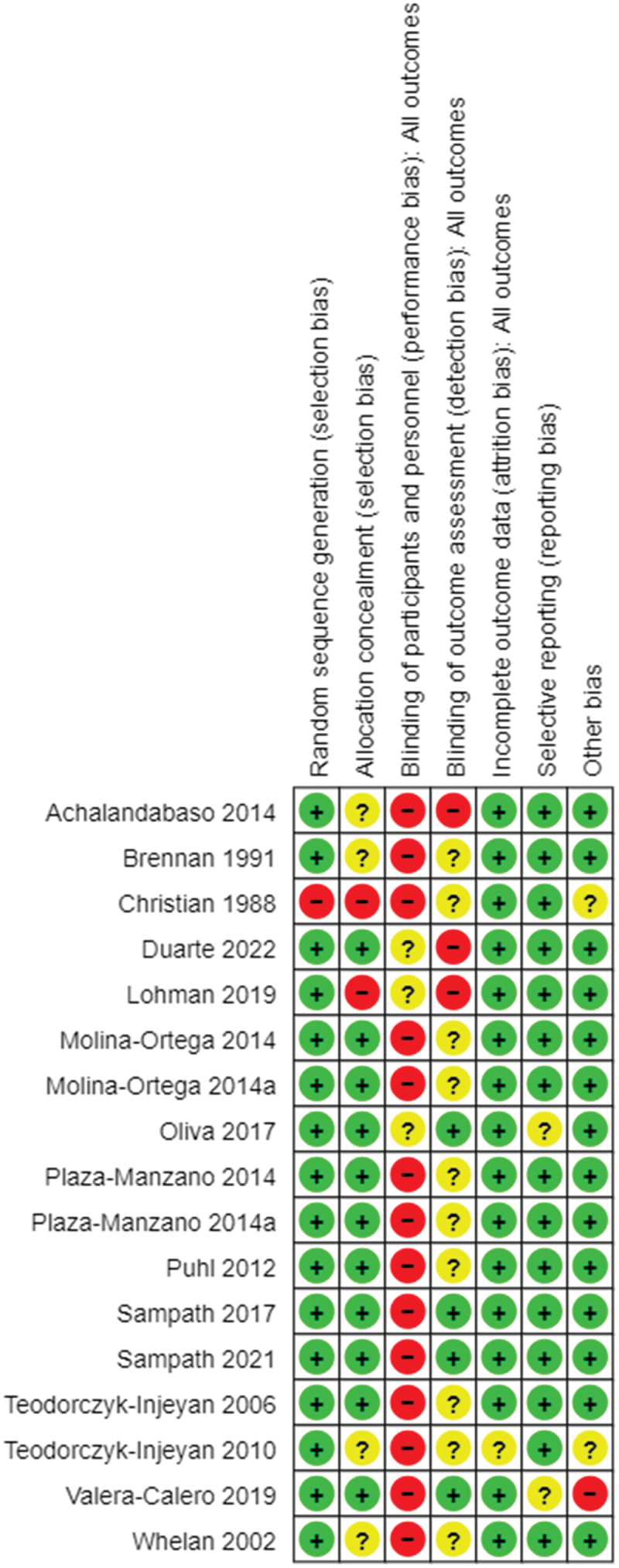
Risk of bias in included studies.
Note: Molina-Ortega 2014 and 2014a are one study; Plaza-Manzano 2014 and 2014a are one study.
Effects of interventions
A summary of findings table was created to summarize the overall quality of evidence using GRADE (Tables 3–5).
Table 3.
Summary of findings (GRADE).
| Spinal manipulation compared to Control/Sham in influencing biochemical markers | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Patient or population: healthy or symptomatic participantsSettings: Primary care, outpatient, communityIntervention: Spinal Manipulation Comparison: Control/sham | |||||||||||
| Biochemical markers (follow up: mean 2 hours; assessed with: plasma or serum or saliva) | |||||||||||
| ainty assessment |
№ of patients |
Effect |
|||||||||
| № of studies |
Study design |
Risk of bias |
Inconsistency |
Indirectness |
Imprecision |
Other considerations |
[SM] |
[Other Intervention] |
Relative(95% CI) |
Absolute(95% CI) |
Certainty |
| 15 | randomised trials | not serious | seriousa | seriousb | not serious | None | 357 | 380 | - | see comment | 2A01◯◯Low |
Note: CI: confidence interval.
Explanations.
Known Heterogeneity across studies, not pooled.
Different settings/context/outcome measures.
Table 4.
Summary of findings (GRADE).
