Abstract
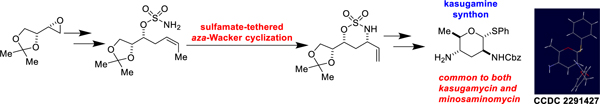
We present our preparation of a kasugamine synthon, which proceeds in 14 steps from a literature epoxide. We expect that this kasugamine derivative can be used for the total syntheses of kasugamycin, minosaminomycin, and analogue antibiotics. A key step in the synthesis is our laboratory’s sulfamate-tethered aza-Wacker cyclization.
Graphical Abstract
Kasugamycin and minosaminomycin are structurally related aminoglycoside antibiotics (Figure 1). Kasugamycin was first isolated in 1965 from a bacterial strain (Streptomyces kasugiensis) found in a soil sample near the Kasuga shrine in Nara, Japan.1–3 Shortly after isolation, empirical testing demonstrated that kasugamycin was active against a variety of bacteria and fungi.4, 5 While its antibacterial activity is much less than that of other members of the aminoglycoside family, its ability to control rice blast disease caused by the fungus Pyricularia oryzae was so remarkable that it continues to be used commercially as an agricultural fungicide (Kasumin).6, 7 Minosaminomycin is a structurally related antibiotic to kasugamycin and has demonstrable activity against a variety of pathogenic bacteria, including mycobacteria.8, 9
Figure 1.

Kasugamycin and minosaminomycin are potent aminoglycoside antibiotics and are structurally related.
Our interest in kasugamycin and minosaminomycin stems from both fundamental and applied considerations. These antibiotics have been synthetically unexamined for close to fifty years.8, 10–16 Our laboratory has a deep interest in the development of new reactions which would enable the syntheses of complex antibiotics. Specifically, we envisioned that our laboratory’s sulfamate-tethered aza-Wacker cyclization could serve as a key step for the assembly of the kasugamine portion of these antibiotics.17–24 Our interest in designing practical synthetic routes to antibiotics with new mechanisms of action25–31 relative to approved agents is motivated by the global rise in resistance to antibacterial and antifungal agents currently in use.32–34 It is our hope that de novo syntheses will allow for the rapid preparation of analogues35–37 and will serve as a start of a drug-discovery effort to replenish worrisome antibacterial and antifungal pipelines. In this account, we describe our preparation of a kasugamine synthon, a common building block for both kasugamycin and minosaminomycin.
There are two general strategies for the syntheses of unusual monosaccharides.38,49–51 One is the classic “heterocycle→heterocycle” approach; in such syntheses, chemists begin with a more readily available monosaccharide and then systematically manipulate it to the end target. A second, less explored approach is to commence with a linear precursor, build functional group complexity, and then effect a late-stage cyclization (“linear→cyclic”). The former approach generally has fewer total step counts; on balance, due to existing complexity, monosaccharides can be very challenging starting materials. For this reason, we prefer the latter approach, because of the greater flexibility it affords and chose kasugamine derivative A as our first target (Scheme 1).
Scheme 1.

Retrosynthetic analysis.
In accord with this plan, our retrosynthesis is depicted in Scheme 1. After a late stage hemiacetalization of heteroatom-rich linear precursor C, deoxygenation and azide reduction would furnish target A. C would be built systematically from epoxide F.39, 40 We envisioned our laboratory’s sulfamate-tethered aza-Wacker cyclization17, 21 would serve as a key step during the transformation of epoxide F into aldehyde C. The successful execution of this strategy would increase the prominence of both classical and tethered aza-Wacker chemistry for the stereoselective assembly of complex targets.19, 41
Our forward synthesis (Scheme 2) commenced with morpholine amide 1, itself prepared in two steps from commercial D-erythrono-1,4-lactone in a procedure developed and disclosed by our laboratory.17 Morpholine amide 1 was converted into diol 2 by heating with NaBH4 in EtOH. Treatment of diol 2 with benzoyl chloride in ice-cold CH2Cl2 allowed for selective esterification of the primary alcohol. The secondary alcohol of benzoate 3 was tosylated using a standard mixture of NEt3, TsCl, and catalytic DMAP. Treatment of 4 with mixture of K2CO3 in MeOH allowed for the hydrolysis of the benzoate ester; the resulting alkoxide anion displaced the tosylate in an intramolecular SN2 reaction to form epoxide 5. CuI mediated the regioselective opening of epoxide 5 by cis-1-propenyl-magnesium bromide. For this transformation, we have found that it is critical to freshly prepare cis-1-propenylmagnesium bromide; for reasons unknown, using commercial solutions of 1-propenyl-magnesium bromide did not allow for clean reactivity. Sulfamoylation of alcohol 6 proceeded smoothly with Johnson and Magolan’s protocol.42
Scheme 2.

