Abstract
High baseline clearance of immune checkpoint inhibitors (ICIs), independent of dose or systemic exposure, is associated with cachexia and poor outcomes in cancer patients. Mechanisms linking ICI clearance, cachexia and ICI therapy failure are unknown. Here, we evaluate in four murine models and across multiple antibodies whether altered baseline catabolic clearance of administered antibody requires a tumor and/or cachexia and whether medical reversal of cachexia phenotype can alleviate altered clearance. Key findings include mild cachexia phenotype and lack of elevated pembrolizumab clearance in the MC38 tumor-bearing model. We also observed severe cachexia and decreased, instead of increased, baseline pembrolizumab clearance in the tumor-free cisplatin-induced cachexia model. Liver Fcgrt expression correlated with altered baseline catabolic clearance, though elevated clearance was still observed with antibodies having no (human IgA) or reduced (human H310Q IgG1) FcRn binding. We conclude cachexia phenotype coincides with altered antibody clearance, though tumor presence is neither sufficient nor necessary for altered clearance in immunocompetent mice. Magnitude and direction of clearance alteration correlated with hepatic Fcgrt, suggesting changes in FcRn expression and/or recycling function may be partially responsible, though factors beyond FcRn also contribute to altered clearance in cachexia.
Keywords: Cachexia, Immune Checkpoint Inhibitor, Clearance, FcRn, LLC, MC38
1. Introduction
Immunotherapy with immune checkpoint inhibition is becoming one of the main treatment options for patients with cancer. Monoclonal antibody (mAb) immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1 (PD-1), programmed cell death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) have demonstrated improved efficacy, either as single agent or as part of combination therapies in both early stage and advanced non–small cell lung cancer (NSCLC) [1-7], advanced urothelial cancer [8], and colorectal carcinoma [9], among many others. Although broadly successful, it remains unclear why overall response rates with ICI monotherapy remain low (~30%) and why only a subset of individuals achieves sustained responses [10]. Significant efforts have focused on characterizing the tumor microenvironment and infiltrating immune cell determinants of response to ICIs [11-16], though awareness and appreciation for broader host factors is increasing [17-22]. Investigations on how to better identify responsive patients and mechanisms of resistance are needed to facilitate effective development of next-generation immunotherapies and improved use of current immunotherapies.
Recent retrospective analyses of clinical pharmacology data revealed high baseline ICI clearance and/or steady or increasing ICI clearance over time are both associated with shorter overall survival in NSCLC, melanoma, and metastatic urothelial carcinoma [23-28]. Importantly, associations between poor response and elevated clearance are decoupled from circulating drug levels and receptor occupancy, suggesting elevated clearance is a biomarker for, and not a cause of, poor response [27]. Notably, in several diseases and with multiple ICIs, high mAb clearance and/or shorter survival are also associated with classical clinical features of cancer cachexia, including increased weight loss and reduced serum albumin levels [29-33]. We recently demonstrated in two common pre-clinical models of cancer cachexia that catabolic clearance of pembrolizumab, a humanized antibody targeting human PD-1, is elevated in tumor-bearing cachectic mice, thus replicating the phenomenon observed in cachectic cancer patients [34]. In this study, we sought to determine whether the observed elevated pembrolizumab clearance in mouse models is in fact dependent on the cachexia phenotype or merely a consequence of tumor burden. Additionally, we investigated how the elevated mAb clearance may be influenced by the neonatal Fc receptor (FcRn) and whether therapeutic interventions to improve cachexia impacted mAb clearance.
To characterize the mechanisms causing elevated pembrolizumab clearance, we utilized two mouse models, the MC38 tumor-bearing non-cachectic model, and the cisplatin-induced cachectic model. MC38 is a murine colon carcinoma model [35], established via subcutaneous injection of MC38 cells to the right flanks of experimental animals. The MC38 colon cancer cell line has revealed mixed results with respect to cachexia, with literature suggesting both non-cachectic and cachectic phenotypes [36, 37]. Within the timeline of our experiments, MC38 tumor-bearing mice were determined to be non-cachectic, similar to published results [34, 38, 39]. Cisplatin is an alkylating antineoplastic agent routinely used in the clinic as first-line therapy to a variety of cancers [40, 41]. Among the severe side effects induced by cisplatin chemotherapy, muscle wasting has been shown to be associated with worsened treatment outcome and increased mortality [42]. Cisplatin-induced muscle wasting in rats and mice has also been described as another model of cachexia atrophy [43-48]. Using MC38 tumor-bearing non-cachectic mice and tumor-free chemotherapy-induced cachectic mice, we aimed to segregate tumor-inducing and cachexia-inducing factors in vivo and evaluate their effect on pembrolizumab clearance. Furthermore, we also expanded our analysis beyond pembrolizumab to other mAbs with divergent immunoglobulin G (IgG) subtypes and specificity to different targets. These studies were carried out in C26 and LLC models of cancer cachexia that we have previously used to characterize pembrolizumab clearance and specifically to investigate whether the observed elevated clearance was unique to pembrolizumab.
All therapeutic mAbs currently used in the clinic are IgGs and are eliminated predominantly via two pathways: 1) target-mediated specific clearance, which happens after specific interaction between therapeutic mAb and its antigen; and 2) catabolic nonspecific clearance, which involves target-independent cellular uptake of mAbs and subsequent protein catabolism. Other possible mAb clearance mechanisms, including Fc-gamma receptor (FcγR)-mediated endocytosis with subsequent proteolysis [49] and antidrug antibody (ADA)-mediated clearance [50] are not thought to significantly contribute to the overall elimination of the investigated mAbs. ADA formation is unlikely with single dose pharmacokinetic (PK) experiments, and while data from FcγR knockout mice suggests FcγR binding does modulate IgG clearance, the contribution of FcγRs is currently considered to be minimal, especially for monomeric IgG that are not part of immune complexes [51-53]. Hence, in our PK experiments, we designed studies focused primarily on catabolic, nonspecific clearance for antibodies that lack a biological mouse target (pembrolizumab, durvalumab and untargeted, wild type (WT) human IgG1), though for a rat, anti-mouse PD-1 antibody (RMP1-14), both non-specific and target-mediated pathways were assumed to contribute to overall clearance.
During the elimination of therapeutic IgG, FcRn provides an efficient IgG recycling mechanism, sustaining plasma concentrations and prolonging circulating half-life, such that the pH-dependent binding of IgG to FcRn has been shown to substantially influence the clearance of mAbs in both animals and humans [54-56]. In line with the well-established role of FcRn in salvaging IgG from lysosomal degradation, we reported previously a significant downregulation of hepatic Fcgrt, the gene encoding for FcRn, in both the C26 and LLC tumor-bearing mice compared to their respective CD2F1 and C57BL/6 tumor-free control groups [34]. Herein we looked at hepatic Fcgrt expression in MC38 tumor-bearing, non-cachectic mice to isolate only cachexia-induced changes. We also studied the effect of altered FcRn salvage on cachexia-associated elevations in the clearance of a mutant mAb with reduced FcRn binding in a mouse model of cancer cachexia.
Finally, we assessed the impact of AR-42 (REC-2282), an investigational histone deacetylase (HDAC) inhibitor with anti-cachectic efficacy in mouse models of cancer cachexia [57], on cachexia-associated elevation of pembrolizumab clearance. Orally administered AR-42 preserved body weight, prevented loss of skeletal muscle and adipose tissue mass, and improved muscle quality and strength [39, 57]. Given its ability to ameliorate cancer-induced wasting, we hypothesized AR-42 administration may reduce or eliminate elevation of mAb clearance in tumor-bearing, cachectic mice.
2. Materials and methods
2.1. Reagents and chemicals
Pembrolizumab (Catalog #A2005, batch A200504) and durvalumab (Catalog #2013) were purchased from Selleckchem (Houston, TX). InVivoMAb rat anti-mouse PD-1 (Clone RMP1-14, Catalog #BE0146) and InVivoPlus rat IgG2a isotype control (Clone 2A3, Catalog #BP0089) were purchased from BioXCell (West Lebanon, NH). Native human IgA protein (hIgA, Catalog #ab91025) was purchased from Abcam (Cambridge, MA). Human IgG1 (hIgG1) kappa isotype control (Catalog #C0001) and its hIgG1 H310Q mutant were supplied by Crown Biosciences (San Diego, CA). Cisplatin (00143950501) was purchased from Covetrus (Portland, ME).
2.2. Animals
Six- to eight-week-old CD2F1 male mice (Envigo, Indianapolis, IN) and C57BL/6 male mice (Envigo, Indianapolis, IN or Jackson Laboratory, Bar Harbor, ME) were group-housed under conditions of constant photoperiod (12hrs light/12hrs dark), temperature, and humidity with ad libitum access to water and standard pelleted chow. Mice were weighed no less than once per week. Tumor volumes were calculated from caliper measurements using a standard formula (length × width2 × π/6). At the end of study, mice were euthanized by CO2 inhalation.
2.3. Cells
The murine MC38 colon carcinoma cells were purchased from Kerafast (Boston, MA). Murine C26 colon carcinoma cells were generously provided by the laboratory of Denis Guttridge (while at The Ohio State University) who obtained the cells through a Material Transfer Agreement with the NCI (Bethesda, MD) in 2001. Cultured murine C26 cells were maintained in RPMI, while LLC, and MC38 cells were maintained in Dulbecco's Modified Eagle Medium (DMEM). All media was supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin, at 37 °C in a humidified incubator with 5% CO2. For injection into mice, cells were harvested with trypsin, pelleted by centrifugation in FBS-supplemented DMEM, and then resuspended in sterile PBS at a concentration of 5 × 106 cells/mL for C26 and MC38 or 10 × 106 cells/mL for LLC. Prior to injection, all cell lines were confirmed negative for mycoplasma using the commercially available Plasmotest kit (Invivogen, San Diego, CA).
2.4. Animal studies
2.4.1. Single-dose pharmacokinetic studies in various mouse models
All studies were conducted in a similar manner to those previously published with pembrolizumab PK in C26 and LLC mouse models [34]. Briefly, one or two experiments were conducted for each combination of antibody and mouse model using a single tail vein injection at dose levels 100 μg, 200 μg, 2 mg/kg, or 10 mg/kg. For all tumor-bearing models, tumors were established via subcutaneous (C26, MC38) or intramuscular injection (LLC) in male CD2F1 (C26) or C57BL/6 (MC38, LLC) mice. For the cisplatin model, mice received three intraperitoneal cisplatin injections, 3 mg/kg every other day, as a modified cisplatin dose-regimen previously described in mice [45, 58]. For the study with AR-42, six days after tumor cell or vehicle inoculation, animals were treated orally by gavage with vehicle or 20 mg/kg AR-42 daily for 12 days, which was demonstrated to provide anti-cachectic effects in C26 tumor-bearing mice [39]. Four to six serial blood samples were collected from each animal post-antibody administration. After CO2 euthanasia, hind limb muscles (gastrocnemius, quadriceps, tibialis anterior), epididymal fat pads, tumor, spleen and liver were carefully dissected from each mouse and weighed. Sections of each tissue were placed in RNAlater (Catalog #AM7021, ThermoFisher) and/or snap frozen in liquid nitrogen and stored at −80 °C until analysis. Additional details are presented as Supplement [see Supplementary Materials and Methods].
2.4.2. Quantification of antibodies in mouse plasma with enzyme-linked immunoassay (ELISA)
Free pembrolizumab (unbound to PD-1) was measured in mouse plasma samples by ELISA as previously described [59]. Plasma concentrations of durvalumab, WT hIgG1, and hIgG1 H310Q antibodies were measured with Invitrogen total human IgG uncoated ELISA kit (Catalog #88-50550), purchased from ThermoFisher (Waltham, MA), and conducted per the manufacturer’s protocol. Plasma concentrations of RMP1-14 and rat IgG2a antibody were measured with Invitrogen total rat IgG uncoated ELISA kit (Catalog # 88-50490), purchased from ThermoFisher (Waltham, MA). The protocol provided with the kit was followed. Additional details are presented as Supplement [see Supplementary Materials and Methods].
2.4.3. Assessment of albumin levels
Plasma samples collected at the terminal timepoint were submitted for albumin analyses to the Comparative Pathology and Digital Imaging Shared Resource (CPDISR) at The Ohio State University.
2.4.4. Atrogin-1, MuRF-1 and Fcgrt Gene Expression Analyses by qRT-PCR
Gene expression of liver Fcgrt and muscle atrogin-1, and MuRF-1 (genes encoding Fcrn, atrogin-1/Fbxo32 and MuRF1/Trim63 proteins, respectively) were determined as previously described [34, 39]. Additional details are presented as Supplement [see Supplementary Materials and Methods].