| Spinal manipulation compared to Control/Sham in influencing neuropeptides and inflammatory biomarkers | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Patient or population: Healthy or symptomatic participants Settings: Primary care, outpatient, communityIntervention: Spinal ManipulationComparison: Control/sham | |||||||||||
| Quality assessment |
№ of patients |
Effect |
|||||||||
| № of studies |
Study design |
Risk of bias |
Inconsistency |
Indirectness |
Imprecision |
Other considerations |
Spinal Manipulation |
Control |
Relative(95% CI) |
Absolute(95% CI) |
Quality |
| Substance-P, immediate changes (assessed with blood) | |||||||||||
| 3 | randomised trials | not serious | not serious | not serious | very serious¹ | none | 56 | 69 | - | SMD 0.71 lower(1.22 lower to 0.22 lower) | ⊕⊕°°LOW |
| Substance-P, short-term changes (assessed with blood) | |||||||||||
| 2 | randomised trials | not serious | serious² | not serious | serious³ | none | 44 | 60 | - | SMD 1.16 fewer(2.53 lower to 0.21 higher) | ⊕°°°VERYLOW |
| Neurotensin (assessed with: Blood) | |||||||||||
| 2 | randomised trials | not serious | not serious | not serious | very serious⁴ | none | 33 | 35 | - | SMD 0.52 lower(1.01 lower to 0.03 lower) | ⊕°°°VERYLOW |
| Oxytocin (assessed with: Blood) | |||||||||||
| 2 | randomised trials | not serious | not serious | not serious | very serious⁴ | none | 33 | 35 | - | SMD 0.47 lower(1 lower to 0.06 higher) | ⊕°°°VERYLOW |
| Orexin-A (assessed with: Blood) | |||||||||||
| 2 | randomised trials | not serious | not serious | not serious | very serious⁴ | none | 33 | 35 | - | SMD 0.47 lower(1 lower to 0.06 higher) | ⊕°°°VERYLOW |
| Inflammatory Biomarkers (TNF, IL-2; assessed with: Blood) | |||||||||||
| 4 | randomised trials | not serious | serious 5 | not serious | not serious | none | 107 | 85 | - | See comment | ⊕⊕°°LOW |
Note: CI: Confidence interval; SMD: Standardised mean difference
1Graphical data retrieved using software and SD imputed.
2Heterogeneity = 86%
3Sample size < 100. Findings based on single study.
4Sample size < 100
5Known Heterogeneity, studies not pooled
Table 5.
Summary of findings (GRADE).
| Spinal manipulation compared to Control in influencing endocrine markers | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Patient or population: Healthy or symptomatic participants Settings: Primary care, outpatient, communityIntervention: Spinal ManipulationComparison: Control | ||||||||||||
| Quality assessment |
№ of patients |
Effect |
||||||||||
| № of studies |
Study design |
Risk of bias |
Inconsistency |
Indirectness |
Imprecision |
Other considerations |
Spinal Manipulation |
Control |
Relative(95% CI) |
Absolute(95% CI) |
Quality |
|
| Cortisol, immediate changes (assessed with blood or saliva) | ||||||||||||
| 7 | randomised trials | not serious | serious¹ | not serious | Serious2 | none | 114 | 115 | - | SMD 0.42 lower(-0.74 lower to -0.1 lower) | ⊕⊕°°LOW | |
| Cortisol, short-term changes (assessed with blood or saliva) | ||||||||||||
| 4 | randomised trials | not serious | Serious3 | not serious | Serious4 | none | 63 | 63 | - | SMD 0.45 lower(-0.79 lower to 0.1 lower) | ⊕⊕°°LOW | |
| Testosterone (assessed with: saliva) | ||||||||||||
| 2 | randomised trials | not serious | serious | not serious | very serious³ | none | 33 | 33 | - | SMD -0.01 lower(-0.14 lower to 0.12 higher) | ⊕°°°VERYLOW | |
| Testosterone (assessed with: saliva) | ||||||||||||
| 2 | randomised trials | not serious | serious | not serious | very serious³ | none | 33 | 33 | - | SMD -0.04 lower(-0.06 lower to 0.14 higher) | ⊕°°°VERYLOW | |
Note: CI: Confidence interval; SMD: Standardized mean difference.
1Sample size < 100.
2Heterogeneity.
3Sample size < 50. Findings based on single study.
Spinal manipulation (vs) control/sham in influencing biochemical markers
Data from 15 studies (total of 737 participants) [15,18–21,35,36,51–58] (not pooled) demonstrated a ‘low’ quality evidence that SM was better than control in eliciting changes in biochemical markers (Table 3).
Spinal manipulation (vs.) control/sham in influencing neuropeptides
Data from three studies (125 participants) [15,35,52] showed (Figure 3) that there was a ‘very low’ quality evidence of no difference that SM is better than control/sham (SMD −0.71, 95% CI − 1.22 to − 0.22; PI: −2.33 to 0.91) in increasing SP levels immediately after intervention. Although, the effect size and associated CIs indicate statistical significance, the prediction intervals are wide and point to lack of clear benefit from SM. Further, there was ‘very low’ quality evidence from two studies (104 participants) of no significant difference that SM is better than control (SMD −01.16, 95% CI − 2.53 to 0.21) (Table 4) in eliciting changes in SP levels at short-term after intervention. Between-study heterogeneity was high (86%).
Figure 3.

Forest plot of comparison: SM vs control/sham, outcome: Substance-P (immediate).
There was ‘very low’ quality evidence from two studies (68 participants) [18,56] of no significant difference that SM is better than control/sham (SMD −0.52, 95% CI − 1.01 to − 0.03; PI − 3.69 to 2.65) in increasing neurotensin after intervention. Although, the effect size and associated CIs indicate statistical significance, the prediction intervals are wide and point to lack of clear benefit from SM. However, ‘very low’ quality evidence from two studies (68 participants) [18,56] demonstrated no significant difference between SM and control/sham (SMD −0.47, 95%CI − 1 to 0.06) in influencing oxytocin and orexin-A (SMD −0.59, 95% CI − 1.48 to 0.29).
Spinal manipulation (vs.) control in influencing inflammatory biomarkers
Data were extracted from four studies (192 participants; not pooled) that compared the effectiveness of SM with control on inflammatory biomarkers such as interleukins. There was ‘low’ quality evidence that SM is better than control in influencing inflammatory markers such as interleukins (Table 4).