Opening sequence of reactions.
As a first step to evaluating the sulfamate-tethered aza-Wacker cyclization with 7, we tested our standard protocol of Pd(OAc)2/Cu(OAc)2 in hot CH3CN and a ~1 atm O2 atmosphere. We were pleased that this delivered desired oxathiazinane 8 in a 36% yield, an excellent starting point for further optimization (Scheme 3, Entry 1). Switching to Pd2(dba)3 at a higher loading increased the yield to 50% (Scheme 3, Entry 2). Using PdCl2(nbd) and Pd(CH3CN)2Cl2 in place of Pd2(dba)3 did not help the reaction (Scheme 3, Entries 3–5). We have previously observed a dramatic improvement in sulfamate-tethered aza-Wacker cyclizations when mono-protected amino acid ligands and 4Å molecular sieves are used. We hypothesize, in accord with the Yu laboratory’s observations,43–45 that mono-protected amino acids (MPAA) ligands help promote the Pd (II) – Pd (0) catalytic cycles operative during aza-Wacker transformations. Indeed, in line with our predictions, the yield of oxathiazinane 8 increased to 63% upon the addition of MPAA ligand Fmoc-Gly-OH and 4Å molecular sieves (Scheme 3, Entry 6); furthermore, starting material was fully consumed. Despite the presence of molecular sieves, it was critical to use anhydrous DMSO for the best reaction yield. X-ray structural analysis of 8 (CCDC: 2289376) allowed us to unambiguously confirm its identity and absolute stereochemistry. We note that this product formed as a single diastereomer (within the limits of 1H NMR analysis). Test reactions were conducted on a 30 mg scale, but our optimized reaction conditions could be applied to batches as large as 1.7 g.
Scheme 3.

Optimization of the key sulfamate-tethered aza-Wacker cyclization.
Moving forward, a Cbz group was appended to the oxathiazinane nitrogen for a subsequent ring-opening with NaN3 (Scheme 4).46, 47 The alkene of 10 was cleaved by dihydroxylation followed by treatment with NaIO4. Aldehyde 12 was unstable to purification and was immediately treated with TsOH to form hemiacetal 13 as a mixture of anomers. This anomeric mixture was bis-acetylated with Ac2O/pyridine, and the anomeric acetate was exchanged with thiophenol using BF3•OEt2. For 15, we have assigned the configuration at the anomeric carbon based on the small coupling constant of the anomeric proton (~1.5 Hz) and by analogy to the crystal structure of a later intermediate (vide infra). The acetate of 15 was removed using K2CO3 in MeOH.
Scheme 4.

Towards the endgame.
Initially, it was our plan to tosylate the alcohol of 16 and to displace it with an appropriate hydride source (Scheme 5A). Accordingly, tosylate 17 was prepared by stirring 16 with NEt3 and TsCl. However, despite multiple trials, including with NaBH4, LiAlH4, and NaBH3CN, we were unable to cleanly obtain 18, likely because of the presence of an azide functionality. We thus sought to develop a protocol which would allow for the clean formation of the methyl group and the primary amine of kasugamine in a single step. Knowing that there was precedent for the Pd-catalyzed hydrogenolysis of azides and alkyl iodides,48 we prepared iodide 19 using a Finkelstein reaction (Scheme 5B). Indeed, stirring 19 with NEt3 and Pd-C under an atmosphere of H2 gas cleanly delivered kasugamine derivative 20. Crystal structure analysis of 20 (CCDC 2291427) allowed us to unambiguously confirm its identity and absolute stereochemistry.
Scheme 5.

(A) Displacement of the tosylate by hydride does not give expected product under multiple conditions. (B) Upon treatment with Pd/C and H2 gas, deiodination and azide reduction occur in one step to furnish the desired target.
In summary, we have completed a concise synthesis of a kasugamine synthon, which we envision can be used in preparations of kasugamycin, minosaminomycin, and related analogues. A highlight of the synthesis is our sulfamate-tethered aza-Wacker cyclization. The march towards total syntheses of these antibiotics continues in our laboratory.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by National Institutes of Health grant R35GM142499 and a CMADP pilot project grant (P30GM145499) awarded to Shyam Sathyamoorthi. Justin Douglas and Sarah Neuenswander (KU NMR Lab) are acknowledged for help with structural elucidation. Lawrence Seib and Anita Saraf (KU Mass Spectrometry Facility) are acknowledged for help acquiring HRMS data. Rasel Mian (KU X-Ray Crystallography Lab) is acknowledged for help with crystal structures.
