Abstract
Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) is currently the leading cause of chronic liver disease worldwide. Metabolic Dysfunction-Associated Steatohepatitis (MASH), an advanced form of MASLD, can progress to liver fibrosis, cirrhosis, and hepatocellular carcinoma. Based on recent findings by our team that liver 5HT2A knockout male mice suppressed steatosis and reduced fibrosis-related gene expression, we developed a peripheral 5HT2A antagonist, compound 11c for MASH. It shows good in vitro activity, stability, and in vivo pharmacokinetics (PK) in rats and dogs. Compound 11c also shows good in vivo efficacy in a diet-induced obesity (DIO) male mice model and in a choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD) male mice model, effectively improving histologic features of MASH and fibrosis. According to the tissue distribution study using [14C]-labeled 11c, the compound was determined to be a peripheral 5HT2A antagonist. Collectively, first-in-class compound 11c shows promise as a therapeutic agent for the treatment of MASLD and MASH.
Subject terms: Drug development, Drug discovery and development, Chemical modification
Metabolic Dysfunction-Associated Steatohepatitis (MASH), an advanced form of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD), can progress to liver fibrosis. Here, the authors develop a peripheral 5HT2A antagonist for the treatment of MASLD and MASH.
Introduction
Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD), formerly known as Nonalcoholic fatty liver disease (NAFLD) is emerging as a leading chronic liver disease worldwide1–3. MASLD is an increasingly common condition defined by the presence of ≥ 5% liver steatosis in the absence of significant alcohol consumption, steatogenic medication, or monogenic hereditary disorders4. Both Metabolic Dysfunction-Associated Steatohepatitis (MASH, formerly known as nonalcoholic steatohepatitis (NASH)) and non-alcoholic fatty liver (NAFL), known as steatosis, are histologically categorized under the umbrella of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD). Steatosis, also called simple fatty liver, is defined as the presence of a fatty liver with little to no evidence of inflammation or damage, whereas MASH is defined as the presence of fatty liver and inflammation with evidence of ballooning, with or without peri-sinusoidal fibrosis5. MASH can progress to more advanced stages of liver disease including cirrhosis, liver failure, and hepatocellular carcinoma (HCC)6,7. Obesity and type-2 diabetes are associated with elevated risk of developing MASH8.
Serotonin (5-hydroxytryptamine (5HT)) is a monoamine neurotransmitter that modulates central and peripheral functions. Peripheral 5HT is emerging as a key regulator of systemic energy metabolism that modulates various physiological roles in multiple metabolic tissues9–13. As 5HT cannot cross the blood brain barrier, central and peripheral 5HT systems function separately14. Interestingly, among the 5HT receptors found to be expressed at detectable levels in the liver, 5HT2A (also known as HTR2A) expression was increased by high-fat diet (HFD) feeding15. Our team reported that HFD-fed liver specific 5HT2A knock-out (5HT2A LKO) mice showed decreased hepatic steatosis as examined by histology, NAFLD Activity Score (NAS), and hepatic triglycerides (TG) concentrations without affecting the body weight, glucose tolerance, insulin sensitivity, and plasma lipid profiles15. Also, inflammation and fibrosis-related gene expressions were decreased in the liver of 5HT2A LKO mice, indicating that 5HT2A signaling is important for inflammation and fibrosis in the liver15. Thus, 5HT2A is a potential target for therapies aiming to prevent the progression of MASLD and MASH. Alarmingly, approximately 25% of MASH patients progress to cirrhosis, a serious premalignant condition that may require liver transplantation or result in liver failure16. Obesity-associated MASH is currently the third leading cause for liver transplantation and is expected to surpass hepatitis C as the principal cause for liver transplantation in the developed world17. The global prevalence of MASLD is estimated to be 25%2 and accounts for 75.1% of all chronic liver disease. The most effective treatments currently for management of MASH are life-style interventions. Healthy diet, physical exercise, and weight loss are advised for MASH patients with an aim to halt disease progression and improve liver function18.
There are currently no approved pharmaceutical therapies for the treatment of MASH. Off-label drugs and functional nutrients use is the only available treatment option. Pharmaceutical therapies could be an important addition and diverse drug candidates are currently in clinical trials. Obeticholic acid (OCA), commercially known as Ocaliva significantly improved hepatic fibrosis and hepatic steatosis, but these therapeutic effects did not outweigh its risk of side effects in clinical trials, and Selonsertib failed to show significantly improved hepatic fibrosis in phase III clinical trial19. We have been working on developing new peripheral 5HT2A antagonists and reported amino-acid containing derivatives for MASLD. The compounds showed good in vitro activities but could not be further developed due to safety issues and deliverability as an oral drug candidate20,21. Therefore, we attempted to identify a new peripheral 5HT2A antagonist suitable for oral treatment of MASH22. Herein, we report the synthesis and biological evolutions of a novel clinical candidate compound 11c for MASLD or MASH treatment. Library screening and molecular docking led to the identification of compound 11c as a potent 5HT2A antagonist. In vitro activity, selectivity, stability, toxicity, and in vivo PK in rats and dogs were conducted for evaluation of druggability and DMPK. The tissue distribution study using [14C]-labeled 11c was performed to determine whether to be a peripheral agent. In vivo efficacy in diet-induced obesity (DIO) mice model and in a choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD) mice model, was evaluated for MASLD or MASH.