Spinal manipulation (vs.) control in influencing endocrine biomarkers
Cortisol
Data was pooled from seven studies (239 participants) to determine the effects of SM on cortisol levels (Figure 4a). Between-study heterogeneity was moderate (I [2] = 63%). Hence a sensitivity analysis was done, and two studies were removed from the meta-analysis, which reduced the heterogeneity (I [2] = 0%) (Figure 4b) There was a ‘low’ quality evidence (Table 5) of statistically significant difference that SM is better than control/sham in eliciting changes in cortisol levels (SMD −0.42, 95% CI − 0.74 to − 0.10; PI − 0.83 to 0.0) immediately after intervention.
Figure 4.

Figure 4.

Forest plot of comparison: SM vs control/sham, outcome: Cortisol (immediate). Forest plot of comparison (sensitivity analysis): SM vs control/sham, outcome: Cortisol (immediate).
Segmental response
A subgroup analysis was undertaken to determine if the response in cortisol was different based on the region of spine manipulated (thoracic vs cervical in this instance). The results demonstrated that cervical spine manipulation cortisol levels compared to a thoracic spine manipulation (SMD- −0.65, 95% CI − 1.10 to − 0.2; PI − 2.01 to 0.7) (refer Figure 5).
Figure 5.

Subgroup analysis (thoracic vs cervical manipulation). Outcome: cortisol (immediate).
Direction of effect
Another subgroup analysis was undertaken to determine the direction of effect (increase or decrease) of cortisol following a spinal manipulation. The subgroup analysis indicates that cortisol levels increase immediately following a spinal manipulation despite the segment being manipulated (SMD −0.65, 95% CI − 1.10 to − 0.2; PI − 2.08 to 0.79) (Figure 6).
Figure 6.

Subgroup analysis (direction of effect – increase or decrease). Outcome: cortisol (immediate).
Healthy vs painful population
Subgroup analysis demonstrated that changes in cortisol following an SM are statistically significant in people with pain (especially neck pain) compared to healthy volunteers (SMD −0.09, 95% CI − 0.12 to − 0.07; PI − 1.4 to 1.2) (Figure 7).
Figure 7.

Sub-group analysis (healthy vs pain). Outcome: cortisol (immediate).
Cortisol (short-term)
Low-quality evidence from four studies (136 participants) demonstrated no significant difference that SM is better than control (SMD −0.45, 95% CI − 0.79 to − 0.1; PI: −1.21 to 0.31) in eliciting changes in cortisol levels at short-term after intervention (Table 5). Although, the effect size and associated CIs indicate statistical significance, the prediction intervals are wide and point to lack of clear benefit from SM in short-term changes in cortisol.
Testosterone
‘Very Low’ quality evidence from two studies (66 participants) demonstrated no significant difference that SM is better than control in eliciting changes in testosterone levels immediately (SMD −0.01, 95% CI − 0.14 to 0.12] and at short-term after intervention (SMD −0.04, 95% CI − 0.06 to 0.14] (Table 5). Findings from single studies indicate no change in epinephrine or nor-epinephrine and urinary pH level following spinal manipulation.
Discussion
Summary of main results
This review updates the previous review published in 2017 [59], comparing spinal manipulation against control in influencing biochemical markers. The updated review now includes 15 studies (737 participants) compared to eight studies (325 participants in the 2017 review). It also includes different types of participants (healthy volunteers, people in pain or disease); various types of spinal manipulation (cervical, thoracic and lumbar); a wide range of outcome measures (inflammatory markers, pain markers, urinary pH and T/C ratio), thus providing a comprehensive analysis of spinal manipulation in influencing biochemical markers. The findings from this review update established ‘low’ level evidence in support of SM in influencing biochemical markers such as cortisol (immediate changes) and inflammatory markers but not for substance-p, neurotensin, testosterone, oxytocin and orexin-A. Further, subgroup analyses established that: (1) cervical SM influences cortisol compared to thoracic SM; (2) cortisol levels increase immediately after intervention despite the segment being manipulated; and (3) response differs in people with pain (especially neck pain) compared to healthy volunteers. The key differences between the original review and this review update have been provided in appendix 1.
Overall completeness and applicability of evidence
The data from this review can be considered relevant to current clinical practice as we found evidence that SM may influence various biochemical markers such as cortisol and inflammatory markers. It is important that these findings are interpreted with caution and in consideration of prediction intervals (discussed later). Further, 10 out of 15 studies [15,19,21,35,36,51,52,54,56,57] have been done on healthy volunteers, which makes it difficult to ascertain the applicability of the evidence in clinical practice. Although four studies [18,20,53,58] included participants with pain, the effect of SM on the magnitude and duration of biochemical responses in symptomatic patients (e.g. pain population or inflammatory disorders) needs further scrutiny and is an ongoing area of investigation [20,35,58]. Cervical or thoracic spinal manipulation are the common techniques utilized in the studies, with a subgroup analysis demonstrating that cervical SM may have more influence on cortisol levels. However, this is based on five studies [18,36,53,56,58] and should be verified by future studies that may have direct comparison between the two techniques. There was no adverse events/harm associated with SM. One study 51measured tissue damage markers and demonstrated that there was no tissue damage associated with SM.