2.4.5. Measurement of murine FcRn affinity by surface plasmon resonance (SPR)
Human IgG1 antibodies binding capacity to murine FcRn was analyzed using surface plasmon resonance by Crown Biosciences (San Diego, CA). In brief, mouse FcRn (FCGRT&B2M heterodimer protein, AcroBio Cat# FCM M52W8) was captured on CM5 sensor chip. Running buffer consisted of 1xPBS, 50 μM EDTA, and 0.05% Tween 20, pH6.0. WT hIgG1, kappa isotype control antibody was injected at 6 different concentrations, 100, 50, 25, 12.5, 6.25, 3.125 nM with 90 s association and 140 s dissociation. H310Q mutant hIgG1 was also injected at 6 different concentrations, 1000, 500, 250, 125, 62.5, 31.25 nM with 90 s association and 140 s dissociation. Binding kinetics was calculated by fitting the curves with 1:1 binding model.
2.5. Pharmacokinetic analysis
Data from each experiment were analyzed using parametric or non-parametric approaches as indicated for each experiment. Pharmacokinetic analyses were performed on antibody plasma concentration data from all single-dose PK studies using nonlinear mixed-effects modeling in NONMEM, Version 7.3, implementing the first-order conditional estimation method with interaction (FOCE-I), as we described previously [34]. Briefly, antibody plasma concentration data was fit to a two-compartment model parameterized in terms of clearance (CL), central compartment volume of distribution (V1), inter-compartmental clearance (Q) and peripheral compartment volume of distribution (V2). Data from each set of combined, replicate experiments were modeled independently using an exponential error model for inter-individual variability (IIV) estimation and an additive error model for estimation of residual variability (ε) for log-transformed data. Mice receiving partial intravenous doses or displaying apparent absorption phases were excluded. R (Version 3.3.1; http://www.r-project.org) was used for visual diagnostics. Additional details are presented in the Supplementary Materials and Methods.
2.6. Statistics
Data from each experiment were analyzed using parametric or non-parametric approaches as indicated for each experiment. Briefly, tissue weight measurements, albumin measurements, qRT-PCR data were analyzed by Mann-Whitney test. For the qRT-PCR, dCT values were used for the analysis and 2^-dCT values as a ratio of control group were used for the display. For the study with AR-42, 2-way ANOVA was used to analyze the effects of both cancer cachexia and AR-42. The influence of tumor or treatment on clearance was estimated as a categorical covariate, and the significance of the effect was judged by the log-likelihood ratio test. For the description of PK parameters, geometric mean and geometric standard deviation (SD) were used as PK parameters generally follow log-normal distribution. Additional details are presented in the Supplementary Materials and Methods.
2.7. Study approval
All animal studies were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee at The Ohio State University (protocol # 2017A00000117), and with responsibilities and procedures outlined in The Ohio State University's Animal Welfare Assurance (D16-00168 [A3261-01]) as approved by the U.S. Public Health Service Office of Laboratory Animal Welfare.
3. Results
3.1. Characterization of cachectic phenotypes in different mouse models
3.1.1. MC38 tumor-bearing mice were non-cachectic
Within the timeline of our experiment, MC38 tumor-bearing mice appeared to be non-cachectic as suggested by examining typical cachexia markers and endpoints in mouse models. As shown in our previous publications [34, 39], both C26 and LLC tumor-bearing mice exhibited typical cachectic phenotypes including significantly reduced tumor-adjusted terminal body weights, lower skeletal muscle mass, based on gastrocnemius and quadriceps weights, and decreased fat mass Figure 1A-D). In contrast, at the time of euthanasia, MC38 tumor-bearing mice showed no significant weight loss, muscle atrophy nor adipose depletion. Splenomegaly was observed in all three tumor models (Figure 1E), in line with reported evidence of induced pro-inflammatory signaling and acute phase response in these models [60]. There was no significant difference in baseline body weights between tumor-free controls and C26, LLC, or MC38 tumor-bearing mice (Mann Whitney p-values 0.78, 0.71, and 0.71, respectively). Changes in body weight and tumor volume over time in various mouse models are presented in Supplemental Figure 1.
Figure 1. Characterization of tumor-induced cachexia in the CD2F1/C26, C57BL/6/LLC, C57BL/6/MC38, and C57BL/6/Cisplatin models.
(A-G) Data from all mice across studies, at the time of euthanasia, presented as mean ratio ± SD (A-E) or as geometric mean ratio ± geometric SD (F,G) of associated control groups (vehicle-treated group for C57BL/6/Cisplatin model, and tumor-free groups for all other models). (A) Terminal body weights (for C57BL/6/Cisplatin model) or tumor-adjusted body weights (for all other models); (B) gastrocnemius mass; (C) quadriceps mass; (D) adipose mass; (E) spleen mass; mRNA expression of (F) Atrogin-1 and (G) MuRF-1. ns, non-significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 by Mann-Whitney test.
To further characterize and compare cachectic phenotypes across these models, we next looked at mRNA expression of muscle-specific E3 ubiquitin ligases, atrogin-1 and MuRF-1 in quadriceps muscles of individual animals from all three models. Increased expression of these genes has been shown to be associated with a variety of muscle wasting diseases and are often upregulated in cachexia models [61-63]. Expression of atrogin-1 and MuRF-1 were significantly elevated in skeletal muscles of both C26 and LLC tumor-bearing mice relative to tumor-free controls (Figure 1F-G), consistent with previously reported results with these models [39, 64]. While the upregulation of these genes is also statistically significant in the skeletal muscles of MC38 tumor-bearing mice, the magnitude of the change is much smaller compared to C26 and LLC. Overall, within the time course of our PK studies where C26 and LLC display characteristics of severe cachexia, MC38 tumor-bearing mice display a significantly reduced cachectic burden. Comparing the two subcutaneous colon cancer models, we note a similar range of tumor sizes, though mean MC38 tumor burden is lower compared to C26 tumor burden in these studies, despite the same number of cells injected and longer survival time, after tumor cell injection, of mice bearing MC38 tumors [Supplemental Figures 1 and 2]. We recognize the data suggest mice bearing MC38 tumors harbor characteristic of pre-cachexia or very mild cachexia, though given the stark differences in MC38 vs. the C26 and LLC models, we will refer to the MC38 model in this manuscript as “non-cachectic”.
3.1.2. Cisplatin-treated mice were severely cachectic
Commonly used models of cisplatin-induced cachexia often involve rat or mouse intraperitoneally treated with cisplatin (1–3 mg/kg) for 3–4 consecutive days, inducing rapid and substantial weight loss and muscle atrophy [43-47]. To accommodate the typical timeline of our PK studies, we extended the model by administrating 3 mg/kg of cisplatin every other day. After about two weeks post first dose, cisplatin-treated mice exhibited approximately 20% weight loss and significant muscle atrophy of both gastrocnemius and quadriceps, as well as severe fat depletion, while vehicle-treated controls showed no reduction in body mass or muscle weights (Figure 1A-D).
There was no significant difference in baseline body weights between cisplatin-treated animals and vehicle-treated controls (Mann Whitney p-value > 0.99). Consistent with previous reports of cisplatin-induced spleen toxicity in animals and humans [65-67], spleens of cisplatin-treated mice were considerably smaller compared to those of healthy, vehicle-treated controls (Figure 1E). We also evaluated E3 ligases, and the results indicated both atrogin-1 and MuRF-1 are upregulated in skeletal muscle of mice receiving cisplatin compared to vehicle-treated controls (Figure 1F-G). In summary, cisplatin-treated mice display a cachectic phenotype similar to the C26 and LLC models, though with even greater magnitude of difference in most of the markers evaluated.
3.2. Investigation of effects of tumor and cachexia on pembrolizumab pharmacokinetics
3.2.1. Pembrolizumab clearance was unchanged in MC38 tumor-bearing non-cachectic mice
Two separate MC38 studies were conducted and mice with or without MC38 tumors received pembrolizumab at dose of 10 mg/kg in the first study and dose of 100 μg in the second study. The average body weight of mice at dosing in the second study was 25.6 g which gives the average dose of 3.9 mg/kg. After exclusion of 19 mice receiving partial intravenous doses or displaying an apparent absorption phase in their individual PK profiles, a total of 30 mice and 150 pembrolizumab plasma concentrations from two independent animal studies were included for nonlinear mixed-effects analysis. Pembrolizumab concentration versus time profiles revealed no obvious differences between MC38 tumor-bearing and tumor-free animals through the entire PK sampling time course of up to 10 days (Figure 2A). Consistently, estimation of pembrolizumab clearance in the MC38 model yielded no difference between tumor-bearing and tumor-free animals (0.21 mL/day in tumor-free mice vs. 0.22 mL/day in MC38 tumor-bearing mice given 10 mg/kg pembrolizumab; 0.30 mL/day in tumor-free mice vs. 0.37 mL/day in MC38 tumor-bearing mice given 100 μg pembrolizumab; P > 0.05 by the log-likelihood ratio test, see Supplemental Table 1 and Supplementary Results). In contrast, significantly elevated clearance was observed in C26 and LLC models of cancer cachexia (Figure 2B). Of note, in our previously published results, we concluded dose proportionality of pembrolizumab PK with doses of 2 mg/kg and 10 mg/kg within the same experiment in CD2F1 tumor free and C26 tumor-bearing mice, and then within the same experiment in C57BL/6 tumor free and LLC tumor-bearing mice [Supplemental Figure 3, A-D]. However, in the current study, when evaluating pembrolizumab PK at doses of 10 mg/kg in one experiment and 100 μg in a second experiment in MC38 tumor-bearing mice [Supplemental Table 1], we observed an apparent difference in pembrolizumab clearance between the two studies and dose levels [Supplemental Figure 3, E-F] [34]. After controlling for this study or dose effect, we evaluated the presence of MC38 tumor on clearance and observed no significant difference between tumor-free and tumor-bearing mice based on the log-likelihood ratio test [Supplementary Results]. These results suggest that the increased pembrolizumab clearance cannot be solely attributed to the presence of tumor burden and may instead be dependent on cachectic signaling.
Figure 2. Pembrolizumab pharmacokinetics in the C57BL/6/MC38 and C57BL/6/Cisplatin models.
(A) Data from 30 C57BL/6 mice without (TF, n = 11) or with MC-38 tumors (TB, n = 19); (B) Comparison of single-dose pembrolizumab clearance (CL) in TF and TB mice in the CD2F1/C26, C57BL/6/LLC, and C57BL/6/MC38 models; (C) Data from 13 C57BL/6 mice treated either with vehicle (n = 5) or with cisplatin (n = 8); (D) Model-estimated single-dose pembrolizumab clearance (CL) in vehicle-treated and cisplatin-treated C57BL/6 mice; All data are shown as mean ± SD (A, C) or as geometric mean ± geometric SD (B, D). ns, non-significant, **, P < 0.01, ****, P < 0.0001 by the log-likelihood ratio test.
3.2.2. Pembrolizumab clearance was reduced in tumor-free mice with chemotherapy-induced cachexia
Chemotherapy-induced cachexia is a relevant experimental model, as chemotherapeutic agents such as cisplatin have been shown to result in the development and progression of cachexia in animals and patients [68-73]. Interestingly, compared to tumor-bearing models of cancer-associated cachexia, pembrolizumab PK characteristics were completely different in cisplatin-induced cachectic mice. After exclusion of 2 mice receiving partial intravenous doses or displaying an apparent absorption phase in their individual PK profiles, a total of 13 mice and 52 pembrolizumab plasma concentrations were included for the PK analysis. Throughout the course of the PK study, circulating pembrolizumab concentrations were higher in cisplatin-treated mice relative to mice receiving vehicle (Figure 2C). Accordingly, estimated pembrolizumab clearance was significantly decreased in cisplatin-treated mice compared to vehicle-treated controls (0.21 mL/day in vehicle-treated mice vs. 0.11 mL/day in cisplatin-treated mice; P = 0.004 by the log-likelihood ratio test; see Supplemental Table 1 and Supplementary Results) (Figure 2D). The geometric mean of maximal concentration (Cmax) was higher in cisplatin-treated mice and the geometric mean of V1 was lower in cisplatin-treated mice, though the differences were not statistically significant (Cmax is 43.4 μg/mL in vehicle-treated mice vs. 58.0 μg/mL in cisplatin-treated mice; P = 0.1274 by Mann-Whitney test; V1 is 2.23 mL in vehicle-treated mice vs. 1.37 mL in cisplatin-treated mice; P = 0.1709 by Mann-Whitney test). Overall, the data in this study reveals how mAb PK may be significantly impacted in chemotherapy-induced wasting, though potentially in ways that are different compared to cancer-induced wasting.
