Abstract
Background
Frey's syndrome is a rare disorder, the symptoms of which include sweating, flushing and warming over the preauricular and temporal areas following a gustatory stimulus. It often occurs in patients who have undergone parotidectomy, submandibular gland surgery, radical neck dissection, infection and traumatic injury in the parotid region, and is caused by the aberrant regrowth of facial autonomic nerve fibres. Currently there are several options used to treat patients with Frey's syndrome; for example, the topical application of anticholinergics and antiperspirants, and the intradermal injection of botulinum toxin. It is uncertain which treatment is most effective and safe.
Objectives
To assess the efficacy and safety of different interventions for the treatment of Frey's syndrome.
Search methods
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; EMBASE; CINAHL; Web of Science; ICTRP and additional sources for published and unpublished trials. The date of the search was 28 April 2014.
Selection criteria
We included randomised or quasi‐randomised controlled trials (RCTs) in participants diagnosed with Frey's syndrome using a clinical standard such as Minor's starch‐iodine test. We planned to include trials in which participants received any intervention versus no treatment (observation) or an alternative intervention, with or without a second active treatment. Our primary outcome measures were success rate (as assessed clinically by Minor's starch‐iodine test, the iodine‐sublimated paper histogram method, blotting paper technique or another method) and adverse events. Our secondary outcome measure was success rate as assessed by patients (disappearance or improvement of symptoms).
Data collection and analysis
We used the standard methodological procedures expected by The Cochrane Collaboration.
Main results
We identified no RCTs or quasi‐RCTs that fulfilled the inclusion criteria. Our searches retrieved eight potentially relevant studies, but after assessment of the full‐text reports we excluded all of them due to the absence of randomisation or because the patients did not have Frey's syndrome. We excluded one randomised controlled trial that compared two different doses of botulinum toxin in patients with Frey's syndrome because the comparator was not an alternative treatment.
Authors' conclusions
We are unable to establish the efficacy and safety of the different methods used for the treatment of Frey's syndrome.
RCTs are urgently needed to assess the effectiveness of interventions for the treatment of Frey's syndrome. Future RCTs should include patients with Frey's syndrome of different ranges of severity and report these patients separately. Studies should investigate all possibly effective treatments (such as anticholinergics, antiperspirants and botulinum toxin) compared to control groups using different treatments or placebo. Subjective assessment of Frey's syndrome should be considered as one of the outcome measures.
Keywords: Humans; Sweating, Gustatory; Sweating, Gustatory/therapy
Plain language summary
Interventions for the treatment of Frey's syndrome
Background
Frey's syndrome is a rare disorder, the symptoms of which include sweating and flushing of the facial skin when eating, smelling, thinking or even dreaming about food. It usually happens in patients who have undergone surgery to the parotid (salivary) gland. This problem may have an impact on quality of life (for example, restricting normal activity such as eating in public). Many methods are currently used to treat Frey's syndrome, including topical application of anticholinergics and antiperspirants, and intradermal (into the skin) injections of botulinum toxin. This systematic review aimed to assess the efficacy and safety of these different methods for the treatment of Frey's syndrome.
Study characteristics
We carried out a comprehensive search for randomised controlled trials (RCTs) in participants diagnosed with Frey's syndrome. We planned to include trials in which participants received any intervention compared to no treatment (observation) or an alternative intervention, with or without a second active treatment. Despite extensive searching, we were unable to identify any studies that met our inclusion criteria.
Key results
There is no high‐quality evidence to establish which type of treatment is most effective for the treatment of Frey's syndrome. High‐quality clinical trials in this area should be urgently conducted. Studies should investigate all possibly effective treatments (such as anticholinergics, antiperspirants and botulinum toxin) compared to control groups using different treatments or placebo. Subjective (patient) assessment of Frey's syndrome should be one of the outcome measures used.
Quality of the evidence
This review is up to date to 28 April 2014.
Background
Description of the condition
Frey's syndrome, or gustatory sweating, is named after Łucja Frey, who first described it as 'auriculotemporal syndrome' in 1923 (Frey 1923). It is a sequela of parotidectomy, submandibular gland surgery, radical neck dissection, infection and traumatic injury in the parotid region, and is caused by the aberrant regrowth of facial autonomic nerve fibres (Bonanno 1992; O'Neill 2008). It is well accepted that Frey's syndrome is the result of aberrant regeneration of cut postganglionic parasympathetic fibres between the otic ganglion and subcutaneous vessels when injury to branches of the auriculotemporal nerve occurs (de Bree 2007). Due to this abnormal communication, the skin glands and vessels are stimulated when eating and masticating (Singh 2011). In response to such nerve impulses, acetylcholine is released from the presynaptic nerve endings to postsynaptic cholinergic receptors, which results in sweating and flushing. As sweating is controlled by sympathetic cholinergic pathways, treatments have traditionally involved anticholinergics (Watkins 1973).
The clinical symptoms of Frey's syndrome include sweating, flushing and warming over the preauricular and temporal areas after a gustatory stimulus (de Bree 2007). This unpleasant phenomenon may occur three to six months or even as long as 14 years after surgery on the parotid gland (Bakke 2006; Wenzel 2004). The incidence of Frey's syndrome varies (O'Neill 2008). A survey has reported patients' self reported incidence of Frey's syndrome to be 23%, while a positive Minor's starch‐iodine test has been observed in 62% of cases following parotidectomy (Neumann 2011). In a questionnaire evaluation of patients who had undergone any type of parotidectomy for benign salivary diseases, Frey's syndrome was identified as the most serious self perceived sequela and was of greatest concern, resulting in discomfort that worsened with time, even longer than five years postoperatively (Baek 2009).