Quality of the evidence
As reflected by the GRADE ratings, the overall quality of the evidence in this review update was ‘low’ to ‘very low’ for all outcomes. This is because included trials studied a wide range of interventions, outcome measure, data collection techniques and post-intervention time points. Therefore, we were unable to pool data due to heterogeneity, especially for inflammatory markers. In addition, the sample size (being low in most studies), wide confidence intervals and prediction intervals led to issues of imprecision and inconsistency. It is important to note that we have downgraded the level of evidence compared to the original review. Although, eight more studies were part of this review update and points to growth in the evidence base, it also has resulted in further heterogeneity. Except for immediate changes in cortisol, the broad prediction intervals for other outcomes may indicate the existence of setting where SM may have suboptimal effects. Ten out of fifteen studies were small-scale RCTs (less than 50 participants) done on healthy volunteers where there is a chance for overly positive trends for interventions due to inflated effect sizes. A review [60] has shown that trials with fewer than 50 participants had effect estimates larger than trials with more participants (48% more on average). Hence, it has been recommended that trials with fewer than 19 participants in each trial arm be excluded from systematic reviews due to risk of bias associated with small RCTs [61]. We did not downgrade the risk of bias for blinding therapists as this is very difficult to achieve in manual therapy setting. While blinding of participants was done in some studies, it was unclear in other studies. Keeping in line with recent recommendations [62], future studies should concentrate on better blinding of participants and also therapists in maintaining blinding including adding a measure of blinding effectiveness. Only one study [58] had reported using the Template for Intervention Description and Replication (TIDieR) guidelines [63]. Therefore, it has to be re-emphasized that the overall quality of reporting of manual therapy studies still requires considerable improvement.
Potential biases in the review process
We consider the review process to be robust and expect minimal biases in extracting and reporting of data. A minimum of two reviewers acted independently through the various phases of the review and a third reviewer was available to resolve any disagreements if required. We undertook extensive search to identify new studies that may be included in this review update. We did not downgrade the risk of bias based on ‘publication’ bias as we had only 15 studies included in the review. It is well noted that existing ways to publication bias are unsatisfactory and funnel plot was not considered appropriate in this instance. Further, only publications done in English language were included in the review, thereby, raising the possibility of language bias [64]. In turn, this may limit the usefulness of the review’s findings as we may miss out important cultural contexts [65]. Hence, recommendations have been made to include studies published in languages other than English (LOTE) [66]. However, due to lack of resources both in terms of funding and/or access to members who can fluently speak/read LOTE, we had to limit our review to studies published only in English, as identified previously [64].
Agreements and disagreements with other studies or reviews
The findings from this review update remain partly consistent with our original systematic review findings. However, we decided to downgrade the quality of evidence from ‘low’ to ‘very low’ compared to the original review, largely due to inconsistency, indirectness and imprecision introduced by the inclusion of these studies.
Our review update established very low evidence that SM does not influence neuropeptides such as SP, neurotensin, oxytocin and orexin-A immediately after intervention. This is in contrast with the previous findings [35,37,52]. These neuropeptides are found in many regions of the CNS and are known to induce analgesia directly or indirectly. Molina-Ortega et al. (2014) further reported a positive correlation between SP levels and pressure pain threshold suggesting that high levels of serum SP before SM are associated with increased pressure pain threshold after SM. Hence, the review findings may be of importance. It has to be noted however that only on a few studies [18,56] have investigated these neuropeptides. Hence, the lack of beneficial effects of SM may be due to low number of studies in this area highlighting the need for further research investigating these biomarkers.
Our review findings indicate the SM may influence cortisol levels immediately (<30 minutes) but not at short-term (many hours) after intervention. This is in agreement with our original review that demonstrated changes in cortisol levels immediately but not at short-term after intervention. The number of studies investigating the effects of SM on cortisol have increased in the last 5 years that may explain the difference. Emerging pattern from the current review update indicates that cortisol level may increase immediately after intervention despite the segment manipulated. However, this is based on only two studies [56,58] that had used a cervical spine manipulation involving rotational thrust. Further, a cervical spine manipulation may influence cortisol levels immediately in people with neck pain. The changes in cortisol were shown to be positively correlated with reduced neck pain and reduced disability in one study [58]. It was noted that recent studies have considered various methodological factors that may influence cortisol levels and have outlined strategies to mitigate these variables, which is consistent with previous recommendations [37,67].
Our review update demonstrated no significant difference that SM is better than control in eliciting changes in testosterone levels immediately and at short-term after intervention. Testosterone was measured in the studies as interactions between the end products of the gonadal (e.g. testosterone) and the adrenal axis (cortisol) have been well documented [68]. Hence, the balance between testosterone and cortisol represented as T/C ratio may therefore provide a better estimation of the HPA axis activity [69]. Although not often used in manual therapy research, T/C ratio has been widely used in sports and exercise science research as valid outcome measure for stress response [69]. Hence, T/C ratio is an area of future research interest.
Findings from our review of four studies indicate that SM is better than control in influencing various inflammatory/immune markers such as interleukins (especially, IL-1, IL-2, IL-6), TNF-α, IgG and IgM. The regulation of inflammation and immunity involve complex interactions between the nervous system and the immune system mediated by the action of numerous neurotransmitters and cytokines [29,30,70]. This is consistent with previous findings and suggest that a central anti-inflammatory mechanism might be activated following a SM. However, it must be noted that some of the studies were done more than 10 years previously indicating a dearth of recent investigation in this area. Hence, our findings must be interpreted with caution.
Implications for clinical practice and research
Two common themes are consistent with our previous systematic review (1) clinical utility: while the changes in endocrine markers (especially cortisol) and inflammatory markers shed light into mechanisms through which SM may work, the clinical utility of such changes (especially short-term) is still largely unknown. Hence, it will be helpful to investigate long-term changes in these biochemical markers and their association with symptom improvement. (2) The mean age of participants explored across studies was 29.2 year (up from 26 years in the original review). Therefore, the generalizability and clinical application of our findings could be questioned. Hence, future studies may target participants across different age groups. The methodology used for collecting hormone samples and the reporting of protocol have improved since our previous review.