3.3. Impact of IgG subclass on mAb clearance in murine cancer cachexia
We consistently observed elevated pembrolizumab clearance across different mouse models of cancer cachexia, replicating the observed clinical phenomenon. As the association between cancer cachexia and clinical outcomes with IgG mAb therapies has been reported with a variety of ICI and other agents [29-33], we next sought to determine if elevated clearance occurs across various IgGs in these murine cachexia models.
3.3.1. Durvalumab and WT human IgG1 exhibit higher clearance in LLC tumor-bearing cachectic mice
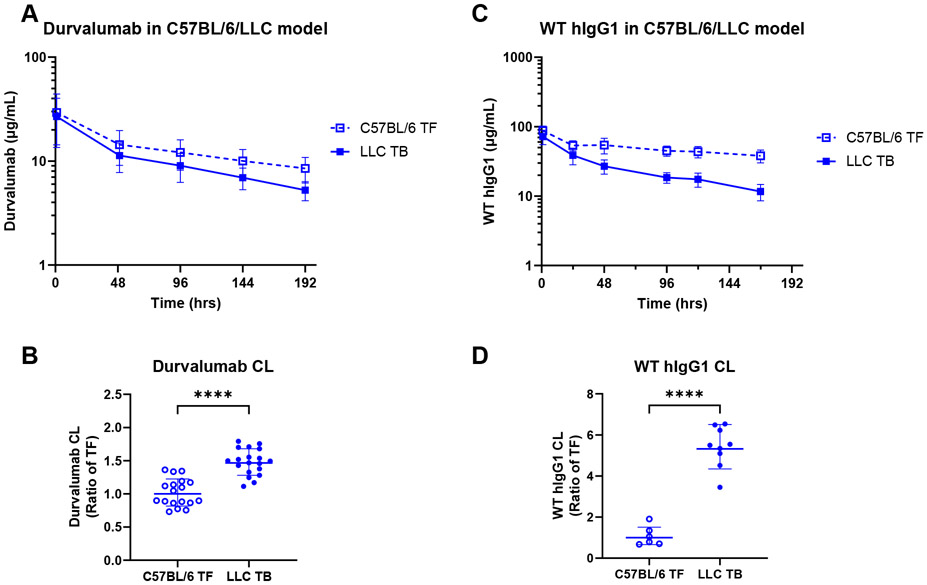
We first sought to evaluate the PK of human mAbs with different IgG backbones and target epitopes in animal models of cancer cachexia. Given that a majority of marketed mAbs utilize the IgG1 subclass [74], we evaluated the PK of durvalumab, an IgG1 anti-human PD-L1 mAb that has high affinity to human but not murine PD-L1. We and others have demonstrated neither pembrolizumab [34, 75] nor durvalumab [76] bind to their respective mouse targets. After exclusion of 3 mice receiving partial intravenous doses or displaying an apparent absorption phase in their individual PK profiles, a total of 185 durvalumab plasma concentrations in 37 C57BL/6 mice with or without cachexia-inducing LLC tumors who received a single, intravenous 200 μg dose of durvalumab, were included in the analysis. Concentration-time curves of durvalumab plasma levels revealed clear differences between LLC cachectic mice and tumor-free controls. Concentrations at one hour after durvalumab intravenous administration were comparable between groups, however, durvalumab concentrations declined more rapidly in LLC tumor-bearing mice compared to tumor-free controls by 192 h (Figure 3A). Estimated durvalumab clearance was significantly higher in LLC tumor-bearing cachectic mice relative to tumor-free animals (1.02 mL/day in tumor-free mice vs. 1.50 mL/day in LLC tumor-bearing mice; P < 0.0001 by the log-likelihood ratio test; see Supplemental Table 1 and Supplementary Results) (Figure 3B).
Figure 3. Pharmacokinetics of durvalumab and WT hIgG1 mAbs in the C57BL/6/LLC model of cancer cachexia.
(A) Durvalumab PK in C57BL/6 mice without (TF, n = 18) or with LLC tumors (LLC TB, n = 19); (B) Model-estimated durvalumab clearance (CL) in C57BL/6 TF and LLC TB mice; (C) WT hIgG1 PK in C57BL/6 TF (n = 6) and LLC TB mice (n = 9); (D) Model-estimated WT hIgG1 clearance (CL) in C57BL/6 TF and LLC TB mice; Data are shown as mean ± SD (A, C) or as geometric mean ± geometric SD (B, D); ****, P ≤ 0.0001 by the log-likelihood ratio test.
Next, we also evaluated the PK of untargeted WT hIgG1 antibody in the LLC model. After exclusion of 5 mice receiving partial intravenous doses or displaying apparent absorption phases in their individual PK profiles, a total of 90 hIgG1 plasma concentrations from 15 animals were included in the analysis. Concentration-time curves revealed the same trends as observed with pembrolizumab and durvalumab with hIgG1 plasma concentrations dropping much more rapidly (Figure 3C) and yielding a significantly elevated clearance of hIgG1 in LLC cachectic mice relative to tumor-free animals (0.16 mL/day in tumor-free mice vs. 0.84 mL/day in LLC tumor-bearing mice; P < 0.0001 by the log-likelihood ratio test; see Supplemental Table 1 and Supplementary Results) (Figure 3D). Together, and combined with our prior data with pembrolizumab in the LLC model, these data suggest the underlying mechanisms driving elevated mAb clearance extend across hIgG subclasses.
3.4. Potential role of FcRn in cachexia-associated changes in mAb clearance
In our previous work, we proposed that elevated pembrolizumab clearance observed in tumor-bearing, cachectic mice could be attributed, at least in part, to reduced FcRn expression or function, and we reported that hepatic Fcgrt mRNA expression was significantly reduced in tumor-bearing, cachectic mice in both C26 and LLC models of cancer cachexia, as compared to tumor-free controls [34]. We therefore sought to validate those results in additional model systems: the MC38 tumor-bearing non-cachectic mouse model and non-tumor-bearing, cisplatin-treated cachectic mouse model.
3.4.1. Fcgrt expression in liver is associated with catabolic clearance changes in tumor-bearing and non-tumor-bearing models of cachexia
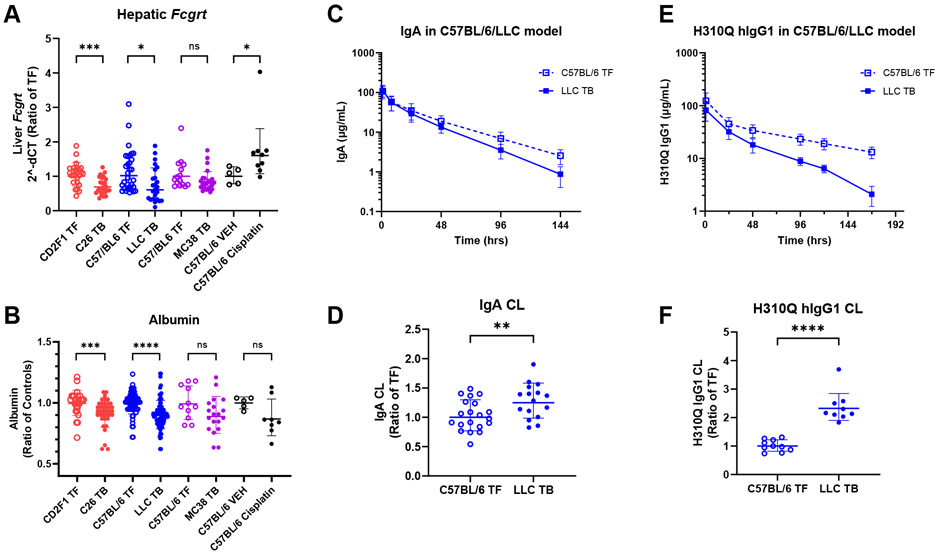
In contrast to the C26 and LLC models of cancer cachexia, hepatic Fcgrt expression trended lower, but was not significantly reduced (p = 0.09) in MC38 tumor-bearing vs. tumor-free mice (Figure 4A). As liver is a major site of mAb catabolic clearance [77, 78], lack of significant change in liver Fcgrt expression is consistent with lack of significant change in pembrolizumab clearance in MC38 tumor-bearing vs. tumor-free mice. However, it is notable that while neither difference in Fcgrt expression nor pembrolizumab clearance are statistically significant, the observed downward trend of Fcgrt expression and upward trend of pembrolizumab clearance suggest a potential role for FcRn.
Figure 4. Probing FcRn modulation in murine cancer cachexia.
(A) Fcgrt mRNA expression in mRNA whole-liver extracts. Observed data are presented as ratio of respective tumor free (TF) groups. (B) Plasma albumin levels between TF and tumor-bearing (TB) mice; (C) hIgA PK in TF C57BL/6 (n = 20) and LLC TB mice (n = 16); (D) Model-estimated hIgA clearance (CL) in C57BL/6 TF and LLC TB mice; I hIgG1 H310Q PK in TF C57BL/6 (n = 10) and LLC TB (n = 9) mice; (F) Model-estimated hIgG1 H310Q CL in C57BL/6 TF and LLC TB mice; Data is shown as geometric mean ± geometric SD (A, D, F) or as mean ± SD (B, C, E); ns, non-significant ns, non-significant; *, P < 0.05; **, P < 0.01, ***, P < 0.001, ****, P < 0.0001 by Mann-Whitney test (A, B) or the log-likelihood ratio test (D, F).
Similar to the trends of Fcgrt and pembrolizumab clearance in MC38 tumor-bearing mice, we observed liver Fcgrt was significantly increased in cisplatin-treated cachectic mice, and these animals displayed a significant decrease in pembrolizumab clearance (Figure 4A). Thus, while these tumor-bearing and tumor-free models of cachexia behave differently in terms of catabolic mAb clearance, data from all models support the hypothesis that cachexia-associated changes in Fcgrt expression in the liver could contribute to changes in mAb clearance observed in cachectic mice.
3.4.2. Plasma albumin levels associate with mAb catabolic clearance and liver Fcgrt expression in tumor-bearing models of cachexia but not in cisplatin-induced cachexia
Since albumin and IgG undergo the same pH-dependent FcRn-mediated cellular recycling that rescues both molecules from intracellular degradation [146], increased albumin clearance might also be expected when increased IgG mAb clearance is observed. We previously reported significantly lower plasma albumin levels in tumor-bearing, cachectic mice than in tumor-free mice in both C26 and LLC models [34]. In the current study we observed a trend toward lower plasma albumin at terminal timepoints in MC38 tumor-bearing, non-cachectic mice vs. tumor-free controls, though this trend was not statistically significant (Figure 4B). Collectively, across the three murine cancer models tested, serum albumin levels appear to correlate with Fcgrt and mAb clearance, such that decreased liver Fcgrt is consistent with lower albumin and higher mAb clearance. Notably, in the non-tumor-bearing cisplatin-induced cachexia model with elevated liver Fcgrt and reduced clearance, we might expect to see albumin levels either remain constant or even slightly increase compared to vehicle treated mice. As Figure 4B displays, we observe no significant difference in albumin between the two groups, though a downward trend is apparent. Notably, cisplatin-treated mice have significantly decreased liver mass [Supplemental Figure 4], and it is possible that the downward trend in albumin is potentially due to a reduction in albumin synthesis in the liver [79].
3.4.3. Elevated clearance of human IgA and reduced FcRn-binding mutant human IgG1 H310Q in cancer cachexia
To further explore the role of FcRn in elevated mAb clearance in mice with cancer cachexia, we assessed PK of hIgA and a custom hIgG1 H310Q mutant in tumor-free and LLC tumor-bearing mice. IgA possesses a similar structure to IgG, but IgA lacks binding sites to FcRn [80], and therefore IgA does not undergo FcRn-mediated recycling. Residues on IgG located in the Fc domain and known to be directly involved in FcRn binding include isoleucine 253, histidine 310, and histidine 435 [81]. It has been shown that a single mutation at H310 is sufficient to significantly reduce binding of hIgG1 to murine FcRn and increase IgG clearance [82]. We therefore utilized the H310Q mutant to evaluate the impact of reduced FcRn binding on IgG clearance in cachectic tumor-bearing animals and tumor-free controls.