Description of the intervention
Several treatments, including topical injection of alcohol (Frey 1923), scopolamine (Laage‐Hellman 1958), glycopyrrolate (Abell 1974), and botulinum toxin A (Clayman 2006; Drobik 1995; Steffen 2012), have been proposed for the treatment of Frey's syndrome and have been widely used. Botulinum toxin A is one of the most commonly used treatments (Arad‐Cohen 2000; Cantarella 2010; Martos 2008). Patients injected intradermally with botulinum toxin type A have been reported to have shown improvement after four to seven days (Pomprasit 2007), flushing has been reported to regress (Tugnoli 2002), and gustatory sweating to decrease (Beerens 2002). According to the results of a questionnaire survey, it has been suggested that botulinum toxin A could also significantly improve patients' functional quality of life (Hartl 2008). However, the effect of the intracutaneous injection of botulinum toxin type A in patients with gustatory sweating does diminish; the one‐, two‐ and three‐year actuarial estimate for symptomatic recurrent gustatory sweating was shown to be 27%, 63% and 92%, respectively (Laccourreye 1999). However, recurrence of Frey's syndrome may still be amenable to reinjection with botulinum toxin A, and investigators have reported that repeated injections are safe, decrease the size of the affected area and increase the duration of the recurrence‐free period (Beerens 2002; de Bree 2009; Laskawi 1998).
How the intervention might work
Sweating is controlled by sympathetic cholinergic pathways, which is why treatment has traditionally involved anticholinergics (Watkins 1973). Topical glycopyrrolate, an antimuscarinic agent, does not cross the blood‐brain barrier and penetrates biological membranes slowly, so this method of administration has few side effects compared to systemic use (Hays 1978; May 1989). Topical application of aluminium chloride hexahydrate directly acts on the sweat glands. Aluminium salt blocks the distal acrosyringium, which leads to functional and structural degeneration of the eccrine acini. However, it may also damage the sweat duct epithelium and cause inflammation (Hölzle 1984).
Botulinum toxin is a polypeptide produced by the bacterium Clostridium botulinum. Seven serological types exist, classified from A to G, with similar structure and distinct antigenic specificity and therapeutic profiles. Injection of botulinum toxin has the effect of an anticholinergic (Khoo 2006), and blocks acetylcholine release at the neuromuscular junction of striated muscles by lysing synaptosomal‐associated protein 25 (SNAP‐25) (Lawrence 2013). It therefore produces chemical denervation and paralysis of the muscles, which is effective both for striated muscle and eccrine glands (Kreyden 2004; Tugnoli 2002). SNAP‐25 is located on the cytoplasmic surface of the plasma membrane and on secretory vesicles (Kreyden 2004; Tugnoli 2002); it is essential for exocytosis, including vesicle docking, priming and fast fusion‐triggering (Hodel 1998; Mohrmann 2013). However, muscle paralysis and synaptic damage could be reversible due to the absorption of denatured SNPA‐25 and recombination of nerve ending and lamina terminalis (Lawrence 2013; Matak 2012). For this reason, recurrence of Frey's syndrome may be observed within three months of the first injection and reinjection may be needed.
Why it is important to do this review
Researchers have studied the potential for the prevention of Frey's syndrome, through the insertion of sternocleidomastoid muscle, temporalis fascia, superficial musculoaponeurotic system or an allograft between the site of surgical trauma and the skin during parotidectomy. However, a recent meta‐analysis has shown that prevention cannot be achieved in 100% of cases: Frey's syndrome still had an incidence of 8.3% in the acellular dermis matrix group and 11.1% in the muscle flap group (Li 2013). Treatment therefore remains necessary.
We aim to determine in this review whether the different treatments mentioned above are effective. Of equal importance is the identification of any adverse effects of treatment, as well as some kind of measurement of how likely it is for Frey's syndrome to re‐occur after treatment. The choice of treatment modality and dosage of agents has been a recent focus of attention (Bakke 2006; Guntinas‐Lichius 2002). However, outcomes such as the duration of freedom from symptoms, the degree of clinical regression, the incidence of recurrence and the seriousness of the crises need careful discussion. Work needs to be done to determine the best evidence for the treatment of Frey's syndrome. Such efforts would help to improve the quality of life of patients suffering from gustatory sweating.
Objectives
To assess the efficacy and safety of different interventions for the treatment of Frey's syndrome.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐RCTs.
Types of participants
Participants diagnosed with Frey's syndrome according to clinical criteria (such as presentation with typical symptoms, proven by Minor's starch‐iodine test, iodine‐sublimated paper histogram method, blotting paper technique).
Types of interventions
The intervention group should receive any possibly effective intervention (such as antiperspirants, anticholinergic agents, alcohol injections or tympanic neurectomy) aiming to treat Frey's syndrome, either alone or combined with another active treatment. The comparison (control group) should receive no treatment (observation) or an alternative intervention, with or without a second active treatment.