The wider prediction interval found in our meta-analysis may have important implication for clinical practice and research. Despite statistically significant findings as demonstrated by effect size and confidence intervals, the wide prediction intervals reduce the confidence in findings. That is, the effects of intervention may vary substantially depending on the setting or population used. This clearly emphasizes the need for more well controlled studies to clarify our findings. The rationale for calculating prediction intervals could be criticized as there are less than ten studies as part of our meta-analysis [47]. However, we decided to calculate prediction intervals for a few reasons (1) there is still no consensus on what a sufficient number of studies would be to generate reliable prediction intervals. Some evidence [46] indicate that a minimum of three studies is enough to calculate prediction intervals (which we meet); (2) it is important to demonstrate the variability/heterogeneity to enable meaningful interpretation of our findings by clinicians and researchers; and (3) it is better to highlight the heterogeneity and therefore the need for further research than to erroneously conclude that the intervention is beneficial (as demonstrated by effect size and CIs alone). Finally, we did not propose GRADE-based recommendations due to the heterogeneity, which can be considered another important limitation.
Author’s Conclusion
This review established low-level evidence that SM influences various inflammatory markers and cortisol. Specifically, we found that SM can increase cortisol levels immediately post-intervention. Hence the beneficial effects of SM such as pain relief and reduced inflammation could potentially be modulated through these mechanistic pathways. However, well-powered trials targeting symptomatic populations are required to validate our review findings.
Supplementary Material
Biographies
Kesava Kovanur Sampath is a Principal Academic Staff Member in the Centre for Health and Social Practice at Waikato Institute of Technology in New Zealand. He is the chair of the osteopathic research steering committee in New Zealand. He is part of the leadership team at the Duke Centre of Excellence in Manual and Manipulative Therapy. He is also a visiting research fellow at the University of Technology, Sydney. Kesava’s research specialties include neurophysiological outcomes, spinal manipulation, manual therapy practice and education.
Dr Loïc Treffel is a Principal Investigator of ALGOS project: Osteopathy in Oncology (NCT05726929 Study Record | Beta ClinicalTrials.gov). He is a Ph.D. Researcher in Space Physiology on vertebral deconditioning. Scientist Principal Investigator supported by CNES (French Space Agency). PhD. Thesis defended in 2017 Life Sciences and Health, Vertebral Dysfunctions and Postural changes after microgravity simulation studies. University of Strasbourg, IPHC-CNRS (UMR7178, ED 414).
Dr Oliver P.Thomson is an Associate Professor at the University College of Osteopathy where he leads the Doctoral Programme, and he works clinically as an osteopath in London. Oliver has a particular interest in qualitative research methodologies to explore the clinical and theoretical aspects of osteopathy and musculoskeletal practice such as the areas of clinical decision-making and the role of evidence-base practice. He has over 50 peer-reviewed publication in these areas. Oliver examines and supervises PhD students in the UK and Australia and collaborates with national and international colleagues on numerous research projects.
Dr Jerry Draper-Rodi is the Director of the National Council for Osteopathic Research (NCOR) in the UK, a Senior Research Fellow at the University College of Osteopathy (UCO), a visiting fellow on the international osteopathic research leadership programme (UTS School of Public Health, Australia) and a clinician. Jerry’s research streams include: knowledge mobilisation; Research methods in complex interventions; and Equity, Diversity and Inclusion (EDI).
Dr Michael Fleischmann has over 25 peer-reviewed publications. His research focus can be conceptualised as 3 main areas: 1. evaluation of education intervention; 2. Epidemiology, particularly in osteopathy and manual therapies; 3. clinical reasoning of manual therapists. Michael’s wide range of experience in educational and clinical settings displays an exceptional ability to work with a high degree of productivity with students as a core focus. He embraces diversity and welcomes opportunities to work with a broad range of disciplines and specialties. His teaching practice focusses on constantly engaging with students and other staff to provide rich learning experiences.
SteveTumilty is one of the few clinician scientists in Physiotherapy Worldwide and one of the few Registered Physiotherapy Specialists in New Zealand. The majority of his clinical experience has been in the outpatient musculoskeletal practice setting in UK, Germany and New Zealand. He also has experience in professional sports and Occupational Health Physiotherapy. In 2002 he came to work at the School of Physiotherapy, Otago University and he has developed and coordinated the specialist Masters degree for Sports and Orthopaedic Manipulative Therapy for which he provides teaching and clinical expertise. He is the author or co-author on 125 research outputs in the field of Physiotherapy and Orthopaedic Manipulative Therapy for musculoskeletal conditions and has been invited to present at international conferences. He has taught manual therapy seminars in New Zealand and Japan. Associate Professor Tumilty’s research interests are in Tendinopathy, modulation of the Hypothalamus-Pituitary Axis using manual interventions, and the influence of the autonomic nervous system on musculoskeletal pain and healing.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10669817.2023.2252187.