3.4.4. Human IgA
After exclusion of 4 mice receiving partial intravenous doses or displaying apparent absorption phases in their individual PK profiles, a total of 215 plasma samples from 36 mice who received a single, intravenous 100 μg dose of hIgA, were included in the analysis (Figure 4C). Mixed-effects modeling and estimation of hIgA PK parameters revealed a modest (1.25-fold), but statistically significant elevation of clearance in tumor-bearing compared to tumor-free mice (0.89 mL/day in tumor-free mice vs. 1.11 mL/day in LLC tumor-bearing mice; P = 0.009 by the log-likelihood ratio test; see Supplemental Table 1 and Supplementary Results) (Figure 4D).
3.4.5. Human IgG1 H310Q
To confirm reduced binding of hIgG1 H310Q in our model systems, the binding affinity to murine FcRn was determined using SPR. Our results demonstrated a diminished FcRn binding capacity of hIgG1 H310Q compared to WT hIgG1 control [Supplemental Figure 5]. WT hIgG1 exhibited a dissociation constant (KD) of 88.1 nM, however, the dissociation constant of H310Q mutant could not be measured under the same assay conditions [Supplemental Table 2]. For the study with hIgG1 H310Q, a total of 114 hIgG1 plasma concentrations from 19 mice who received 200 μg of hIgG1 H310Q were included in the analysis, after exclusion of 1 early dead mouse (Figure 4E). Similar to previous observations with other IgG antibodies in the LLC model, significantly higher clearance of H310Q was observed in LLC cachectic mice as compared to tumor-free controls (0.77 mL/day in tumor-free mice vs. 1.80 mL/day in LLC tumor-bearing mice; P < 0.0001 by the log-likelihood ratio test; see Supplemental Table 1 and Supplementary Results) (Figure 4F).
Collectively, the Fcgrt expression and serum albumin data are consistent with our hypothesis that Fcgrt/FcRn expression and/or FcRn recycling function are altered in cachexia. Furthermore, the hIgA and hIgG1 H310Q data provide evidence that there are indeed factors beyond FcRn that are contributing to changes in mAb clearance in our models of cancer cachexia.
3.5. Impact of Fab domain targeting and mAb species on mAb clearance in mouse models of cancer cachexia
3.5.1. Rat IgG2a anti-murine PD-1 (RMP1-14) and IgG2a Isotype Control
Our initial experiments evaluated cachexia-associated changes in clearance of human antibodies without a high affinity target in mice, thus focusing entirely on catabolic clearance and excluding contributions of target-mediated clearance. Next, we focused our studies to include evaluation of the PK behavior of the targeted antibody, RMP1-14, a rat IgG2a monoclonal antibody that targets the murine PD-1 protein and has been used extensively to explore the effects of PD-1 inhibition in preclinical murine models, and its isotype control, non-targeted rat IgG2a [83-86]. RMP1-14 PK was evaluated at 2 mg/kg and 10 mg/kg in C26 mouse models and at 10 mg/kg in LLC mouse models of cancer cachexia. A total of 96 RMP1-14 plasma concentrations from 24 CD2F1 mice with or without C26 tumors and a total of 56 RMP1-14 plasma concentrations from 14 C57BL/6 mice with or without LLC tumors were included in the analysis, after exclusion of 15 and 5 mice, respectively. Dose proportionality of pembrolizumab PK for 2 mg/kg and 10 mg/kg was observed in C26 models [Supplemental Figure 6, A-B]. For both models, RMP1-14 plasma levels visually separated with more rapid decrease in tumor-bearing, cachectic mice (Figure 5A and 5C), and estimated clearance was significantly higher in tumor-bearing mice relative to tumor-free controls for both models of cancer cachexia (0.14 mL/day in tumor-free mice vs. 0.26 mL/day in C26 tumor-bearing mice given 2 mg/kg RMP1-14; 0.13 mL/day in tumor-free mice vs. 0.33 mL/day in C26 tumor-bearing mice given 10 mg/kg RMP1-14; 0.09 mL/day in tumor-free mice vs. 0.42 mL/day in LLC tumor-bearing mice given 10 mg/kg RMP1-14; P < 0.001 for CD2F1 mice and P < 0.0001 for C57BL/6 mice by the log-likelihood ratio test, see Supplemental Table 1 and Supplementary Results) (Figure 5B and 5D). Rat IgG2a isotype control was evaluated at 2 mg/kg and 10 mg/kg in 15 CD2F1 mice with or without C26 tumors. After exclusion of 5 mice receiving partial intravenous doses or displaying apparent absorption phases in their individual PK profiles, a total of 60 rat IgG2a plasma concentrations from 15 mice were included in the analysis. Dose normalized Cmax was slightly higher with 10 mg/kg than 2 mg/kg, but this was not statistically significant in either dose normalized Cmax and clearance between the two doses [Supplemental Figure 6 C-D]. The data revealed a clear separation in antibody plasma concentrations between tumor-free and tumor-bearing mice over time (Figure 5E) and significantly elevated clearance in tumor-bearing cachectic mice compared to controls (0.13 mL/day in tumor-free mice vs. 0.27 mL/day in C26 tumor-bearing mice given 2 mg/kg rat IgG2a; 0.08 mL/day in tumor-free mice vs. 0.23 mL/day in C26 tumor-bearing mice given 10 mg/kg rat IgG2a; P = 0.002 by the log-likelihood ratio test; see Supplemental Table 1 and Supplementary Results) (Figure 5F). These data indicate the increased catabolic clearance observed in cachectic mouse models extends beyond human immunoglobulins and remains when the mAb has an endogenous target, as with RMP1-14 and murine PD-1.
Figure 5. Pharmacokinetics of RMP1-14 and Rat IgG2a in the CD2F1/C26 and C57BL/6/LLC models of cancer cachexia.
(A) RMP1-14 PK in CD2F1 mice without (TF, n = 9) or with C26 tumors (C26 TB, n = 15); (B) Model-estimated RMP1-14 clearance (CL) in CD2F1 TF and C26 TB mice; (C) RMP1-14 PK in C57BL/6 mice without (TF, n = 7) or with LLC tumors (LLC TB, n = 7); (D) Model-estimated RMP1-14 clearance (CL) in C57BL/6 TF and LLC TB mice; (E) Rat IgG2a PK in CD2F1 mice without (TF, n = 7), or with C26 tumors (C26 TB, n = 8); (F) Model-estimated RatIgG2a CL in CD2F1 TF and C26 TB mice; Data are shown as mean ± SD (A, C, E) or as geometric mean ± geometric SD (B, D, F); **, P < 0.01; ***, P < 0.001, ****, P < 0.0001 by the log-likelihood ratio test.
3.6. Impact of anti-cachectic effects of AR-42 on pembrolizumab clearance in C26 tumor-bearing mice
Our prior data demonstrated the HDAC inhibitor, AR-42, could reduce or eliminate cachexia burden in CD2F1 mice bearing C26 tumors [39, 57]. We therefore sought to determine if pembrolizumab clearance would be impacted by the anti-cachectic effects of AR-42 in C26 tumor-bearing mice. As anticipated in this study, cachexia-related endpoints demonstrated AR-42 reduced cachectic burden across most cachexia endpoints measured [Supplemental Figure 7]. Daily administration of AR-42 at 20 mg/kg orally was able to significantly reduce cachexia-associated MuRF-1 induction, though decrease in Atrogin-1 expression was not statistically significant. AR-42 administration also increased the spleen mass significantly in C26 tumor-bearing mice.
Given the overall anti-cachectic response of AR-42 treatment, we hypothesized that AR-42 would prevent cachexia-associated elevation in pembrolizumab clearance. Two separate studies were conducted and tumor-free or C26 tumor-bearing CD2F1 mice received pembrolizumab at dose of 2 mg/kg in the first study and dose of 100 μg in the second study, with or without oral treatment of 20 mg/kg AR-42. The average body weight of mice at dosing in the second study was 23.1 g which gives the average dose of 4.4 mg/kg. After exclusion of 20 mice receiving partial intravenous doses or displaying apparent absorption phases in their individual PK profiles, a total of 176 pembrolizumab plasma concentrations from 44 tumor-free or C26 tumor-bearing CD2F1 mice were included in the analysis. We observed statistically significant difference in dose normalized Cmax and clearance from C26 tumor-bearing with AR-42 treatment group between the two studies, and similar to the MC38 data, these differences could be due to study-to-study variability or to dose-dependent PK [Supplemental Figure 3, G-H]. After controlling for study/dose, pembrolizumab clearance was observed to be significantly increased in C26 tumor-bearing mice compared to tumor-free mice, and this increased clearance was independent of AR-42 treatment [See Supplemental Table 1 and Supplementary Results] (Figure 6A-C). These data indicate that AR-42 treatment significantly reduced cachectic burden but did not alter the cancer cachexia-associated increase in pembrolizumab catabolic clearance. Interestingly, in tumor-free mice, AR-42 significantly reduced Fcgrt expression, whereas AR-42 did not have an effect in Fcgrt expression in tumor-bearing mice (Figure 6D). However, we note the relatively low number of samples available from vehicle-treated animals in these experiments and acknowledge additional studies would be needed to verify these results. Overall, these data suggest either the mechanisms by which AR-42 reduces cachectic burden are distinct from the mechanisms driving increased mAb clearance in cancer cachexia or that the magnitude of reduction in cachectic burden is not adequate to cause a measurable change in mAb clearance.
Figure 6. Pharmacokinetics of pembrolizumab in the CD2F1/C26 model of cancer cachexia, with or without AR-42 treatment.
(A) Pembrolizumab 2 mg/kg PK in TF CD2F1 (n = 11) or C26 tumor-bearing (TB) mice (n = 13) receiving either AR-42 (PO, 20 mg/kg daily, n=17) or vehicle (n=7); (B) Pembrolizumab 100 μg PK in TF CD2F1 (n = 7) or C26 TB mice (n = 13) receiving either AR-42 (PO, 20 mg/kg daily, n=15) or vehicle (n=5); (C) Model-estimated pembrolizumab clearance (CL) in TF and TB mice, with or without AR-42 treatment; (D) Fcgrt expression in liver; ns, non-significant, *, P<0.05; **, P<0.01, by the log-likelihood ratio test (C) or Mann-Whitney test (D).
4. Discussion
We previously reported that cachectic mice display a significantly higher clearance of pembrolizumab compared to tumor-free mice, replicating the clinically observed phenomenon [34]. In this study we characterized the effects of antibody isotype, target specificity and affinity for murine FcRn on antibody clearance in experimental mouse models of cachexia. In addition, we tested how clearance may be altered in tumor-bearing mice without cachexia or with reduced cachectic burden (MC38 tumor-bearing mice and C26 tumor-bearing mice treated with AR-42), and in tumor-free mice with chemotherapy-induced cachexia (cisplatin-treated mice).
In our studies, besides RMP1-14, all other antibodies have no target in our mouse models. As a result, they are not subject to a saturable, target-mediated clearance pathway, and thus non-specific catabolism is presumed to be the main clearance pathway. Even with its endogenous target in our mouse models, RMP1-14 exhibited apparent linear PK at doses of 2 mg/kg and 10 mg/kg [Supplemental Figure 6, A and B]. We observed statistically significant difference in pembrolizumab PK between experiments where different dose levels were used in MC38 tumor-bearing mice (higher dose correlated with lower CL) and C26 tumor-bearing mice treated with AR-42 (higher dose correlated with higher CL). We conclude these differences were due to variability between experiments, though we acknowledge the unlikely possibility that there is a lack of dose proportionality, and in opposite directions, with these two mouse models and at the dose levels evaluated. Nevertheless, we were able to control for the observed differences in clearance between experiments to test the primary hypotheses regarding impact of tumor and cachectic burden on pembrolizumab clearance.
When evaluating human IgG4 (pembrolizumab, hIgG4), IgG1 (durvalumab, hIgG1), and rat IgG2a (IgG2a isotype and RMP1-14), we observed consistently elevated clearance in LLC and C26 tumor-bearing compared to tumor-free C57BL/6 and CD2F1 mice (Figures 2, 3, 5). This demonstrated that elevated mAb clearance in mouse models of cancer cachexia was not specific to pembrolizumab, but rather a phenomenon generalizable to other IgG antibodies and possibly, to a much smaller extent, IgA antibodies (Figure 4), expanding the utility of these models in studying altered PK and resistance to various therapeutic mAbs. Furthermore, the Fab domains of these mAbs are all different, with only one recognizing a murine target, PD-1 (RMP1-14). Though there were some distinct differences among the various antibodies, all displayed altered clearance in tumor-bearing, cachectic mice compared to tumor-free mice.