Footnotes
Supporting Information Placeholder
ASSOCIATED CONTENT
Supporting Information Statement
Contains additional experimental details including NMR Spectra and X-ray Crystallographic Information.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
REFERENCES
- 1.Umezawa H; Hamada M; Suhara Y; Hashimoto T; Ikekawa T, Kasugamycin, a new antibiotic. Antimicrob. Agents Chemother 1965, 5, 753–757. [PubMed] [Google Scholar]
- 2.Umezawa H; Okami Y; Hashimoto T; Suhara Y; Hamada M; Takeuchi T, A New Antibiotic, Kasugamycin. J. Antibiot., Ser. A 1965, 18, 101–103. [PubMed] [Google Scholar]
- 3.Suhara Y; Maeda K; Umezawa H; Ohno M, Kasugamycin. In Deoxy Sugars, American Chemical Society; 1968; Vol. 74, pp 15–40. [DOI] [PubMed] [Google Scholar]
- 4.Hamada M; Hashimoto T; Takahashi T; Yokoyama S; Miyake M; Takeuchi T; Okami Y; Umezawa H, Antimicrobial Activity of Kasugamycin. J. Antibiot. Ser. A 1965, 18, 104–106. [PubMed] [Google Scholar]
- 5.Takeuchi T; Ishizuka M; Takayama H; Kureha K; Hamada M; Umezawa H, Pharmacology Of Kasugamycin And The Effect On Pseudomonas Infection. J. Antibiot. (Tokyo) 1965, 18, 107–110. [PubMed] [Google Scholar]
- 6.Ishiyama T; Hara I; Matsuoka M; Satō K; Shimada S; Izawa R; Hashimoto T; Hamada M; Okami Y; Takeuchi T; Umezawa H, Studies on the Preventive Effect of Kasugamycin on Rice Blast. J. Antibiot. Ser. A 1965, 18, 115–119. [PubMed] [Google Scholar]
- 7.Yoshii A; Moriyama H; Fukuhara T, The Novel Kasugamycin 2′-N-Acetyltransferase Gene aac(2′)-IIa, Carried by the IncP Island, Confers Kasugamycin Resistance to Rice-Pathogenic Bacteria. Appl. Environ. Microbiol 2012, 78, 5555–5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsuharu I; Shinichi K; Kenji M; Hamao U, Total Synthesis of Minosaminomycin. Bull. Chem. Soc. Jpn 1977, 50, 1850–1854. [Google Scholar]
- 9.Hamada M; Kondo S; Yokoyama T; Miura K; Iinuma K; Yamamoto H; Maeda K; Takeuchi T; Umezawa H, Minosaminomycin A New Antibiotic Containing Myo-Inosamine. J. Antibiot. (Tokyo) 1974, 27, 81–83. [DOI] [PubMed] [Google Scholar]
- 10.Suhara Y; Sasaki F; Maeda K; Umezawa H; Ohno M, The total synthesis of kasugamycin. J. Am. Chem. Soc 1968, 90, 6559–6560. [DOI] [PubMed] [Google Scholar]
- 11.Suhara Y; Sasaki F; Koyama G; Maeda K; Umezawa H; Ohno M, Total synthesis of kasugamycin. J. Am. Chem. Soc 1972, 94, 6501–6507. [DOI] [PubMed] [Google Scholar]
- 12.Nii Y; Okano K; Kobayashi S; Ohno M, Synthesis of amidinoformic acids using benzyl cyanoformate as a synthon. Tetrahedron Lett 1979, 20, 2517–2520. [Google Scholar]
- 13.Juji Y; Ken-ichi S; Hironobu H; Kuniaki S, Aminosugars. XXVIII. A Facile Synthesis of Benzyl α- and β-Kasugaminides via the Corresponding Abequosides. Bull. Chem. Soc. Jpn 1977, 50, 3305–3309. [Google Scholar]
- 14.Hanessian S; Masse R, Synthetic approaches to kasugamine. Carbohydr. Res 1974, 35, 175–185. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda S; Ogasawara T; Kawabata S; Iwataki I; Matsumoto T, The synthesis of methyl N,N′-diacetyl-α-d-kasugaminide. Tetrahedron 1973, 29, 3141–3147. [Google Scholar]
- 16.Suhara Y; Maeda K; Umezawa H; Ohno M, Chemical studies on kasugamycin. V. The structure of kasugamycin. Tetrahedron Lett 1966, 7, 1239–1244. [DOI] [PubMed] [Google Scholar]