References
- [1].Coronado RA, Gay CW, Bialosky JE, et al. Changes in pain sensitivity following spinal manipulation: a systematic review and meta-analysis. J Electromyogr Kinesiol. 2012;22(5):752–767. doi: 10.1016/j.jelekin.2011.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bonic EE, Stockwell CA, Kettner NW.. Brain stem compression and atlantoaxial instability secondary to chronic rheumatoid arthritis in a 67-year-old female. J Manipulative Physiol Ther. 2010;33(4):315–320. doi: 10.1016/j.jmpt.2010.03.008 [DOI] [PubMed] [Google Scholar]
- [3].Brosseau L, Wells GA, Poitras S, et al. Ottawa panel evidence-based clinical practice guidelines on therapeutic massage for low back pain. J Bodyw Mov Ther. 2012;16(4):424–455. doi: 10.1016/j.jbmt.2012.04.002 [DOI] [PubMed] [Google Scholar]
- [4].Gross A, Miller J, D’Sylva J, et al. Manipulation or mobilisation for neck pain: a Cochrane review. Manual Ther. 2010;15(4):315–333. doi: 10.1016/j.math.2010.04.002 [DOI] [PubMed] [Google Scholar]
- [5].Martin SL, Kerr KL, Bartley EJ, et al. Respiration-induced hypoalgesia: exploration of potential mechanisms. J Pain. 2012;13(8):755–763. doi: 10.1016/j.jpain.2012.05.001 [DOI] [PubMed] [Google Scholar]
- [6].Coppieters MW, Butler DS. Do ‘sliders’ slide and ‘tensioners’ tension? An analysis of neurodynamic techniques and considerations regarding their application. Manual Ther. 2008;13(3):213–221. doi: 10.1016/j.math.2006.12.008 [DOI] [PubMed] [Google Scholar]
- [7].Bialosky JE, Bishop MD, Price DD, et al. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Manual Ther. 2009;14(5):531–538. doi: 10.1016/j.math.2008.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wellens F. The traditional mechanistic paradigm in the teaching and practice of manual therapy: time for a reality check. 2010.
- [9].Bakhtadze MA, Vernon H, Karalkin AV, et al. Cerebral perfusion in patients with chronic neck and upper back pain: preliminary observations. J Manipulative Physiol Ther. 2012;35(2):76–85. doi: 10.1016/j.jmpt.2011.12.006 [DOI] [PubMed] [Google Scholar]
- [10].Chu J, Allen DD, Pawlowsky S, et al. Peripheral response to cervical or thoracic spinal manual therapy: an evidence-based review with meta analysis. J Manual Manipulative Ther. 2014;22(4):220–229. doi: 10.1179/2042618613y.0000000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Eisold S, Mehrabi A, Konstantinidis L, et al. Experimental study of cardiorespiratory and stress factors in esophageal surgery using robot-assisted thoracoscopic or open thoracic approach. Arch Surg. 2008;143(2):156–163. doi: 10.1001/archsurg.2007.56 [DOI] [PubMed] [Google Scholar]
- [12].Kingston L, Claydon L, Tumilty S. The effects of spinal mobilizations on the sympathetic nervous system: a systematic review. Manual Ther. 2014;19(4):281–287. doi: 10.1016/j.math.2014.04.004 [DOI] [PubMed] [Google Scholar]
- [13].Matus S, Valenzuela V, Medinas DB, et al. ER dysfunction and protein folding stress in ALS. Int J Cell Biol. 2013;2013:1–12. doi: 10.1155/2013/674751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moutzouri M, Joanna P, Eudokia B. Investigation of the effects of a centrally applied lumbar sustained natural apophyseal glide mobilization on lower limb sympathetic nervous system activity in asymptomatic subjects. J Manipulative Physiol Ther. 2012;35(4):286–294. doi: 10.1016/j.jmpt.2012.04.016 [DOI] [PubMed] [Google Scholar]
- [15].Puhl AA, Injeyan HS. Short-term effects of manipulation to the upper thoracic spine of asymptomatic subjects on plasma concentrations of epinephrine and norepinephrine—A Randomized and controlled Observational study. J Manipulative Physiol Ther. 2012;35(3):209–215. doi: 10.1016/j.jmpt.2012.01.012 [DOI] [PubMed] [Google Scholar]
- [16].Schmid A, Brunner F, Wright A, et al. Paradigm shift in manual therapy? Evidence for a central nervous system component in the response to passive cervical joint mobilisation. Manual Ther. 2008;13(5):387–396. doi: 10.1016/j.math.2007.12.007 [DOI] [PubMed] [Google Scholar]
- [17].Wingenfeld K, Heim C, Schmidt I, et al. HPA axis reactivity and lymphocyte glucocorticoid sensitivity in fibromyalgia syndrome and chronic pelvic pain. Psychosom Med. 2008;70(1):65–72. doi: 10.1097/PSY.0b013e31815ff3ce [DOI] [PubMed] [Google Scholar]
- [18].Lohman EB, Pacheco GR, Gharibvand L, et al. The immediate effects of cervical spine manipulation on pain and biochemical markers in females with acute non-specific mechanical neck pain: a randomized clinical trial. J Manual Manipulative Ther. 2019;27(4):186–196. doi: 10.1080/10669817.2018.1553696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sampath KK, Botnmark E, Mani R, et al. Neuroendocrine response following a thoracic spinal manipulation in healthy men. J Orthop Sports Phys Ther. 2017;47(9):617–627. doi: 10.2519/jospt.2017.7348 [DOI] [PubMed] [Google Scholar]
- [20].Sampath KK, Mani R, Katare R, et al. Thoracic spinal manipulation effect on neuroendocrine response in people with Achilles tendinopathy: a randomized crossover trial. J Manipulative Physiol Ther. 2021;44(5):420–431. doi: 10.1016/j.jmpt.2021.06.001 [DOI] [PubMed] [Google Scholar]