Types of outcome measures
We analysed the following outcomes in the review, but they were not used as a basis for including or excluding studies.
Primary outcomes
Success rate assessed clinically (Minor's starch‐iodine test, the iodine‐sublimated paper histogram method, blotting paper technique etc.) Success rate was defined as the proportion of patients with complete resolution of symptoms/signs as measured by the various tests.
Adverse events.
Secondary outcomes
Success rate assessed by participants (disappearance or improvement of symptoms).
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials. There were no language, publication year or publication status restrictions. The date of the last search was 28 April 2014.
Electronic searches
We searched the following databases from their inception for published, unpublished and ongoing trials: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 3); PubMed; EMBASE; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; CNKI; ISRCTN; ClinicalTrials.gov; ICTRP; OpenSigle; Sciencepaper Online; CBM; VIP; Google and Google Scholar.
We modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by The Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Higgins 2011)). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, we searched PubMed, TRIPdatabase, The Cochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. We searched for conference abstracts using the Cochrane Ear, Nose and Throat Disorders Group Trials Register.
Reference lists and contacts
We screened the references of the included articles for studies. We contacted authors and experts in the field to identify unpublished RCTs.
Data collection and analysis
Two review authors (Chunjie Li and Qi Zhang) carried out the selection of studies, data extraction and management, and assessment of risk of bias in the included studies. We resolved any disagreement by discussion or by referral to an arbiter (Longjiang Li).
Selection of studies
We used two steps in the selection of studies. First, we screened the titles and abstracts from the search results in order to find any studies that needed further assessment. We then retrieved the full text of possibly eligible studies and assessed them to judge whether they met the inclusion criteria. We classified studies into three categories: included, excluded or awaiting classification. We recorded the reasons for the exclusion of full‐text studies.
Data extraction and management
For data extraction and management, we extracted and recorded all of the following data using a data extraction form, which we pilot‐tested using three studies before any formal data extraction took place.
Source: study ID; review author ID; citation and contact details.
Eligibility: reasons for inclusion or exclusion.
Methods of the study: centres and their location; study duration; inclusion and exclusion criteria for participants; study design, sequence generation, allocation concealment, blinding and statistical methods.
Participants: total number; age and sex; characteristics of participants in each group, etc.
Interventions: number of intervention groups; intervention details of the treatment; control treatment and other active treatment; time, frequency, dose and usage if drugs administered.
Outcomes: definition of outcome measures and units of measurement; time points of measurement; measurement methods; sample size calculation; number of participants allocated to each group; number lost follow‐up and reasons; detailed summary data for each group.
Miscellaneous: funding; key conclusions of each article; correspondence required and miscellaneous comments from review authors.
Assessment of risk of bias in included studies
CJ Li and Q Zhang carried out assessment of the risk of bias of the included trials independently with the following taken into consideration, as guided by theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011):
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
other bias that is not covered by the first six, such as confounding bias, co‐intervention and contamination.
We used the Cochrane 'Risk of bias' tool in RevMan 5.3 (RevMan 2014), which involves describing each of these domains as reported in the trial and then assigning a judgement about the adequacy of each entry: 'low', 'high' or 'unclear' risk of bias.
We further classified the risk of bias in the included studies as 'high risk of bias', 'unclear risk of bias' or 'low risk of bias' according to the criteria presented in Table 1.
1. Criteria for summary 'Risk of bias' assessment.
| Risk of bias | Interpretation | In outcome | In included studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
The 'Risk of bias' assessment of outcomes was to be based on the key domains. For each outcome, we derived a series of key domains from the seven domains listed above. The key domains for success rate by clinical assessment are domain 1, 2, 4, 5, 6 and 7; the key domains for adverse events and success rate assessed by participants are domains 1, 2, 3, 5, 6 and 7. We also classified the risk of bias for each outcome into 'high risk of bias', 'unclear risk of bias' or 'low risk of bias' according to the criteria presented in Table 1.
Measures of treatment effect
The measures of treatment effect differed according to the data type and outcome variables. We treated success rate (either assessed clinically or by the participants) as dichotomous data. Adverse events can be ordinal or dichotomous data. However, ordinal data can also be treated as dichotomous data. We planned to express all dichotomous data as risk ratios (RR) with 95% confidence intervals (CIs).
There were no continuous data anticipated, considering the nature of the planned outcomes.
Unit of analysis issues
The unit of analysis was individual participants. It was not possible for cross‐over or cluster‐randomised controlled trials to be designed to study treatments for Frey's syndrome.
Studies with multiple treatment groups
As each meta‐analysis addresses only a single pair‐wise comparison, we considered two approaches. For those trials with multiple treatment groups, we planned to first try to combine groups to create a single pair‐wise comparison (groups would be combined in one outcome and they might not be combined in another outcome), or if this failed we planned to select the most related pair of interventions.
Dealing with missing data
We tried to obtain any missing data from the original study authors by email and letter.
Assessment of heterogeneity
Clinical heterogeneity might be due to different participant types (participants with different kinds of surgery or trauma, etc.), or different interventions and comparisons.
To assess statistical heterogeneity we planned to use the I² statistic to determine the range as follows:
0% to 40% slight heterogeneity;
30% to 60% moderate heterogeneity;
50% to 90% substantial heterogeneity;
75% to 100% considerable heterogeneity.