One of our primary objectives was to investigate the role of FcRn as one possible mechanism for altered mAb clearance in murine models of cachexia. FcRn is an MHC class I–like molecule that is essential to maintaining IgG and albumin levels via salvage from lysosomal degradation. Given its well-studied function directly affecting IgG PK and clearance, FcRn is a logical initial candidate in our effort to identify potential drivers of cachexia-associated changes in clearance. Considering the high affinity binding of hIgG to murine FcRn [87, 88], we were able to utilize human antibodies to probe the potential role of FcRn in our murine PK studies. Hepatic Fcgrt mRNA expression data were supportive of a role for FcRn, showing significantly reduced Fcgrt levels in both C26 and LLC cachectic tumor-bearing animals compared to tumor-free mice, which corresponded with elevated mAb clearance observed in these models. Such changes in Fcgrt appeared to be independent of mere tumor presence, as Fcgrt mRNA expression in liver lysates of MC38 tumor-bearing, non-cachectic mice were not different than tumor-free controls, and this further corresponded to non-significant changes in pembrolizumab clearance in these mice. Similarly, in cisplatin-treated mice, we observed an increase in liver Fcgrt expression and a corresponding decrease in pembrolizumab clearance. Overall, these data demonstrate consistency between observed differences in mAb clearance and changes in liver Fcgrt expression. These results also support the hypothesis that changes in FcRn expression and/or recycling function may be partially responsible for observed changes in mAb clearance in cachexia. It is also notable that circulating albumin, which is also recycled by FcRn, decreased in tumor-bearing cachectic mice. However, the decreasing trend in albumin in cisplatin-treated mice vs. vehicle-treated controls, though non-significant, was not consistent with the increase in hepatic Fcgrt expression and decreased pembrolizumab clearance in this model. Our data shows that liver weight was significantly smaller in cisplatin-treated mice as compared to vehicle-treated controls, suggesting a potential damage in liver function [Supplemental Figure 4]. Since albumin is synthesized in the liver and hypoalbuminemia is a well-known indicator of liver dysfunction[79], the reduction in circulating albumin in cisplatin-treated mice can potentially be explained by cisplatin-induced liver damage and impaired albumin synthesis [89]. It is also important to note that impaired liver function would be expected to impact mAb clearance, as liver is a major site of mAb clearance [77, 90]. Though this would suggest smaller livers and general hepatotoxicity may lead to decreased mAb clearance, we actually see either no alteration in clearance or even increased clearance of mAb therapies in cancer patients with mild or moderate hepatic impairment [91, 92]. Additional work is necessary to better understand how various forms of hepatic dysfunction alter mAb disposition.
To further evaluate the role FcRn plays in driving altered therapeutic antibody clearance in cachectic mice, the PK of hIgA was studied in the LLC mouse model of cancer cachexia. Like IgG, IgA is also a member of the immunoglobulin family and has a similar structure, comprising two identical heavy chains and two identical light chains. In humans, the majority of human plasma cells are committed to producing IgA, though IgA represents only ~15% of total serum immunoglobulin [93]. IgA is the most prevalent in secretions at mucosal sites, acting as a defense by preventing invasion of pathogens. A major structural difference between IgA and IgG is that Fc receptor binding sites are not present in IgA, which thus do not bind to FcRn or Fcγ receptors (FcγRs). IgA endocytosis is facilitated by a specific IgA receptor such that IgA does not compete for Fc receptor binding with IgG or albumin [94]. Without FcRn-mediated recycling, IgA has a relatively short circulating half-life (<1 day to ~4 days) in multiple species [95]. In our study, an increase in hIgA clearance was observed in the LLC tumor-bearing mice compared to tumor-free animals. While the magnitude of increase in hIgA clearance in LLC tumor-bearing vs. tumor-free animals is much less than that observed with all evaluated IgG mAbs, this data does suggest that other mechanisms independent of FcRn salvage contribute to altered immunoglobulin clearance. Furthermore, the stark difference in altered catabolic clearance of IgG compared to IgA in cancer cachexia reinforces a role for the IgG Fc domain binding to Fc receptors, such as FcRn or even FcγRs, as a key factor in the observed elevation of catabolic clearance of IgG mAbs in cancer cachexia.
In addition to evaluating hIgA as a non-mFcRn binding antibody, we also evaluated H310Q, an engineered hIgG1 antibody specifically designed to have reduced binding to FcRn which we confirmed with SPR. We hypothesized that cachexia-associated elevations in IgG clearance would be limited following compromised FcRn-mediated IgG salvage. Indeed, following reduced binding affinity to FcRn, the degree of tumor/cachexia-associated elevation in clearance of H310Q was ~2-fold compared to ~5-fold for hIgG1 wild-type control. This result further supports a potential link between FcRn activity and elevated mAb clearance observed in cancer cachexia. Notably, although the binding capacity of H310Q hIgG1 to mouse FcRn was not measurable in our assay, H310Q does still bind to murine FcRn with much lower affinity, as has been shown previously [96]. Therefore, the ~2-fold increase in clearance of H310Q hIgG1 could potentially still be due to FcRn-mediated salvage and could be further reduced with other mutations thought to completely abrogate binding to FcRn [96]. Nevertheless, the residual elevations in clearance that remain despite limited FcRn binding suggest increased catabolic IgG clearance in cachexia is not solely due to reduced FcRn-mediated IgG salvage.
Our data indicate no significant change in pembrolizumab clearance in MC38 tumor-bearing animals relative to tumor-free controls, suggesting that the presence of tumor alone is not sufficient to cause increased catabolic clearance of pembrolizumab. As indicated, MC38 tumor size is smaller on overage, but similar in range, compared to C26 tumors, and over the time course of MC38 tumor growth in our studies, we do not observe the clear composite markers of cachexia in these mice. Therefore, this data further supports a common underlying driver, different than mere presence of tumor, for both cachexia and elevation in catabolic clearance of IgG mAbs in our tumor-bearing mouse models and potentially in cancer patients, as has been proposed [97-99].
In this study we also evaluated mAb clearance in a “non-cachectic” tumor-bearing mouse model as well as in tumor-free mice with chemotherapy-induced cachexia. Contrary to our tumor-bearing cancer cachexia models, we observed a striking difference in mAb clearance in the chemotherapy-induced model of cachexia. Here instead of elevated clearance, we saw a significant reduction in pembrolizumab clearance in cisplatin-induced cachectic mice compared to vehicle-treated controls. Therefore, despite cisplatin treatment serving as an established model of cachexia [45, 70], and despite our data clearly illustrating the typical phenotypes of cachexia in these animals (weight loss, muscle loss, fat loss, increased muscle E3 ligase expression), this form of cachexia by itself does not produce the elevated IgG mAb clearance observed in our tumor-bearing cachectic models. Notably, while splenomegaly was observed with all evaluated models of cancer cachexia, treatment with immunosuppressive cisplatin significantly reduced spleen mass in tumor-free mice. With the reduced spleen mass and presumed reduction in circulating immune cell numbers, cisplatin treatment may eliminate immune cell populations either directly responsible for increased catabolic clearance of IgG mAbs or those that may be responsible for contributing to underlying tumor/cachexia-associated signaling that leads to elevated pembrolizumab clearance in tumor-bearing, cachectic mice. It is also notable that this same cisplatin treatment regimen is used to model acute kidney injury [45, 58, 100, 101], and we might have expected increased clearance due to leakage of pembrolizumab in urine, as is observed with other large proteins, such as albumin, in patients [102] and in mice treated with cisplatin [58]. Although we did not measure albumin in urine in our studies, albumin loss in urine would align with our observation of a downward trend in plasma albumin levels in cisplatin-treated mice compared to vehicle-treated mice, despite an increase in liver Fcgrt expression and decreased pembrolizumab clearance. Similarly, although we do not consider kidney to be a significant contributor to IgG clearance, and while we speculate the presence of a tumor and cachexia may be essential for producing the elevated IgG mAb clearance phenotype, we acknowledge that reduced immune cell quantities, including key phagocytic cell populations such as macrophages known to contribute to IgG clearance [103], reduced liver size [Supplemental Figure 4], and kidney injury may all be contributing to the observation of reduced, and not elevated, clearance of pembrolizumab in our studies with cisplatin-treated mice. The reduced spleen and liver size in cisplatin-treated mice was reflected by the smaller V1 estimates, as the smaller spleen and liver size would have smaller volume of sinusoids that have leaky junctions through which mAbs can freely travel. Overall, this data reveals how mAb clearance may be significantly impacted, but in different ways, in cancer-induced wasting compared to chemotherapy-induced wasting. Therefore, different non-tumor bearing cachexia models will be needed to investigate the elevation of mAb clearance in cancer-induced wasting. These observations also highlight a gap in our understanding of how chemotherapy may fundamentally alter the clinically observed link between elevated immune checkpoint inhibitor mAb clearance, cachexia, and outcomes.
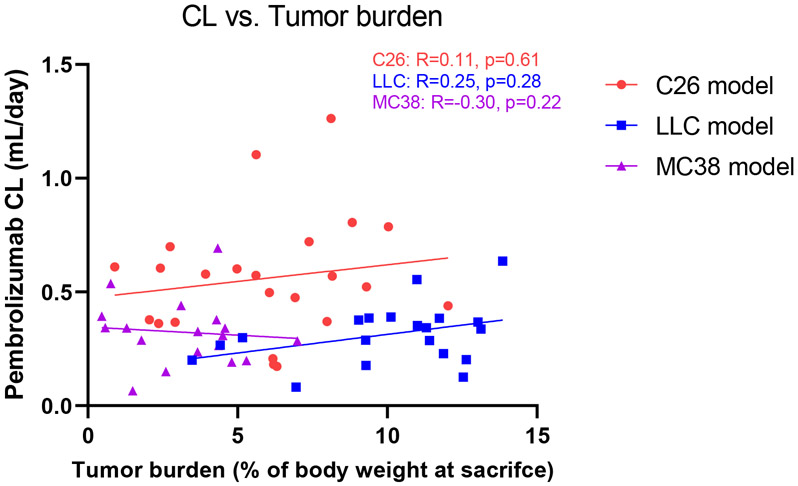
While the MC38 studies demonstrated that tumor burden does not necessarily increase the catabolism of mAb drugs, the possibility of tumor burden influencing mAb catabolism in a tumor-specific manner cannot be ruled out. Accordingly, the relationship between pembrolizumab clearance and tumor burden was investigated (Figure 7). Spearman’s correlation analysis indicated that clearance is not significantly related to tumor burden in any of the tumor models, despite the considerable variability in tumor sizes (ranging 0.24-2.31 g in the C26 model, 0.98-3.51 g in the LLC model, and 0.35-1.73 g in the MC38 model). This does not support a direct contribution of tumor burden as a site for mAb catabolism and elevation of CL. Instead, the physiological and immunological changes resulting from tumor development may be altering mAb catabolism.
Figure 7. Correlation plot of pembrolizumab clearance vs. tumor burden.
The relationship between pembrolizumab clearance and tumor burden in each tumor model. Tumor burden was calculated as the percentage of tumor weight relative to body weight at the time of sacrifice. R = Spearman’s correlation coefficient.