- [21].Teodorczyk-Injeyan JA, McGregor M, Ruegg R, et al. Interleukin 2-regulated in vitro antibody production following a single spinal manipulative treatment in normal subjects. Chiropr Man Therap. 2010;18(1):1. doi: 10.1186/1746-1340-18-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gevers-Montoro C, Provencher B, Descarreaux M, et al. Neurophysiological mechanisms of chiropractic spinal manipulation for spine pain. Eur J Pain. 2021;25(7):1429–1448. doi: 10.1002/ejp.1773 [DOI] [PubMed] [Google Scholar]
- [23].Haavik H, Kumari N, Holt K, et al. The contemporary model of vertebral column joint dysfunction and impact of high-velocity, low-amplitude controlled vertebral thrusts on neuromuscular function. Eur J Appl Physiol. 2021;121(10):2675–2720. doi: 10.1007/s00421-021-04727-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lutke Schipholt IJ, Coppieters MW, Meijer OG, et al. Effects of joint and nerve mobilisation on neuroimmune responses in animals and humans with neuromusculoskeletal conditions: a systematic review and meta-analysis. Pain Rep. 2021;6(2):e927. doi: 10.1097/pr9.0000000000000927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wirth B, Gassner A, de Bruin ED, et al. Neurophysiological effects of high velocity and low amplitude spinal manipulation in symptomatic and asymptomatic humans: a systematic literature review. Spine. 2019;44(15):E914–E926. doi: 10.1097/BRS.0000000000003013 [DOI] [PubMed] [Google Scholar]
- [26].Takuwa H, Matsuura T, Bakalova R, et al. Contribution of nitric oxide to cerebral blood flow regulation under hypoxia in rats. J Physiol Sci. 2010;60(6):399–406. doi: 10.1007/s12576-010-0108-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dobner PR. Neurotensin and pain modulation. Peptides. 2006;27(10):2405–2414. doi: 10.1016/j.peptides.2006.04.025 [DOI] [PubMed] [Google Scholar]
- [28].Nicoletti M, Neri G, Maccauro G, et al. Impact and neuropeptide substance P an inflammatory compound on arachidonic acid compound generation. Int J Immunopathol Pharmacol. 2012;25(4):849–857. doi: 10.1177/039463201202500403 [DOI] [PubMed] [Google Scholar]
- [29].Lotz M, Vaughan JH, Carson DSA. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988;241(4870):1218–1221. doi: 10.1126/science.2457950 [DOI] [PubMed] [Google Scholar]
- [30].Suffredini AF, Fantuzzi G, Badolato R, et al. New insights into the biology of the acute phase response. J Clin Immunol. 1999;19(4):203–214. doi: 10.1023/A:1020563913045 [DOI] [PubMed] [Google Scholar]
- [31].Elenkov IJ, Wilder RL, Chrousos GP, et al. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- [32].Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106 [DOI] [PubMed] [Google Scholar]
- [33].Priftis KN, Papadimitriou A, Anthracopoulos MB, et al. Endocrine-immune interactions in adrenal function of asthmatic children on inhaled corticosteroids. Neuroimmunomodulation. 2009;16(5):333–339. doi: 10.1159/000216191 [DOI] [PubMed] [Google Scholar]
- [34].Degenhardt BF, Darmani NA, Johnson JC, et al. Role of osteopathic manipulative treatment in altering pain biomarkers: a pilot study. J Am Osteopath Assoc. 2007;107(9):387–400. http://www.scopus.com/inward/record.url?eid=2-s2.0-34948818710&partnerID=40&md5=257097abc17ab76422809bfb21c045e3 [PubMed] [Google Scholar]
- [35].Molina-Ortega F, Lomas-Vega R, Hita-Contreras F, et al. Immediate effects of spinal manipulation on nitric oxide, substance P and pain perception. Manual Ther. 2014;19(5):411–417. doi: 10.1016/j.math.2014.02.007 [DOI] [PubMed] [Google Scholar]
- [36].Whelan TL, Dishman JD, Burke J, et al. The effect of chiropractic manipulation on salivary cortisol levels. J Manipulative Physiol Ther. 2002;25(3):149–153. http://search.ebscohost.com/login.aspx?direct=true&db=cin20&AN=2002089945&site=ehost-live&scope=site [DOI] [PubMed] [Google Scholar]
- [37].Sampath KK, Mani R, Cotter J, et al. Reply to letter to the editor ‘changes in biochemical markers following spinal manipulation – a systematic review and meta-analysis’. Musculoskeletal Sci Pract. 2017;30:e90. doi: 10.1016/j.msksp.2017.04.004 [DOI] [PubMed] [Google Scholar]
- [38].Garner P, Hopewell S, Chandler J, et al. When and how to update systematic reviews: consensus and checklist. BMJ. 2016;354:i3507. doi: 10.1136/bmj.i3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Smith GD, Ho KHM. Systematic reviews: when should they be updated? J Clin Nurs. 2023;32(9–10):e17–e18. doi: 10.1111/jocn.16547 [DOI] [PubMed] [Google Scholar]
- [40].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann internal med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- [41].Conway PJW, Herzog W, Zhang Y, et al. Forces required to cause cavitation during spinal manipulation of the thoracic spine. Clin Biomech. 1993;8(4):210–214. doi: 10.1016/0268-0033(93)90016-B [DOI] [PubMed] [Google Scholar]
- [42].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- [44].Version 1.22.0: The Cochrane Collaboration ; 2020.