If there was considerable heterogeneity in one outcome, we would not carry out the meta‐analysis.
Assessment of reporting biases
We planned to assess any reporting bias for each outcome if there were more than 10 studies per outcome, by drawing funnel plots initially. Asymmetric funnel plots would indicate that there might be reporting bias. We would then conduct statistical analysis. We planned to test the asymmetry of the funnel plot using the methods introduced by Begg 1994 (using STATA 11.0) at the level of α = 0.10.
Data synthesis
We planned to consider two types of analysis model: we would adopt a random‐effects model if the I² statistic was > 50% and P value ≤ 0.10. If not, we would choose a fixed‐effect model. The exact statistical methods for the meta‐analysis would be the Mantel‐Haenszel (M‐H) method for dichotomous data and the inverse variance (IV) method for continuous data. Statistical significance for the hypothesis test would be set at P value < 0.05 (two‐tailed z tests).
Subgroup analysis and investigation of heterogeneity
We planned to carry out subgroup analysis according to the different interventions and types of participants. Such methods would mainly be adopted to reduce the clinical heterogeneity in each outcome.
Sensitivity analysis
We planned to carry out sensitivity analysis in order to test the stability of each outcome. We planned two sensitivity analyses:
including high‐quality studies only; and
intention‐to‐treat (ITT) analysis ('worst‐case scenario' analysis versus 'best‐case scenario' analysis).
We would report the results of sensitivity analysis and analyse the stability of the outcome.
'Summary of findings' tables
The quality of the combined data might be downgraded for several reasons, including study limitations (risk of bias at study level), directness of the evidence, heterogeneity, precision of effect estimates and risk of publication bias (see Assessment of reporting biases). To assess the quality of the body of evidence, we adopted the GRADE system using the software GRADEprofiler (Atkins 2004; Guyatt 2008; Higgins 2011). We planned to classify the quality of the evidence into four categories: high, moderate, low and very low. We would then consider the strength of recommendations derived from the quality of the evidence as strong or weak (Guyatt 2008). We planned to produce a 'Summary of findings' table for each of the primary outcomes of this review.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
After searching (databases and other sources) we identified 913 records, which reduced to 687 once duplicates were removed. Of these, we discarded 679 as irrelevant after reading the titles and abstracts. We retrieved the full texts of the remaining eight publications. After reading the full texts, we excluded all eight studies (see Excluded studies). The reasons for the exclusion of studies can be seen in the Characteristics of excluded studies table.
There were no ongoing studies identified. The study selection flow diagram is shown in Figure 1.
1.
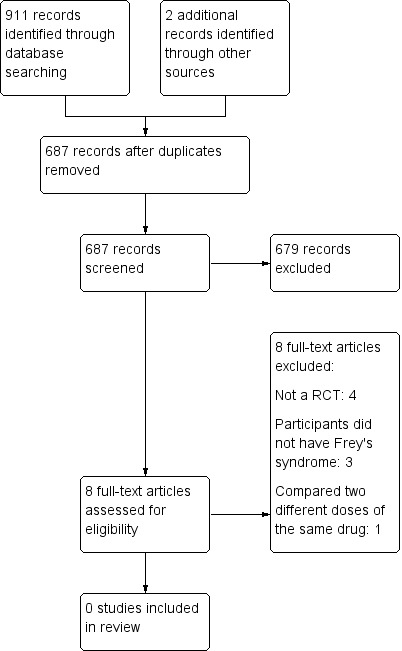
Study flow diagram.
Included studies
No studies were included in this review.
Excluded studies
See Characteristics of excluded studies table.
We excluded eight studies because they did not satisfy the inclusion criteria. We excluded most studies because of the absence of randomisation or because the patients did not have Frey's syndrome.
Nolte 2004 was a parallel‐group randomised controlled trial involving 20 adult participants with Frey's syndrome. The intervention group received a topical injection of botulinum toxin A at a dose of 3 MU/cm², while the control group received the same intervention at a dose of 2 MU/cm². However, two weeks after the injection, the control group participants received another 3 MU/cm² botulinum toxin A injection because of low treatment effect, which led to contamination of the trial. Nonetheless, the study did not meet our inclusion criteria because it did not compare alternative interventions for Frey's syndrome, but compared two different doses of the same intervention.
Risk of bias in included studies
There were no studies that met the review inclusion criteria.
Effects of interventions
No studies met the review inclusion criteria.
Discussion
Summary of main results
Although Frey's syndrome can be prevented, no procedure is 100% effective (Li 2013). This systematic review, which aimed to assess the efficacy and safety of different interventions for the treatment of Frey's syndrome, did not identify any randomised controlled trials (RCTs) that met the inclusion criteria.
Our search retrieved eight potentially relevant studies, which we examined in detail. However, all were ultimately excluded from the review for the reasons shown in Characteristics of excluded studies. We identified one parallel‐group randomised controlled trial involving 20 adult participants with Frey's syndrome (Nolte 2004). The intervention group received a topical injection of botulinum toxin A at a dose of 3 MU/cm², while the control group received the same intervention at a dose of 2 MU/cm². However, the study did not compare alternative interventions for Frey's syndrome (rather it compared two different doses of the same intervention) therefore it did not meet the inclusion criteria. Moreover, two weeks after the injection, the control group participants received another 3 MU/cm² botulinum toxin A injection because of low treatment effect, therefore the trial was contaminated.