In this report we presented one attempt to pharmacologically alter cachexia and observe its impact on mAb clearance. Though AR-42 significantly reduced cachectic burden in C26 tumor-bearing mice, we did not observe a change in pembrolizumab clearance between tumor-bearing animals receiving oral AR-42 daily for 12 days vs. those receiving vehicle. It is possible that AR-42 exerted its anti-cachectic effect through molecular mechanisms distinct from those factors that drive elevated mAb clearance in C26 tumor-bearing mice with cachexia. Additionally, though AR-42 was able to significantly improve bodyweights and skeletal muscle mass in C26 tumor-bearing mice, it was not able to completely eliminate cachectic burden. Hence, it is also possible the cancer cachexia-associated factors were still present at adequate levels to drive elevated pembrolizumab clearance. Finally, we note AR-42 decreased liver Fcgrt relative to vehicle in tumor-free, but not in tumor-bearing mice, and AR-42 increased spleen mass relative to vehicle treatment in both tumor-free and tumor-bearing mice. Similar to liver Fcgrt, differences in spleen mass mostly coincide with differences in clearance between control and experimental groups (i.e., larger/smaller spleen associates with higher/lower clearance), with MC38 being the notable exception. While AR-42 does not appear to alter mAb clearance, more studies are required to determine if other therapies capable of modulating cachexia are also capable of modulating mAb clearance. Given the significant correlation between elevated ICI clearance and efficacy, improved understanding of factors governing increased ICI clearance may help to determine early in therapy if a patient will respond or if resistance is developing within a patient. It is tempting to speculate that cachexia-dependent changes in FcRn underlie changes in both ICI disposition and ICI response given the established role of dysfunctional antigen presentation in primary resistance to ICIs [104] and the evidence that normal FcRn function is critical for both proper antigen presentation [105] and anti-tumor immunity [106]. Future studies will need to focus on understanding FcRn’s role in both ICI disposition and ICI response and extend to characterizing this interaction in cancer patients receiving ICI therapy. The FcγR family represents another potential driver of poor ICI response and elevated CL. Though FcγR-mediated processes impacting mAb PK and response are not well understood, FcγRs are involved in endocytosis, phagocytosis, and to some extent, clearance of mAbs [107-109]. FcγRs also play a critical role in eliciting antibody effector functions like antibody-dependent cytotoxicity and complement-dependent cytotoxicity, and thus ultimately contribute substantially to the biologic and therapeutic activity of many mAbs. There are potential limitations of our current models that should be addressed in future studies interrogating mechanisms driving elevated therapeutic mAb clearance in cachectic patients. As mentioned above, human IgG binds with higher affinity to murine FcRn than to human FcRn, and this must be taken into account when interpreting data derived in mouse models where complex interactions between exogenous IgG and various Fc receptors may be contributing to the observed phenotypes. Alternative animal models may be considered for future studies, including transgenic mice that express human FcRn [110, 111] or cynomolgus monkeys, in which human IgG binding affinity to FcRn is similar to human FcRn [87, 88]. Our pre-clinical mouse models of cancer cachexia, while well-characterized and frequently used in translational cachexia research, are aggressive with large tumors and severe cachexia present within two weeks post tumor engraftment. This does not replicate the conditions in the vast majority of cachectic patients nor allow for study of changes in mAb clearance over time. Furthermore, it renders our assessment of mAb clearance through only about 1-2 elimination half-lives of any administered IgG. Therefore, longer-term models with slower development of tumor and cachectic burden could be useful for studying mechanisms over a longer time period. Also, while we have thus far evaluated the chemotherapy-induced model of cachexia, other non-cancer cachexia models, such as the adeno-associated viral model inducing muscle IL-6/activin A [112], should also be evaluated.
Supplementary Material
ACKNOWLEDGMENTS
Study was supported by The Ohio State University Comprehensive Cancer Center (OSUCCC) and the National Cancer Institute, grants P30CA016058, R01CA273924 and R01CA201382; TTV and MC were supported by Eli Lilly Fellowships; KK, AMC and MM were supported by Pelotonia Fellowships; JT was supported by a Dennis Feller Fellowship; BR was supported by a Cheng-Yok and Kai-King Chow Pharmaceutics Fellowship; DHO is supported by The LUNGevity Career Development Award. This research was made possible through resources, expertise, and support provided by the Pelotonia Institute for Immuno-Oncology (PIIO), which is funded by the Pelotonia community and the OSUCCC.
Abbreviations:
- mAb
monoclonal antibody
- ICI
immune checkpoint inhibitor
- PD-1
programmed cell death protein 1
- PD-L1
programmed cell death-ligand 1
- CTLA-4
cytotoxic T-lymphocyte associated protein 4
- NSCLC
non-small cell lung cancer
- CL
clearance
- FcRn
neonatal Fc receptor
- IgG
immunoglobulin G
- FcγR
Fc-gamma receptor
- ADA
antidrug antibody
- PK
pharmacokinetic
- WT
wild type
- RMP1-14
rat anti-mouse PD-1 antibody
- HDAC
histone deacetylase
- hIgA
human immunoglobulin A
- hIgG1
human immunoglobulin G 1
- DMEM
Dulbecco's Modified Eagle Medium
- FBS
fetal bovine serum
- ELISA
enzyme-linked immunoassay
- SPR
surface plasmon resonance
- qRT-PCR
quantitative real-time polymerase chain reaction
- CT
delta cycle threshold
- ANOVA
analysis of variance
Footnotes
Conflict-of-interests: DHO reports research funding to institution from BMS, Merck, Genentech, Palobiofarma, Onc.AI, which is outside the scope of the current work. All other authors declare they have no conflict of interest.
REFERENCES
- [1].Martinez P, Peters S, Stammers T, Soria JC, Immunotherapy for the First-Line Treatment of Patients with Metastatic Non-Small Cell Lung Cancer, Clin Cancer Res 25(9) (2019) 2691–2698. [DOI] [PubMed] [Google Scholar]
- [2].Segal EM, Immunotherapy in the frontline management of advanced and metastatic NSCLC, Am J Manag Care 27(18 Suppl) (2021) S323–S332. [DOI] [PubMed] [Google Scholar]
- [3].Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, K.-. Investigators, Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer, N Engl J Med 375(19) (2016) 1823–1833. [DOI] [PubMed] [Google Scholar]
- [4].Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC, K.-. Investigators, Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer, N Engl J Med 378(22) (2018) 2078–2092. [DOI] [PubMed] [Google Scholar]
- [5].Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeno J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Ozguroglu M, Investigators P, Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer, N Engl J Med 377(20) (2017) 1919–1929. [DOI] [PubMed] [Google Scholar]
- [6].Felip E, Altorki N, Zhou C, Csoszi T, Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A, Kenmotsu H, Chen YM, Chella A, Sugawara S, Voong D, Wu F, Yi J, Deng Y, McCleland M, Bennett E, Gitlitz B, Wakelee H, Investigators IM, Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial, Lancet 398(10308) (2021) 1344–1357. [DOI] [PubMed] [Google Scholar]
- [7].Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson SJ, Kerr K, Wang C, Ciuleanu TE, Saylors GB, Tanaka F, Ito H, Chen KN, Liberman M, Vokes EE, Taube JM, Dorange C, Cai J, Fiore J, Jarkowski A, Balli D, Sausen M, Pandya D, Calvet CY, Girard N, CheckMate I, Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer, N Engl J Med 386(21) (2022) 1973–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Grivas P, Plimack ER, Balar AV, Castellano D, O'Donnell PH, Bellmunt J, Powles T, Hahn NM, de Wit R, Bajorin DF, Ellison MC, Frenkl TL, Godwin JL, Vuky J, Pembrolizumab as First-line Therapy in Cisplatin-ineligible Advanced Urothelial Cancer (KEYNOTE-052): Outcomes in Older Patients by Age and Performance Status, Eur Urol Oncol 3(3) (2020) 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Casak SJ, Marcus L, Fashoyin-Aje L, Mushti SL, Cheng J, Shen YL, Pierce WF, Her L, Goldberg KB, Theoret MR, Kluetz PG, Pazdur R, Lemery SJ, FDA Approval Summary: Pembrolizumab for the First-line Treatment of Patients with MSI-H/dMMR Advanced Unresectable or Metastatic Colorectal Carcinoma, Clin Cancer Res 27(17) (2021) 4680–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schoenfeld AJ, Hellmann MD, Acquired Resistance to Immune Checkpoint Inhibitors, Cancer Cell 37(4) (2020) 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bullock BL, Kimball AK, Poczobutt JM, Neuwelt AJ, Li HY, Johnson AM, Kwak JW, Kleczko EK, Kaspar RE, Wagner EK, Hopp K, Schenk EL, Weiser-Evans MC, Clambey ET, Nemenoff RA, Tumor-intrinsic response to IFNgamma shapes the tumor microenvironment and anti-PD-1 response in NSCLC, Life Sci Alliance 2(3) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Daud AI, Loo K, Pauli ML, Sanchez-Rodriguez R, Sandoval PM, Taravati K, Tsai K, Nosrati A, Nardo L, Alvarado MD, Algazi AP, Pampaloni MH, Lobach IV, Hwang J, Pierce RH, Gratz IK, Krummel MF, Rosenblum MD, Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma, J Clin Invest 126(9) (2016) 3447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li HY, McSharry M, Bullock B, Nguyen TT, Kwak J, Poczobutt JM, Sippel TR, Heasley LE, Weiser-Evans MC, Clambey ET, Nemenoff RA, The Tumor Microenvironment Regulates Sensitivity of Murine Lung Tumors to PD-1/PD-L1 Antibody Blockade, Cancer Immunol Res 5(9) (2017) 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ngiow SF, Young A, Jacquelot N, Yamazaki T, Enot D, Zitvogel L, Smyth MJ, A Threshold Level of Intratumor CD8(+) T-cell PD1 Expression Dictates Therapeutic Response to Anti-PD1, Cancer Res 75(18) (2015) 3800–3811. [DOI] [PubMed] [Google Scholar]
- [15].Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, Engblom C, Pfirschke C, Siwicki M, Gungabeesoon J, Freeman GJ, Warren SE, Ong S, Browning E, Twitty CG, Pierce RH, Le MH, Algazi AP, Daud AI, Pai SI, Zippelius A, Weissleder R, Pittet MJ, Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-gamma and IL-12 (vol 49, 1148.e1, 2018), Immunity 55(9) (2022) 1749–1749. [DOI] [PubMed] [Google Scholar]
- [16].Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A, PD-1 blockade induces responses by inhibiting adaptive immune resistance, Nature 515(7528) (2014) 568-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gunjur A, Manrique-Rincon AJ, Klein O, Behren A, Lawley TD, Welsh SJ, Adams DJ, 'Know thyself' - host factors influencing cancer response to immune checkpoint inhibitors, J Pathol 257(4) (2022) 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chat V, Ferguson R, Simpson D, Kazlow E, Lax R, Moran U, Pavlick A, Frederick D, Boland G, Sullivan R, Ribas A, Flaherty K, Osman I, Weber J, Kirchhoff T, Autoimmune genetic risk variants as germline biomarkers of response to melanoma immune-checkpoint inhibition, Cancer Immunol Immun 68(6) (2019) 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, Lane S, Lee DY, Kaper M, McKean M, Beckermann KE, Rubinstein SM, Rooney I, Musib L, Budha N, Hsu J, Nowicki TS, Avila A, Haas T, Puligandla M, Lee S, Fang SY, Wargo JA, Gershenwald JE, Lee JE, Hwu P, Chapman PB, Sosman JA, Schadendorf D, Grob JJ, Flaherty KT, Walker D, Yan YB, McKenna E, Legos JJ, Carlino MS, Ribas A, Kirkwood JM, Long GV, Johnson DB, Menzies AM, Davies MA, Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis, Lancet Oncol 19(3) (2018) 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N, Rotin D, Anafi L, Avivi C, Melnichenko J, Steinberg-Silman Y, Mamtani R, Harati H, Asher N, Shapira-Frommer R, Brosh-Nissimov T, Eshet Y, Ben-Simon S, Ziv O, Khan MAW, Amit M, Ajami NJ, Barshack I, Schachter J, Wargo JA, Koren O, Markel G, Boursi B, Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients, Science 371(6529) (2021) 602-+. [DOI] [PubMed] [Google Scholar]