- [45].Cohen J. Statistical power analysis for the behavioral sciences. revised ed. New York: Academic Press; 1977. [Google Scholar]
- [46].Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25(6):646–654. doi: 10.1177/0272989x05282643 [DOI] [PubMed] [Google Scholar]
- [47].Borenstein M. Research note: in a meta-analysis, the I2 index does not tell us how much the effect size varies across studies. J Physiother. 2020;66(2):135–139. doi: 10.1016/j.jphys.2020.02.011 [DOI] [PubMed] [Google Scholar]
- [48].Joanna I, John PAI, Maroeska MR, et al. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7):e010247. doi: 10.1136/bmjopen-2015-010247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172(1):137–159. doi: 10.1111/j.1467-985X.2008.00552.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Achalandabaso A, Plaza-Manzano G, Lomas-Vega R, et al. Tissue damage markers after a spinal manipulation in healthy subjects: a preliminary report of a randomized controlled trial. Dis Markers. 2014;2014:1–7. doi: 10.1155/2014/815379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Brennan PC, Kokjohn K, Kaltinger CJ, et al. Enhanced phagocytic cell respiratory burst induced by spinal manipulation: potential role of substance P. J Manipulative Physiol Ther. 1991;14(7):399–408. [PubMed] [Google Scholar]
- [53].Christian GF, Stanton GJ, Sissons D, et al. Immunoreactive ACTH, β-endorphin, and cortisol levels in plasma following spinal Manipulative therapy. Spine. 1988;13(12):1411–1417. doi: 10.1097/00007632-198812000-00014 [DOI] [PubMed] [Google Scholar]
- [54].Duarte FCK, Funabashi M, Starmer D, et al. Effects of distinct force magnitude of spinal manipulative therapy on blood biomarkers of inflammation: a proof of principle study in healthy young adults. J Manipulative Physiol Ther. 2022;45(1):20–32. doi: 10.1016/j.jmpt.2022.03.012 [DOI] [PubMed] [Google Scholar]
- [55].Pascual-Vaca Á O, Punzano-Rodríguez R, Escribá-Astaburuaga P, et al. Short-term changes in algometry, inclinometry, stabilometry, and urinary pH Analysis after a thoracolumbar junction manipulation in patients with kidney stones. J Altern Complementary Med. 2017;23(8):639–647. http://ezproxy.canberra.edu.au/login?url=https://search.ebscohost.com/login.aspx?direct=true&db=asn&AN=124584232. [DOI] [PubMed] [Google Scholar]
- [56].Plaza-Manzano G, Molina F, Lomas-Vega R, et al. Changes in biochemical markers of pain perception and stress response after spinal manipulation. J Orthop Sports Phys Ther. 2014;44(4):231–239. doi: 10.2519/jospt.2014.4996 [DOI] [PubMed] [Google Scholar]
- [57].Teodorczyk-Injeyan JA, Injeyan HS, Ruegg R. Spinal manipulative therapy reduces inflammatory cytokines but not substance P production in normal subjects. J Manipulative Physiol Ther. 2006;29(1):14–21. doi: 10.1016/j.jmpt.2005.10.002 [DOI] [PubMed] [Google Scholar]
- [58].Valera-Calero A, Lluch Girbes E, Gallego-Izquierdo T, et al. Endocrine response after cervical manipulation and mobilization in people with chronic mechanical neck pain: a randomized controlled trial. Eur J Phys Rehabil Med. 2019;55(6):792–805. doi: 10.23736/S1973-9087.19.05475-3 [DOI] [PubMed] [Google Scholar]
- [59].Sampath KK, Mani R, Cotter JD, et al. Measureable changes in the neuro-endocrinal mechanism following spinal manipulation. Med Hypotheses. 2015;85(6):819–824. doi: 10.1016/j.mehy.2015.10.003 [DOI] [PubMed] [Google Scholar]
- [60].Dechartres A, Trinquart L, Boutron I, et al. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ. 2013;346:f2304. doi: 10.1136/bmj.f2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].de C Williams AC, Fisher E, Hearn L, et al. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2020;2020(8). doi: 10.1002/14651858.CD007407.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hohenschurz-Schmidt D, Vase L, Scott W, et al. Recommendations for the development, implementation, and reporting of control interventions in efficacy and mechanistic trials of physical, psychological, and self-management therapies: the CoPPS statement. BMJ. 2023;381:e072108. doi: 10.1136/bmj-2022-072108 [DOI] [PubMed] [Google Scholar]
- [63].Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348(mar07 3):g1687. doi: 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- [64].Neimann Rasmussen L, Montgomery P. The prevalence of and factors associated with inclusion of non-English language studies in campbell systematic reviews: a survey and meta-epidemiological study. Syst Rev. 2018;7(1):129. doi: 10.1186/s13643-018-0786-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Walpole SC. Including papers in languages other than English in systematic reviews: important, feasible, yet often omitted. J Clin Epidemiol. 2019;111:127–134. doi: 10.1016/j.jclinepi.2019.03.004 [DOI] [PubMed] [Google Scholar]
- [66].Kugley S, Wade A, Thomas J, et al. Searching for studies: a guide to information retrieval for campbell. Campbell Syst Rev. 2016;13(1):1–73. doi: 10.4073/cmg.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Stalder T, Kirschbaum C, Kudielka BM, et al. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010 [DOI] [PubMed] [Google Scholar]
- [68].Bedgood D, Boggiano MM, Turan B. Testosterone and social evaluative stress: The moderating role of basal cortisol. Psychoneuroendocrinology. 2014;47:107–115. doi: 10.1016/j.psyneuen.2014.05.007 [DOI] [PubMed] [Google Scholar]
- [69].Hayes LD, Grace FM, Baker JS, et al. Exercise-induced responses in Salivary testosterone, cortisol, and their ratios in men: a meta-analysis. Sports Med. 2015;45(5):713–726. doi: 10.1007/s40279-015-0306-y [DOI] [PubMed] [Google Scholar]
- [70].Goebel MU, Mills PJ, Irwin MR, et al. Interleukin-6 and tumor necrosis factor-α production after acute psychological stress, exercise, and infused isoproterenol: differential effects and pathways. Psychosom Med. 2000;62(4):591–598. doi: 10.1097/00006842-200007000-00019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