Overall completeness and applicability of evidence
In this systematic review we have not been able to achieve the objectives set out in our protocol. Many of the studies of the treatment of Frey's syndrome that we found during our review process were not RCTs. The evidence from the studies that we screened was inconclusive: defects in study design mean that there is no strong evidence to support treatment choice for patients with Frey's syndrome.
Quality of the evidence
No studies were included in the review.
Potential biases in the review process
The risk of potential bias could not be totally avoided during the review process but we made efforts to reduce this. Firstly, no systematic review can state that all the studies that might meet the inclusion criteria have definitely been identified. However, we searched 21 databases (both English and Chinese) and we also searched for grey literature and ongoing studies. Studies reported in languages other than Chinese or English were translated and two review authors carefully judged them independently. We made great efforts to minimise the possibility of missing any studies. Secondly, the search, which was carried out on 28 April 2014, is up to date. Thirdly, where data were missing, we sent emails and letters to the original authors to see if they could provide detailed information. However, although we made these efforts, we received no response from the authors six months after the emails and letters were sent. We have identified no other risks of bias in the review process.
Agreements and disagreements with other studies or reviews
A review was published in the journal Laryngoscope in 1978 (Hays 1978). At that time, clinicians treated patients with the local use of anticholinergic agents such as scopolamine (Laage‐Hellman 1958) and glycopyrrolate (Abell 1974). Agreement was reached on the topical injection of agents.
A study published in the journal Head & Neck in 2003 reported the injection of botulinum toxin A in patients who had undergone superficial parotidectomy because of adenoma. The authors reported that the symptoms of gustatory sweating and flushing completely disappeared within one week after the first botulinum toxin A injection with no side effects (Eckardt 2003). Unfortunately, this study was not conducted as a RCT.
Many systematic reviews and clinical trials have focused on the prevention of Frey's syndrome (Durgut 2013; Li 2013; Sanabria 2012), however the focus of this review is treatment therefore these studies are not discussed here.
Authors' conclusions
Implications for practice.
This review identified no randomised controlled trials (RCTs) that compared different methods for the treatment of Frey's syndrome, therefore we are unable to establish the efficacy and safety of any of the methods currently in use.
Implications for research.
Clinicians need high‐quality RCTs with large sample sizes on this topic to guide their clinical practice. Trials should include patients with Frey's syndrome of different ranges of severity and report these groups separately. Studies should investigate all possibly effective treatments (such as anticholinergics, antiperspirants and botulinum toxin) compared to control groups using different treatments or placebo. Subjective assessment of Frey's syndrome should be considered as one of the outcome measures. Trials should clearly report not only their results but also their methods, according to the CONSORT statement (Moher 2010).
Acknowledgements
The authors would like to thank Mrs Gemma Sandberg, the Trials Search Co‐ordinator of the Cochrane ENT Group, for the search strategy design and database searches. We would also like to thank Mrs Jenny Bellorini, Managing Editor of the Cochrane ENT Group, for her help during the preparation of the review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Cochrane ENT Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
| CENTRAL | PubMed | EMBASE (Ovid) |
| #1 frey* AND syndrome #2 MeSH descriptor "Sweating, Gustatory" explode all trees #3 MeSH descriptor "Facial Nerve Injuries" explode all trees #4 MeSH descriptor "Taste" explode all trees #5 MeSH descriptor "Taste Disorders" explode all trees #6 gustatory #7 #3 OR #4 OR #5 OR #6 #8 MeSH descriptor "Sweat" explode all trees #9 MeSH descriptor "Sweat Glands" explode all trees #10 MeSH descriptor "Sweating" explode all trees #11 MeSH descriptor "Hyperhidrosis" explode all trees #12 sweat* OR hyperhidrosis #13 #8 OR #9 OR #10 OR #11 OR #12 #14 #7 AND #13 #15 #1 OR #2 OR #14 | #1 frey* AND syndrome #2 "Sweating, Gustatory"[Mesh] #3 "Facial Nerve Injuries"[Mesh] #4 "Taste"[Mesh] #5 "Taste Disorders"[Mesh] #6 gustatory #7 #3 OR #4 OR #5 OR #6 #8 "Sweat"[Mesh] #9 "Sweat Glands"[Mesh] #10 "Sweating"[Mesh] #11 "Hyperhidrosis"[Mesh] #12 sweat* OR hyperhidrosis #13 #8 OR #9 OR #10 OR #11 OR #12 #14 #7 AND #13 #15 #1 OR #2 OR #14 |