- [21].Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding QQ, Pagliano O, Zidi B, Zhang SW, Badger JH, Vetizou M, Cole AM, Fernandes MR, Prescott S, Costa RGF, Balaji AK, Morgun A, Vujkovic-Cvijin I, Wang H, Borhani AA, Schwartz MB, Dubner HM, Ernst SJ, Rose A, Najjar YG, Belkaid Y, Kirkwood JM, Trinchieri G, Zarour HM, Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients, Science 371(6529) (2021) 595-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang ZM, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, Mirsoian A, Minnar CM, Stoffel KM, Sturgill IR, Grossenbacher SK, Withers SS, Rebhun RB, Hartigan-O'Connor DJ, Mendez-Lagares G, Tarantal AF, Isseroff RR, Griffith TS, Schalper KA, Merleev A, Saha A, Maverakis E, Kelly K, Aljumaily R, Ibrahimi S, Mukherjee S, Machiorlatti M, Vesely SK, Longo DL, Blazar BR, Canter RJ, Murphy WJ, Monjazeb AM, Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade, Nat Med 25(1) (2019) 141-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chatterjee MS, Elassaiss-Schaap J, Lindauer A, Turner DC, Sostelly A, Freshwater T, Mayawala K, Ahamadi M, Stone JA, de Greef R, Kondic AG, de Alwis DP, Population Pharmacokinetic/Pharmacodynamic Modeling of Tumor Size Dynamics in Pembrolizumab-Treated Advanced Melanoma, CPT: pharmacometrics & systems pharmacology 6(1) (2017) 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang X, Feng Y, Bajaj G, Gupta M, Agrawal S, Yang A, Park JS, Lestini B, Roy A, Quantitative Characterization of the Exposure-Response Relationship for Cancer Immunotherapy: A Case Study of Nivolumab in Patients With Advanced Melanoma, CPT: pharmacometrics & systems pharmacology 6(1) (2017) 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stroh M, Winter H, Marchand M, Claret L, Eppler S, Ruppel J, Abidoye O, Teng SL, Lin WT, Dayog S, Bruno R, Jin J, Girish S, Clinical Pharmacokinetics and Pharmacodynamics of Atezolizumab in Metastatic Urothelial Carcinoma, Clin Pharmacol Ther 102(2) (2017) 305–312. [DOI] [PubMed] [Google Scholar]
- [26].Feng Y, Roy A, Masson E, Chen TT, Humphrey R, Weber JS, Exposure-response relationships of the efficacy and safety of ipilimumab in patients with advanced melanoma, Clin Cancer Res 19(14) (2013) 3977–86. [DOI] [PubMed] [Google Scholar]
- [27].Turner DC, Kondic AG, Anderson KM, Robinson AG, Garon EB, Riess JW, Jain L, Mayawala K, Kang J, Ebbinghaus SW, Sinha V, de Alwis DP, Stone JA, Pembrolizumab Exposure-Response Assessments Challenged by Association of Cancer Cachexia and Catabolic Clearance, Clin Cancer Res 24(23) (2018) 5841–5849. [DOI] [PubMed] [Google Scholar]
- [28].Mayu Ohuchi SY, Jo Hitomi, Akagi Kazumasa, Higashiyama Ryoko Inaba, Masuda Ken, Shinno Yuki, Okuma Yusuke, Yoshida Tatsuya, Goto Yasushi, Horinouchi Hidehito, Makino Yoshinori, Yamamoto Noboru, Ohe Yuichiro, Hamada Akinobu, Early change in the clearance of pembrolizumab reflects the survival and therapeutic response: A population pharmacokinetic analysis in real-world non-small cell lung cancer patients, Lung Cancer 173 (2022) 35–42. [DOI] [PubMed] [Google Scholar]
- [29].Daly LE, Power DG, O'Reilly A, Donnellan P, Cushen SJ, O'Sullivan K, Twomey M, Woodlock DP, Redmond HP, Ryan AM, The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma, Br J Cancer 116(3) (2017) 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shiroyama T, Nagatomo I, Koyama S, Hirata H, Nishida S, Miyake K, Fukushima K, Shirai Y, Mitsui Y, Takata S, Masuhiro K, Yaga M, Iwahori K, Takeda Y, Kida H, Kumanogoh A, Impact of sarcopenia in patients with advanced non-small cell lung cancer treated with PD-1 inhibitors: A preliminary retrospective study, Sci Rep 9(1) (2019) 2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nishioka N, Uchino J, Hirai S, Katayama Y, Yoshimura A, Okura N, Tanimura K, Harita S, Imabayashi T, Chihara Y, Tamiya N, Kaneko Y, Yamada T, Takayama K, Association of Sarcopenia with and Efficacy of Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer, J Clin Med 8(4) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chu MP, Li Y, Ghosh S, Sass S, Smylie M, Walker J, Sawyer MB, Body composition is prognostic and predictive of ipilimumab activity in metastatic melanoma, J Cachexia Sarcopenia Muscle 11(3) (2020) 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Roch B, Coffy A, Jean-Baptiste S, Palaysi E, Daures JP, Pujol JL, Bommart S, Cachexia - sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors, Lung Cancer 143 (2020) 19–26. [DOI] [PubMed] [Google Scholar]
- [34].Castillo AMM, Vu TT, Liva SG, Chen M, Xie Z, Thomas J, Remaily B, Guo Y, Subrayan UL, Costa T, Helms TH, Irby DJ, Kim K, Owen DH, Kulp SK, Mace TA, Phelps MA, Coss CC, Murine cancer cachexia models replicate elevated catabolic pembrolizumab clearance in humans, JCSM Rapid Commun 4(2) (2021) 232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Corbett TH, Griswold DP Jr., Roberts BJ, Peckham JC, Schabel FM Jr., Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure, Cancer Res 35(9) (1975) 2434–9. [PubMed] [Google Scholar]
- [36].Huot JR, Pin F, Essex AL, Bonetto A, MC38 Tumors Induce Musculoskeletal Defects in Colorectal Cancer, Int J Mol Sci 22(3) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Miao C, Zhang W, Feng L, Gu X, Shen Q, Lu S, Fan M, Li Y, Guo X, Ma Y, Liu X, Wang H, Zhang X, Cancer-derived exosome miRNAs induce skeletal muscle wasting by Bcl-2-mediated apoptosis in colon cancer cachexia, Mol Ther Nucleic Acids 24 (2021) 923–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Talbert EE, Cuitino MC, Ladner KJ, Rajasekerea PV, Siebert M, Shakya R, Leone GW, Ostrowski MC, Paleo B, Weisleder N, Reiser PJ, Webb A, Timmers CD, Eiferman DS, Evans DC, Dillhoff ME, Schmidt CR, Guttridge DC, Modeling Human Cancer-induced Cachexia, Cell Rep 28(6) (2019) 1612–1622 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Liva SG, Tseng YC, Dauki AM, Sovic MG, Vu T, Henderson SE, Kuo YC, Benedict JA, Zhang X, Remaily BC, Kulp SK, Campbell M, Bekaii-Saab T, Phelps MA, Chen CS, Coss CC, Overcoming resistance to anabolic SARM therapy in experimental cancer cachexia with an HDAC inhibitor, EMBO Mol Med 12(2) (2020) e9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ghosh S, Cisplatin: The first metal based anticancer drug, Bioorg Chem 88 (2019) 102925. [DOI] [PubMed] [Google Scholar]
- [41].CISPLATIN for injection, for intravenous use [package insert], WG Critical Care, LLC, Paramus, NJ, 2019. [Google Scholar]
- [42].Conte E, Bresciani E, Rizzi L, Cappellari O, De Luca A, Torsello A, Liantonio A, Cisplatin-Induced Skeletal Muscle Dysfunction: Mechanisms and Counteracting Therapeutic Strategies, Int J Mol Sci 21(4) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Conte E, Camerino GM, Mele A, De Bellis M, Pierno S, Rana F, Fonzino A, Caloiero R, Rizzi L, Bresciani E, Ben Haj Salah K, Fehrentz JA, Martinez J, Giustino A, Mariggio MA, Coluccia M, Tricarico D, Lograno MD, De Luca A, Torsello A, Conte D, Liantonio A, Growth hormone secretagogues prevent dysregulation of skeletal muscle calcium homeostasis in a rat model of cisplatin-induced cachexia, J Cachexia Sarcopenia Muscle 8(3) (2017) 386–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bresciani E, Rizzi L, Molteni L, Ravelli M, Liantonio A, Ben Haj Salah K, Fehrentz JA, Martinez J, Omeljaniuk RJ, Biagini G, Locatelli V, Torsello A, JMV2894, a novel growth hormone secretagogue, accelerates body mass recovery in an experimental model of cachexia, Endocrine 58(1) (2017) 106–114. [DOI] [PubMed] [Google Scholar]
- [45].Sakai H, Sagara A, Arakawa K, Sugiyama R, Hirosaki A, Takase K, Jo A, Sato K, Chiba Y, Yamazaki M, Matoba M, Narita M, Mechanisms of cisplatin-induced muscle atrophy, Toxicol Appl Pharmacol 278(2) (2014) 190–9. [DOI] [PubMed] [Google Scholar]
- [46].Dickey DT, Muldoon LL, Doolittle ND, Peterson DR, Kraemer DF, Neuwelt EA, Effect of N-acetylcysteine route of administration on chemoprotection against cisplatin-induced toxicity in rat models, Cancer Chemother Pharmacol 62(2) (2008) 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Garcia JM, Cata JP, Dougherty PM, Smith RG, Ghrelin prevents cisplatin-induced mechanical hyperalgesia and cachexia, Endocrinology 149(2) (2008) 455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kim GT, Kim EY, Shin SH, Lee H, Lee SH, Park K, Sohn KY, Yoon SY, Kim JW, PLAG alleviates cisplatin-induced cachexia in lung cancer implanted mice, Transl Oncol 20 (2022) 101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tax WJ, van de Winkel JG, Human Fc gamma receptor II: a standby receptor activated by proteolysis?, Immunol Today 11(9) (1990) 308–10. [DOI] [PubMed] [Google Scholar]
- [50].Liu L, Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins, Protein & cell 9(1) (2018) 15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Abuqayyas L, Balthasar JP, Application of knockout mouse models to investigate the influence of FcgammaR on the tissue distribution and elimination of 8C2, a murine IgG1 monoclonal antibody, Int J Pharm 439(1-2) (2012) 8–16. [DOI] [PubMed] [Google Scholar]
- [52].Oldham RJ, Mockridge CI, James S, Duriez PJ, Chan HTC, Cox KL, Pitic VA, Glennie MJ, Cragg MS, Fc gamma RII (CD32) modulates antibody clearance in NOD SCID mice leading to impaired antibody-mediated tumor cell deletion, J Immunother Cancer 8(1) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].James BH, Papakyriacou P, Gardener MJ, Gliddon L, Weston CJ, Lalor PF, The Contribution of Liver Sinusoidal Endothelial Cells to Clearance of Therapeutic Antibody, Front Physiol 12 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Robbie GJ, Criste R, Dall'acqua WF, Jensen K, Patel NK, Losonsky GA, Griffin MP, A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults, Antimicrob Agents Chemother 57(12) (2013) 6147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Deng R, Loyet KM, Lien S, Iyer S, DeForge LE, Theil FP, Lowman HB, Fielder PJ, Prabhu S, Pharmacokinetics of humanized monoclonal anti-tumor necrosis factor-{alpha} antibody and its neonatal Fc receptor variants in mice and cynomolgus monkeys, Drug Metab Dispos 38(4) (2010) 600–5. [DOI] [PubMed] [Google Scholar]
- [56].Yeung YA, Leabman MK, Marvin JS, Qiu J, Adams CW, Lien S, Starovasnik MA, Lowman HB, Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates, J Immunol 182(12) (2009) 7663–71. [DOI] [PubMed] [Google Scholar]
- [57].Tseng YC, Kulp SK, Lai IL, Hsu EC, He WA, Frankhouser DE, Yan PS, Mo X, Bloomston M, Lesinski GB, Marcucci G, Guttridge DC, Bekaii-Saab T, Chen CS, Preclinical Investigation of the Novel Histone Deacetylase Inhibitor AR-42 in the Treatment of Cancer-Induced Cachexia, J Natl Cancer Inst 107(12) (2015) djv274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sharp CN, Doll MA, Megyesi J, Oropilla GB, Beverly LJ, Siskind LJ, Subclinical kidney injury induced by repeated cisplatin administration results in progressive chronic kidney disease, Am J Physiol Renal Physiol 315(1) (2018) F161–F172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Basak EA, Wijkhuijs AJM, Mathijssen RHJ, Koolen SLW, Schreurs MWJ, Development of an Enzyme-Linked Immune Sorbent Assay to Measure Nivolumab and Pembrolizumab Serum Concentrations, Ther Drug Monit 40(5) (2018) 596–601. [DOI] [PubMed] [Google Scholar]
- [60].Deboer MD, Animal models of anorexia and cachexia, Expert Opin Drug Discov 4(11) (2009) 1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL, Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression, FASEB J 18(1) (2004) 39–51. [DOI] [PubMed] [Google Scholar]
- [62].Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ, Identification of ubiquitin ligases required for skeletal muscle atrophy, Science 294(5547) (2001) 1704–8. [DOI] [PubMed] [Google Scholar]