- 17.Paul D; Mague JT; Sathyamoorthi S, Sulfamate-Tethered Aza-Wacker Cyclization Strategy for the Syntheses of 2-Amino-2-deoxyhexoses: Preparation of Orthogonally Protected d-Galactosamines. J. Org. Chem 2023, 88, 1445–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagamalla S; Johnson DK; Sathyamoorthi S, Sulfamate-tethered aza-Wacker approach towards analogs of Bactobolin A. Med. Chem. Res 2021, 30, 1348–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas AA; Nagamalla S; Sathyamoorthi S, Salient features of the aza-Wacker cyclization reaction. Chem. Sci 2020, 11, 8073–8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagamalla S; Mague JT; Sathyamoorthi S, Progress towards the syntheses of Bactobolin A and C4-epi-Bactobolin A using a sulfamate-tethered aza-Wacker cyclization strategy. Tetrahedron 2022, 133112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shinde AH; Sathyamoorthi S, Oxidative Cyclization of Sulfamates onto Pendant Alkenes. Org. Lett 2020, 22, 896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinde AH; Sathyamoorthi S, Large Scale Oxidative Cyclization of (E)-hex-3-en-1-yl (4-methoxyphenyl)sulfamate. Org. Synth 2022, 99, 286–304. [Google Scholar]
- 23.Joshi H; Sathyamoorthi S, Hydroxyselenylation and Tethered Silanoxyselenylation of Allylic Silanols. J. Org. Chem 2022, 87, 5017–5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinde AH; Thomas AA; Mague JT; Sathyamoorthi S, Highly Regio- and Diastereoselective Tethered Aza-Wacker Cyclizations of Alkenyl Phosphoramidates. J. Org. Chem 2021, 86, 14732–14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka N; Yamaguchi H; Umezawa H, Mechanism of Kasugamycin Action on Polypeptide Synthesis. J. Biochem 1966, 60, 429–434. [DOI] [PubMed] [Google Scholar]
- 26.Schluenzen F; Takemoto C; Wilson DN; Kaminishi T; Harms JM; Hanawa-Suetsugu K; Szaflarski W; Kawazoe M; Shirouzu M; Nierhaus KH; Yokoyama S; Fucini P, The antibiotic kasugamycin mimics mRNA nucleotides to destabilize tRNA binding and inhibit canonical translation initiation. Nat. Struct. Mol. Biol 2006, 13, 871–878. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka N; Yoshida Y; Sashikata K; Yamaguchi H; Umezawa H, Inhibition of polypeptide synthesis by kasugamycin, an aminoglycosidic antibiotic. J. Antibiot. (Tokyo) 1966, 19, 65–68. [PubMed] [Google Scholar]
- 28.Zhang Y; Aleksashin NA; Klepacki D; Anderson C; Vázquez-Laslop N; Gross CA; Mankin AS, The context of the ribosome binding site in mRNAs defines specificity of action of kasugamycin, an inhibitor of translation initiation. Proc. Natl. Acad. Sci. USA 2022, 119, e2118553119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poldermans B; Goosen N; Van Knippenberg PH, Studies on the function of two adjacent N6,N6-dimethyladenosines near the 3’ end of 16 S ribosomal RNA of Escherichia coli. I. The effect of kasugamycin on initiation of protein synthesis. J. Biol. Chem 1979, 254, 9085–9089. [PubMed] [Google Scholar]
- 30.van Buul CPJJ; Visser W; van Knippenberg PH, Increased translational fidelity caused by the antibiotic kasugamycin and ribosomal ambiguity in mutants harbouring the ksgA gene. FEBS Lett 1984, 177, 119–124. [DOI] [PubMed] [Google Scholar]
- 31.Okuyama A; Machiyama N; Kinoshita T; Tanaka N, Inhibition by kasugamycin of initiation complex formation on 30S ribosomes. Biochem. Biophys. Res. Commun 1971, 43, 196–199. [DOI] [PubMed] [Google Scholar]
- 32.Ventola CL, The antibiotic resistance crisis: part 1: causes and threats. P T 2015, 40, 277–83. [PMC free article] [PubMed] [Google Scholar]
- 33.Lucas JA; Hawkins NJ; Fraaije BA, Chapter Two - The Evolution of Fungicide Resistance. In Advances in Applied Microbiology, Academic Press; 2015; Vol. 90, pp 29–92. [DOI] [PubMed] [Google Scholar]