1 (frey* and syndrome).tw. 2 (gustatory and sweat*).tw. 3 exp facial nerve injury/ 4 exp taste/ 5 exp taste disorder/ 6 gustatory.tw. 7 3 or 4 or 5 or 6 8 exp sweat/ 9 exp sweat gland/ 10 exp sweating/ 11 exp hyperhidrosis/ 12 (sweat* or hyperhidrosis).tw. 13 8 or 9 or 10 or 11 or 12 14 7 and 13 15 1 or 2 or 14 |
| CINAHL (EBSCO) | Web of Science (Web of Knowledge) | ICTRP |
| S1 TX frey* AND TX syndrome S2 (MH "Frey's Syndrome") S3 (MH "Facial Nerve/IN") S4 (MH "Taste") S5 (MH "Taste Disorders+") S6 TX gustatory S7 S3 or S4 or S5 or S6 S8 (MH "Sweat") S9 (MH "Sweat Glands") S10 (MH "Sweating") S11 (MH "Hyperhidrosis+") S12 TX sweat* OR hyperhidrosis S13 S8 or S9 or S10 or S11 or S12 S14 S7 and S13 S15 S1 or S2 or S14 |
#1 TS=(frey* AND syndrome) #2 TS=(sweat* AND gustatory) #3 TS=("facial nerve*" AND (injur* OR damage OR trauma)) #4 TS=tast* #5 TS=gustatory #6 #5 OR #4 OR #3 #7 TS=(sweat* OR hyperhidrosis) #8 #7 AND #6 #9 #8 OR #2 OR #1 |
frey* AND syndrome OR gustatory AND sweat* OR hyperhidrosis AND gustatory OR facial nerve AND sweat* OR facial nerve AND hyperhidrosis OR taste AND sweat* OR taste AND hyperhidrosis |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Guntinas‐Lichius 2003 | ALLOCATION: not a RCT, review article |
| Hays 1978 | ALLOCATION: not a RCT |
| Heckmann 2001 | ALLOCATION: randomised PARTICIPANTS: patients did not have Frey's syndrome |
| Laccourreye 1990 | ALLOCATION: not a RCT |
| May 1989 | ALLOCATION: not a RCT |
| Nesathurai 1996 | ALLOCATION: not a RCT PARTICIPANTS: patients did not have Frey's syndrome |
| Nolte 2004 | ALLOCATION: parallel‐group RCT PARTICIPANTS: 20 adult patients with Frey's syndrome INTERVENTIONS: intervention group: topical injection of botulinum toxin A at a dose of 3 MU/cm²; control group: topical injection of botulinum toxin A at a dose of 2 MU/cm²*. Study did not compare botulinum toxin A with an alternative treatment for Frey's syndrome *2 weeks after the injection, the patients in the control group received another 3 MU/cm² botulinum toxin A injection because of low treatment effect, which led to contamination of the trial |
| Shaw 1997 | ALLOCATION: randomised PARTICIPANTS: patients did not have Frey's syndrome |
RCT: randomised controlled trial
Differences between protocol and review
We added a clarifying sentence to Types of outcome measures: "We analysed these outcomes in the review, but they were not used as a basis for including or excluding studies." This was done at the suggestion of the Cochrane ENT Group.
In Primary outcomes, we have added the definition of success rate.
Contributions of authors
Both Chunjie Li and Fanglong Wu were first authors of this review.
Chunjie Li proposed the title, registered the review, did the analysis and participated in the writing of the protocol and review.
Both Chunjie Li and Fanglong Wu selected studies, assessed risk of bias, extracted data and wrote the manuscript.
Qi Zhang participated in the writing of the review.
Longjiang Li proposed the title, registered the review, provided advice on the clinical and policy perspective for the review and revised the text.
Zongdao Shi and Qinghong Gao provided advice on the methodological perspective for the review, guided the review and revised the text.
Sources of support
Internal sources
West China School of Stomatology, Sichuan University, China.
State Key Laboratory of Oral Diseases, Sichuan University, China.
External sources
UK Cochrane Centre, UK.
Cochrane Ear, Nose and Throat Disorders Group, UK.
-
National Institute for Health Research, UK.
Infrastructure funding for the Cochrane Ear, Nose and Throat Disorders Group
Declarations of interest
Chunjie Li: none known.
Fanglong Wu: none known.
Qi Zhang: none known.
Qinghong Gao: none known.
Zongdao Shi: none known.
Longjiang Li: none known.
New
References
References to studies excluded from this review
Guntinas‐Lichius 2003 {published data only}
- Guntinas‐Lichius O. Management of Frey's syndrome and hypersialorrhea with botulinum toxin. Facial Plastic Surgery Clinics of North America 2003;11(4):503‐13. [DOI] [PubMed] [Google Scholar]
Hays 1978 {published data only}
- Hays LL. The Frey syndrome: a review and double blind evaluation of the topical use of a new anticholinergic agent. Laryngoscope 1978;88(11):1796‐824. [DOI] [PubMed] [Google Scholar]
Heckmann 2001 {published data only}
- Heckmann M, Ceballos‐Baumann AO, Plewig G, Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). New England Journal of Medicine 2001;344(7):488‐93. [DOI] [PubMed] [Google Scholar]