- [63].Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL, Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy, Proc Natl Acad Sci U S A 98(25) (2001) 14440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhang G, Lin RK, Kwon YT, Li YP, Signaling mechanism of tumor cell-induced up-regulation of E3 ubiquitin ligase UBR2, FASEB J 27(7) (2013) 2893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wen SW, Everitt SJ, Bedo J, Chabrot M, Ball DL, Solomon B, MacManus M, Hicks RJ, Moller A, Leimgruber A, Spleen Volume Variation in Patients with Locally Advanced Non-Small Cell Lung Cancer Receiving Platinum-Based Chemo-Radiotherapy, PLoS One 10(11) (2015) e0142608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wang Y, Juan LV, Ma X, Wang D, Ma H, Chang Y, Nie G, Jia L, Duan X, Liang XJ, Specific hemosiderin deposition in spleen induced by a low dose of cisplatin: altered iron metabolism and its implication as an acute hemosiderin formation model, Curr Drug Metab 11(6) (2010) 507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Milicevic Z, Slepcevic V, Nikolic D, Zivanovic V, Milicevic NM, Effects of cis-diamminedichloroplatinum II (cisplatin) on the splenic tissue of rats: a histoquantitative study, Exp Mol Pathol 61(2) (1994) 77–81. [DOI] [PubMed] [Google Scholar]
- [68].Coletti D, Chemotherapy-induced muscle wasting: an update, Eur J Transl Myol 28(2) (2018) 7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Barreto R, Waning DL, Gao H, Liu Y, Zimmers TA, Bonetto A, Chemotherapy-related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs, Oncotarget 7(28) (2016) 43442–43460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Garcia JM, Scherer T, Chen JA, Guillory B, Nassif A, Papusha V, Smiechowska J, Asnicar M, Buettner C, Smith RG, Inhibition of cisplatin-induced lipid catabolism and weight loss by ghrelin in male mice, Endocrinology 154(9) (2013) 3118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chen JA, Splenser A, Guillory B, Luo J, Mendiratta M, Belinova B, Halder T, Zhang G, Li YP, Garcia JM, Ghrelin prevents tumour- and cisplatin-induced muscle wasting: characterization of multiple mechanisms involved, J Cachexia Sarcopenia Muscle 6(2) (2015) 132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Damrauer JS, Stadler ME, Acharyya S, Baldwin AS, Couch ME, Guttridge DC, Chemotherapy-induced muscle wasting: association with NF-kappaB and cancer cachexia, Eur J Transl Myol 28(2) (2018) 7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Le Bricon T, Gugins S, Cynober L, Baracos VE, Negative impact of cancer chemotherapy on protein metabolism in healthy and tumor-bearing rats, Metabolism 44(10) (1995) 1340–8. [DOI] [PubMed] [Google Scholar]
- [74].Ryman JT, Meibohm B, Pharmacokinetics of Monoclonal Antibodies, CPT: pharmacometrics & systems pharmacology 6(9) (2017) 576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lindauer A, Valiathan CR, Mehta K, Sriram V, de Greef R, Elassaiss-Schaap J, de Alwis DP, Translational Pharmacokinetic/Pharmacodynamic Modeling of Tumor Growth Inhibition Supports Dose-Range Selection of the Anti-PD-1 Antibody Pembrolizumab, CPT: pharmacometrics & systems pharmacology 6(1) (2017) 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Magiera-Mularz K, Kocik J, Musielak B, Plewka J, Sala D, Machula M, Grudnik P, Hajduk M, Czepiel M, Siedlar M, Holak TA, Skalniak L, Human and mouse PD-L1: similar molecular structure, but different druggability profiles, iScience 24(1) (2021) 101960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Eigenmann MJ, Fronton L, Grimm HP, Otteneder MB, Krippendorff BF, Quantification of IgG monoclonal antibody clearance in tissues, MAbs 9(6) (2017) 1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].England CG, Ehlerding EB, Hernandez R, Rekoske BT, Graves SA, Sun H, Liu G, McNeel DG, Barnhart TE, Cai W, Preclinical Pharmacokinetics and Biodistribution Studies of 89Zr-Labeled Pembrolizumab, J Nucl Med 58(1) (2017) 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tufoni M, Zaccherini G, Caraceni P, Bernardi M, Albumin: Indications in chronic liver disease, United European Gastroenterol J 8(5) (2020) 528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].van Tetering G, Evers M, Chan C, Stip M, Leusen J, Fc Engineering Strategies to Advance IgA Antibodies as Therapeutic Agents, Antibodies (Basel) 9(4) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Grevys A, Bern M, Foss S, Bratlie DB, Moen A, Gunnarsen KS, Aase A, Michaelsen TE, Sandlie I, Andersen JT, Fc Engineering of Human IgG1 for Altered Binding to the Neonatal Fc Receptor Affects Fc Effector Functions, J Immunol 194(11) (2015) 5497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yip V, Palma E, Tesar DB, Mundo EE, Bumbaca D, Torres EK, Reyes NA, Shen BQ, Fielder PJ, Prabhu S, Khawli LA, Boswell CA, Quantitative cumulative biodistribution of antibodies in mice: effect of modulating binding affinity to the neonatal Fc receptor, MAbs 6(3) (2014) 689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Curran MA, Montalvo W, Yagita H, Allison JP, PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors, Proc Natl Acad Sci U S A 107(9) (2010) 4275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zander RA, Obeng-Adjei N, Guthmiller JJ, Kulu DI, Li J, Ongoiba A, Traore B, Crompton PD, Butler NS, PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity, Cell Host Microbe 17(5) (2015) 628–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Doleschel D, Hoff S, Koletnik S, Rix A, Zopf D, Kiessling F, Lederle W, Regorafenib enhances anti-PD1 immunotherapy efficacy in murine colorectal cancers and their combination prevents tumor regrowth, J Exp Clin Cancer Res 40(1) (2021) 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Jin Y, An X, Mao B, Sun R, Kumari R, Chen X, Shan Y, Zang M, Xu L, Muntel J, Beeler K, Bruderer R, Reiter L, Guo S, Zhou D, Li QX, Ouyang X, Different syngeneic tumors show distinctive intrinsic tumor-immunity and mechanisms of actions (MOA) of anti-PD-1 treatment, Sci Rep 12(1) (2022) 3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ober RJ, Radu CG, Ghetie V, Ward ES, Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies, Int Immunol 13(12) (2001) 1551–9. [DOI] [PubMed] [Google Scholar]
- [88].Andersen JT, Daba MB, Berntzen G, Michaelsen TE, Sandlie I, Cross-species binding analyses of mouse and human neonatal Fc receptor show dramatic differences in immunoglobulin G and albumin binding, J Biol Chem 285(7) (2010) 4826–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Habib SA, Suddek GM, Abdel Rahim M, Abdelrahman RS, The protective effect of protocatechuic acid on hepatotoxicity induced by cisplatin in mice, Life Sci 277 (2021) 119485. [DOI] [PubMed] [Google Scholar]
- [90].Fan YY, Farrokhi V, Caiazzo T, Wang M, O'Hara DM, Neubert H, Human FcRn Tissue Expression Profile and Half-Life in PBMCs, Biomolecules 9(8) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Sun Q, Seo S, Zvada S, Liu C, Reynolds K, Does Hepatic Impairment Affect the Exposure of Monoclonal Antibodies?, Clin Pharmacol Ther 107(5) (2020) 1256–1262. [DOI] [PubMed] [Google Scholar]
- [92].Zhao D, Long X, Fan H, Wang J, Effects of hepatic or renal impairment on the pharmacokinetics of immune checkpoint inhibitors, Am J Cancer Res 12(11) (2022) 4892–4903. [PMC free article] [PubMed] [Google Scholar]
- [93].Schroeder HW Jr., Cavacini L, Structure and function of immunoglobulins, J Allergy Clin Immunol 125(2 Suppl 2) (2010) S41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Daeron M, Nimmerjahn F, Fc Receptors, Current Topics in Microbiology and Immunology,, Springer International Publishing : Imprint: Springer,, Cham, 2014, pp. 1 online resource (XV, 424 pages 55 illustrations, 44 illustrations in color. [Google Scholar]
- [95].Lombana TN, Rajan S, Zorn JA, Mandikian D, Chen EC, Estevez A, Yip V, Bravo DD, Phung W, Farahi F, Viajar S, Lee S, Gill A, Sandoval W, Wang J, Ciferri C, Boswell CA, Matsumoto ML, Spiess C, Production, characterization, and in vivo half-life extension of polymeric IgA molecules in mice, MAbs 11(6) (2019) 1122–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Burvenich IJ, Farrugia W, Lee FT, Catimel B, Liu Z, Makris D, Cao D, O'Keefe GJ, Brechbiel MW, King D, Spirkoska V, Allan LC, Ramsland PA, Scott AM, Cross-species analysis of Fc engineered anti-Lewis-Y human IgG1 variants in human neonatal receptor transgenic mice reveal importance of S254 and Y436 in binding human neonatal Fc receptor, MAbs 8(4) (2016) 775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Guo Y, Wei L, Patel SH, Lopez G, Grogan M, Li M, Haddad T, Johns A, Ganesan LP, Yang Y, Spakowicz DJ, Shields PG, He K, Bertino EM, Otterson GA, Carbone DP, Presley C, Kulp SK, Mace TA, Coss CC, Phelps MA, Owen DH, Serum Albumin: Early Prognostic Marker of Benefit for Immune Checkpoint Inhibitor Monotherapy But Not Chemoimmunotherapy, Clin Lung Cancer 23(4) (2022) 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Jain R, Coss C, Whooley P, Phelps M, Owen DH, The Role of Malnutrition and Muscle Wasting in Advanced Lung Cancer, Curr Oncol Rep 22(6) (2020) 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Coss CC, Clinton SK, Phelps MA, Cachectic Cancer Patients: Immune to Checkpoint Inhibitor Therapy?, Clin Cancer Res 24(23) (2018) 5787–5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Sears SM, Orwick A, Siskind LJ, Modeling Cisplatin-Induced Kidney Injury to Increase Translational Potential, Nephron (2022) 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Rabe M, Schaefer F, Non-Transgenic Mouse Models of Kidney Disease, Nephron 133(1) (2016) 53–61. [DOI] [PubMed] [Google Scholar]
- [102].George B, Joy MS, Aleksunes LM, Urinary protein biomarkers of kidney injury in patients receiving cisplatin chemotherapy, Exp Biol Med (Maywood) 243(3) (2018) 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Challa DK, Wang X, Montoyo HP, Velmurugan R, Ober RJ, Ward ES, Neonatal Fc receptor expression in macrophages is indispensable for IgG homeostasis, MAbs 11(5) (2019) 848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Syn NL, Teng MWL, Mok TSK, Soo RA, De-novo and acquired resistance to immune checkpoint targeting, Lancet Oncol 18(12) (2017) e731–e741. [DOI] [PubMed] [Google Scholar]
- [105].Flint TR, Fearon DT, Janowitz T, Connecting the Metabolic and Immune Responses to Cancer, Trends Mol Med 23(5) (2017) 451–464. [DOI] [PubMed] [Google Scholar]
- [106].Baker K, Rath T, Flak MB, Arthur JC, Chen Z, Glickman JN, Zlobec I, Karamitopoulou E, Stachler MD, Odze RD, Lencer WI, Jobin C, Blumberg RS, Neonatal Fc receptor expression in dendritic cells mediates protective immunity against colorectal cancer, Immunity 39(6) (2013) 1095–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kasturirangan S, Rainey GJ, Xu L, Wang X, Portnoff A, Chen T, Fazenbaker C, Zhong H, Bee J, Zeng Z, Jenne C, Wu H, Gao C, Targeted Fcgamma Receptor (FcgammaR)-mediated Clearance by a Biparatopic Bispecific Antibody, J Biol Chem 292(10) (2017) 4361–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Mannik M, Arend MP, Hall AP, Gilliland BC, Studies on antigen-antibody complexes. I. Elimination of soluble complexes from rabbit circulation, J Exp Med 133(4) (1971) 713–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kurlander RJ, Ellison DM, Hall J, The blockade of Fc receptor-mediated clearance of immune complexes in vivo by a monoclonal antibody (2.4G2) directed against Fc receptors on murine leukocytes, J Immunol 133(2) (1984) 855–62. [PubMed] [Google Scholar]
- [110].Avery LB, Wang M, Kavosi MS, Joyce A, Kurz JC, Fan YY, Dowty ME, Zhang M, Zhang Y, Cheng A, Hua F, Jones HM, Neubert H, Polzer RJ, O'Hara DM, Utility of a human FcRn transgenic mouse model in drug discovery for early assessment and prediction of human pharmacokinetics of monoclonal antibodies, MAbs 8(6) (2016) 1064–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Proetzel G, Roopenian DC, Humanized FcRn mouse models for evaluating pharmacokinetics of human IgG antibodies, Methods 65(1) (2014) 148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Chen JL, Walton KL, Qian H, Colgan TD, Hagg A, Watt MJ, Harrison CA, Gregorevic P, Differential Effects of IL6 and Activin A in the Development of Cancer-Associated Cachexia, Cancer Res 76(18) (2016) 5372–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.