- 34.Vitiello A; Ferrara F; Boccellino M; Ponzo A; Cimmino C; Comberiati E; Zovi A; Clemente S; Sabbatucci M, Antifungal Drug Resistance: An Emergent Health Threat. Biomedicines 2023, 11, 1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Z-C; Boger DL, Maxamycins: Durable Antibiotics Derived by Rational Redesign of Vancomycin. Acc. Chem. Res 2020, 53, 2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright PM; Seiple IB; Myers AG, The Evolving Role of Chemical Synthesis in Antibacterial Drug Discovery. Angew. Chem. Int. Ed 2014, 53, 8840–8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Privalsky TM; Soohoo AM; Wang J; Walsh CT; Wright GD; Gordon EM; Gray NS; Khosla C, Prospects for Antibacterial Discovery and Development. J. Am. Chem. Soc 2021, 143, 21127–21142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skarbek K; Milewska MJ, Biosynthetic and synthetic access to amino sugars. Carbohydr. Res 2016, 434, 44–71. [DOI] [PubMed] [Google Scholar]
- 39.White JD; Badger RA; Kezar HS; Pallenberg AJ; Schiehser GA, Structure, Sythesis and Absolute Configuration of Leptosphaerin, a Metabolite of the Marine Ascomycete Leptosphaeria oraemaris. Tetrahedron 1989, 45, 6631–6644. [Google Scholar]
- 40.Vargeese C; Abushanab E, Chemistry of L-ascorbic and D-isoascorbic acids. 4. An efficient synthesis of 2-deoxypentofuranoses. J. Org. Chem 1990, 55, 4400–4403. [Google Scholar]
- 41.Weinstein AB; Schuman DP; Tan ZX; Stahl SS, Synthesis of Vicinal Aminoalcohols by Stereoselective Aza-Wacker Cyclizations: Access to (−)-Acosamine by Redox Relay. Angew. Chem. Int. Ed 2013, 52, 11867–11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sguazzin MA; Johnson JW; Magolan J, Hexafluoroisopropyl Sulfamate: A Useful Reagent for the Synthesis of Sulfamates and Sulfamides. Org. Lett 2021, 23, 3373–3378. [DOI] [PubMed] [Google Scholar]
- 43.Shao Q; Wu K; Zhuang Z; Qian S; Yu J-Q, From Pd(OAc)2 to Chiral Catalysts: The Discovery and Development of Bifunctional Mono-N-Protected Amino Acid Ligands for Diverse C–H Functionalization Reactions. Acc. Chem. Res 2020, 53, 833–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engle KM; Wang D-H; Yu J-Q, Ligand-Accelerated C−H Activation Reactions: Evidence for a Switch of Mechanism. J. Am. Chem. Soc 2010, 132, 14137–14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi B-F; Maugel N; Zhang Y-H; Yu J-Q, PdII-Catalyzed Enantioselective Activation of C(sp2)-H and C(sp3)-H Bonds Using Monoprotected Amino Acids as Chiral Ligands. Angew. Chem. Int. Ed 2008, 47, 4882–4886. [DOI] [PubMed] [Google Scholar]
- 46.Espino CG; Wehn PM; Chow J; Du Bois J, Synthesis of 1,3-Difunctionalized Amine Derivatives through Selective C−H Bond Oxidation. J. Am. Chem. Soc 2001, 123, 6935–6936. [Google Scholar]
- 47.Paradine SM; Griffin JR; Zhao J; Petronico AL; Miller SM; Christina White M, A manganese catalyst for highly reactive yet chemoselective intramolecular C(sp3)–H amination. Nat. Chem 2015, 7, 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weerapana E; Glover KJ; Chen MM; Imperiali B, Investigating Bacterial N-Linked Glycosylation: Synthesis and Glycosyl Acceptor Activity of the Undecaprenyl Pyrophosphate-Linked Bacillosamine. J. Am. Chem. Soc 2005, 127, 13766–13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dhara D; Bouchet M; Mulard LA Scalable Synthesis of Versatile Rare Deoxyamino Sugar Building Blocks from D -Glucosamine. J. Org. Chem 2023, 88, 6645–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pragani R; Stallforth P; Seeberger PH De Novo Synthesis of a 2-Acetamido-4-Amino-2,4,6-Trideoxy-D-Galactose (AAT) Building Block for the Preparation of a Bacteroides Fragilis A1 Polysaccharide Fragment. Org. Lett 2010, 12, 1624–1627. [DOI] [PubMed] [Google Scholar]
- 51.Vasquez O; Alibrandi A; Bennett CS, De Novo Synthetic Approach to 2,4-Diamino-2,4,6-trideoxyhexoses (DATDH): Bacterial and Rare Deoxy-Amino Sugars. Org. Lett 2023, 25, 7873–7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.