Laccourreye 1990 {published data only}
- Laccourreye O, Bonan B, Brasnu D, Laccourreye H. Treatment of Frey's syndrome with topical 2% diphemanil methylsulfate (Prantal): a double‐blind evaluation of 15 patients. Laryngoscope 1990;100(6):651‐3. [DOI] [PubMed] [Google Scholar]
May 1989 {published data only}
- May JS, McGuirt WF. Frey's syndrome: treatment with topical glycopyrrolate. Head and Neck 1989;11(1):85‐9. [DOI] [PubMed] [Google Scholar]
Nesathurai 1996 {published data only}
- Nesathurai S, Harvey DT. Clonidine in the management of asymmetrical gustatory facial sweating: an N‐of‐1 trial. Archives of Physical Medicine and Rehabilitation 1996;77(9):906‐8. [DOI] [PubMed] [Google Scholar]
Nolte 2004 {published data only}
- Nolte D, Gollmitzer I, Loeffelbein DJ, Hölzle F, Wolff KD. Botulinum toxin for treatment of gustatory sweating. A prospective randomized study [Botulinumtoxin zur Behandlung des gustatorischen Schwitzens]. Mund‐, Kiefer‐ und Gesichtschirurgie 2004;8(6):369‐75. [DOI] [PubMed] [Google Scholar]
Shaw 1997 {published data only}
- Shaw JE, Abbott CA, Tindle K, Hollis S, Boulton AJ. A randomised controlled trial of topical glycopyrrolate, the first specific treatment for diabetic gustatory sweating. Diabetologia 1997;40(3):299‐301. [DOI] [PubMed] [Google Scholar]
Additional references
Abell 1974
- Abell E, Morgan K. The treatment of idiopathic hyperhidrosis by glycopyrronium bromide and tap water iontophoresis. British Journal of Dermatology 1974;91(1):87‐91. [DOI] [PubMed] [Google Scholar]
Arad‐Cohen 2000
- Arad‐Cohen A, Blitzer A. Botulinum toxin treatment for symptomatic Frey's syndrome. Otolaryngology ‐ Head and Neck Surgery 2000;122(2):237‐40. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baek 2009
- Baek CH, Chung MK, Jeong HS, Son YI, Jung SC, Jeon HK, et al. Questionnaire evaluation of sequelae over 5 years after parotidectomy for benign diseases. Journal of Plastic, Reconstructive & Aesthetic Surgery 2009;62(5):633‐8. [DOI] [PubMed] [Google Scholar]
Bakke 2006
- Bakke M, Max Thorsen N, Bardow A, Dalager T, Eckhart Thomsen C, Regeur L. Treatment of gustatory sweating with low‐dose botulinum toxin A: a case report. Acta Odontologica Scandinavica 2006;64(3):129‐33. [DOI] [PubMed] [Google Scholar]
Beerens 2002
- Beerens AJ, Snow GB. Botulinum toxin A in the treatment of patients with Frey syndrome. British Journal of Surgery 2002;89(1):116‐9. [DOI] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PubMed] [Google Scholar]
Bonanno 1992
- Bonanno PC, Casson PR. Frey's syndrome: a preventable phenomenon. Plastic and Reconstructive Surgery 1992;89(3):452‐6; discussion 457‐8. [PubMed] [Google Scholar]
Cantarella 2010
- Cantarella G, Berlusconi A, Mele V, Cogiamanian F, Barbieri S. Treatment of Frey's syndrome with botulinum toxin type B. Otolaryngology ‐ Head and Neck Surgery 2010;143(2):214‐8. [DOI] [PubMed] [Google Scholar]
Clayman 2006
- Clayman MA, Clayman SM, Seagle MB. A review of the surgical and medical treatment of Frey syndrome. Annals of Plastic Surgery 2006;57(5):581‐4. [DOI] [PubMed] [Google Scholar]
de Bree 2007
- Bree R, Waal I, Leemans CR. Management of Frey syndrome. Head and Neck 2007;29(8):773‐8. [DOI] [PubMed] [Google Scholar]
de Bree 2009
- Bree R, Duyndam JE, Kuik DJ, Leemans CR. Repeated botulinum toxin type A injections to treat patients with Frey syndrome. Archives of Otolaryngology ‐ Head and Neck Surgery 2009;135(3):287‐90. [DOI] [PubMed] [Google Scholar]
Drobik 1995
- Drobik C, Laskawi R. Frey's syndrome: treatment with botulinum toxin. Acta Oto‐Laryngologica 1995;115(3):459‐61. [DOI] [PubMed] [Google Scholar]
Durgut 2013
- Durgut O, Basut O, Demir UL, Ozmen OA, Kasapoglu F, Coskun H. Association between skin flap thickness and Frey's syndrome in parotid surgery. Head and Neck 2013;35(12):1781‐6. [DOI] [PubMed] [Google Scholar]
Eckardt 2003
- Eckardt A, Kuettner C. Treatment of gustatory sweating (Frey's syndrome) with botulinum toxin A. Head and Neck 2003;25(8):624‐8. [DOI] [PubMed] [Google Scholar]
Frey 1923
- Frey L. Le syndrome du nerf auriculo‐temporal. Revue Neurologique 1923;2:92‐104. [Google Scholar]
Guntinas‐Lichius 2002
- Guntinas‐Lichius O. Increased botulinum toxin type A dosage is more effective in patients with Frey's syndrome. Laryngoscope 2002;112(4):746‐9. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartl 2008
- Hartl DM, Julieron M, LeRidant AM, Janot F, Marandas P, Travagli JP. Botulinum toxin A for quality of life improvement in post‐parotidectomy gustatory sweating (Frey's syndrome) . Journal of Laryngology and Otology 2008;122(10):1100‐4. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hodel 1998
- Hodel A. SNAP‐25. International Journal of Biochemistry and Cell Biology 1998;30(10):1069‐73. [DOI] [PubMed] [Google Scholar]
Hölzle 1984
- Hölzle E, Braun‐Falco O. Structural changes in axillary eccrine glands following long‐term treatment with aluminium chloride hexahydrate solution. British Journal of Dermatology 1984;110(4):399‐403. [DOI] [PubMed] [Google Scholar]
Khoo 2006
- Khoo SG, Keogh IJ, Timon C. The use of botulinum toxin in Frey's syndrome. Irish Medical Journal 2006;99(5):136‐7. [PubMed] [Google Scholar]
Kreyden 2004
- Kreyden OP, Scheidegger EP. Anatomy of the sweat glands, pharmacology of botulinum toxin, and distinctive syndromes associated with hyperhidrosis. Clinics in Dermatology 2004;22(1):40‐4. [DOI] [PubMed] [Google Scholar]
Laage‐Hellman 1958
- Laage‐Hellman JE. Treatment of gustatory sweating and flushing. Acta Oto‐Laryngologica 1958;49(2):132‐43. [DOI] [PubMed] [Google Scholar]
Laccourreye 1999
- Laccourreye O, Akl E, Gutierrez‐Fonseca R, Garcia D, Brasnu D, Bonan B. Recurrent gustatory sweating (Frey syndrome) after intracutaneous injection of botulinum toxin type A: incidence, management, and outcome. Archives of Otolaryngology ‐‐ Head and Neck Surgery 1999;125(3):283‐6. [DOI] [PubMed] [Google Scholar]
Laskawi 1998
- Laskawi R, Drobik C, Schönebeck C. Up‐to‐date report of botulinum toxin A treatment in patients with gustatory sweating (Frey's syndrome). Laryngoscope 1998;108(3):361‐84. [DOI] [PubMed] [Google Scholar]
Lawrence 2013
- Lawrence GW, Ovsepian SV, Wang J, Aoki KR, Dolly JO. Therapeutic effectiveness of botulinum neurotoxin A: potent blockade of autonomic transmission by targeted cleavage of only the pertinent SNAP‐25. Neuropharmacology 2013;70:287‐95. [DOI] [PubMed] [Google Scholar]
Li 2013
- Li C, Yang X, Pan J, Shi Z, Li L. Graft for prevention of Frey syndrome after parotidectomy: a systematic review and meta‐analysis of randomized controlled trials. Journal of Oral and Maxillofacial Surgery 2013;71(2):419‐27. [DOI] [PubMed] [Google Scholar]
Martos 2008
- Martos Díaz P, Bances del Castillo R, Mancha de la Plata M, Naval Gías L, Martínez Nieto C, Lee GY, et al. Clinical results in the management of Frey's syndrome with injections of botulinum toxin. Medicina Oral, Patologia Oral, Cirugia Bucal 2008;13(4):E248‐52. [PubMed] [Google Scholar]
Matak 2012
- Matak I, Riederer P, Lacković Z. Botulinum toxin's axonal transport from periphery to the spinal cord. Neurochemistry International 2012;61(2):236‐9. [DOI] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohrmann 2013
- Mohrmann R, Wit H, Connell E, Pinheiro PS, Leese C, Bruns D, et al. Synaptotagmin interaction with SNAP‐25 governs vesicle docking, priming, and fusion triggering. Journal of Neuroscience 2013;33(36):14417‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neumann 2011
- Neumann A, Rosenberger D, Vorsprach O, Dazert S. The incidence of Frey syndrome following parotidectomy: results of a survey and follow‐up. HNO 2011;59(2):173‐8. [DOI] [PubMed] [Google Scholar]
O'Neill 2008
- O'Neill JP, Condron C, Curran A, Walsh A. Lucja Frey‐‐historical relevance and syndrome review. Surgeon 2008;6(3):178‐81. [DOI] [PubMed] [Google Scholar]
Pomprasit 2007
- Pomprasit M, Chintrakarn C. Treatment of Frey's syndrome with botulinum toxin. Journal of the Medical Association of Thailand 2007;90(11):2397‐402. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sanabria 2012
- Sanabria A, Kowalski LP, Bradley PJ, Hartl DM, Bradford CR, Bree R, et al. Sternocleidomastoid muscle flap in preventing Frey's syndrome after parotidectomy: a systematic review. Head and Neck 2012;34(4):589‐98. [DOI] [PubMed] [Google Scholar]
Singh 2011
- Singh N, Kohli M, Kohli H. Innovative technique to reduce incidence of Frey's syndrome after parotid surgery. The American Surgeon 2011;77(3):351‐4. [PubMed] [Google Scholar]
Steffen 2012
- Steffen A, Rotter N, König IR, Wollenberg B. Botulinum toxin for Frey's syndrome: a closer look at different treatment responses. Journal of Laryngology and Otology 2012;126(2):185‐9. [DOI] [PubMed] [Google Scholar]
Tugnoli 2002
- Tugnoli V, Marchese Ragona R, Eleopra R, Quatrale R, Capone JG, et al. The role of gustatory flushing in Frey's syndrome and its treatment with botulinum toxin type A. Clinical Autonomic Research 2002;12(3):174‐8. [DOI] [PubMed] [Google Scholar]
Watkins 1973
- Watkins PJ. Facial sweating after food: a new sign of diabetic autonomic neuropathy. British Medical Journal 1973;1(5853):583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wenzel 2004
- Wenzel GI, Draf W. Unusually long latency before the appearance of Frey's syndrome after parotidectomy. HNO 2004;52(6):554‐6. [DOI] [PubMed] [Google Scholar]