Results
Discovery of a potent 5HT2A antagonist
Based on previous 5HT2A LKO results by our team15, we have been synthesizing new (peripheral) 5HT2A antagonists. In this paper, we tried to identify new hits through the drug library and/or rational design from FDA approved neuronal drugs. Therefore, we searched for a new scaffold and found compound 2 that is known as Clarinex (Desloratadine), which is used to treat allergies (Supplementary Fig. 1). Compound 2 is a tricyclic compound with a peripheral H1-antagonist action, was identified as a hit with IC50 value of 232 nM for 5HT2A. According to the initial finding, we attempted to develop a new peripheral 5HT2A antagonist. Based on molecular docking support, diverse derivatives were synthesized (Supplementary Figs. 2, 3), and the inhibitory potency of the synthesized compounds towards 5HT2A was evaluated, and the results were summarized in Tables 1–4. Therefore, we decided to explore different substituents on nitrogen of piperidine moiety of compound 2 by introducing N-acetyl (3), N-methylsulfonyl (4), isopropyl thiourea (5) and methyl (6) groups. Compounds 3-5 (Table 1) showed significantly decrease in activity compared to compound 2, on the other hand the methyl substituent derivative 6 showed improved activity (IC50 = 119 nM).
Table 1.
SAR Exploration of compounds 1-6
| Compound | Structure | % Inhibition at 10 μM (SD) | IC50 (nM) |
|---|---|---|---|
| 1 | 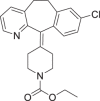 |
2 | ND |
| 2 | 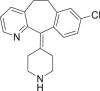 |
88 ± 2.60 | 232 |
| 3 | 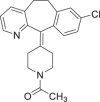 |
-1 | ND |
| 4 | 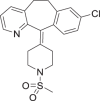 |
2 | ND |
| 5 | 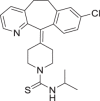 |
8 | ND |
| 6 | 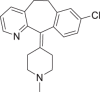 |
95 ± 0.31 | 119 |
SD Standard Deviation, ND Not determined.
Table 4.
Pharmacokinetic Study of Compound 11c in rat and dog models
| Parameters | In vivo PK (rat) | In vivo PK (dog) | |||
|---|---|---|---|---|---|
| IV (5 mpk) | PO (5 mpk) | PO (10 mpk) | IV (5 mpk) | PO (5 mpk) | |
|
T1/2 (h) AUC (μg·h/mL) CL (L/h/kg) V (L/kg) F (%) |
4.4 1.55 2.82 8.86 - |
2.5 0.98 - - 63 |
2.7 2.88 - - 92.5 |
8.6 16.79 0.42 4.36 - |
10.2 19.11 - - 73 |
(T1/2) Half-life, AUC Area under the curve, CL Clearance, V Volume of Distribution, F Bioavailability, PO per so, by mouth), IV Intravenous, mg/kg (mpk).
To further expand the substituents on N-methyl piperidine derivative series, more substituted derivatives 8a–c were synthesized and evaluated as shown in Table 2. As compared to derivative 6, 8a displayed improved activity with an IC50 value of 81.37 nM. 8b and 8c, which have bicyclic moieties, showed weaker or similar percent inhibition activities (68% and 73% inhibition at 1 uM) Next, we examined whether the reduction of keto to alcohol could further improve the activity. Thus, compounds 9a–c were synthesized as shown in Table 2, and evaluated for their activity. They showed good in vitro activities, but all alcohol derivatives exhibited low liver microsomal stability.
Table 2.
SAR Exploration of compounds 8a-c and 9a-c
| Compound | Structure | % Inhibition at 1 μM (SD) | IC50 (nM) |
|---|---|---|---|
| 6 | 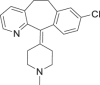 |
90 ± 0.62 | 119 |
| 8a | 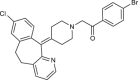 |
83.2 ± 1.98 | 81.37 |
| 8b | 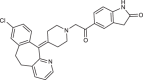 |
68.34 ± 2.78 | ND |
| 8c | 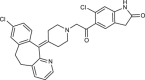 |
73.02 ± 0.51 | ND |
| 9a | 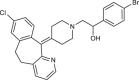 |
92 ± 1.07 | 31.95 |
| 9b | 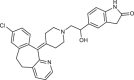 |
92.18 ± 1.59 | 21.35 |
| 9c | 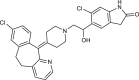 |
93.85 ± 3.13 | 22.19 |
SD Standard Deviation, ND Not determined.
Based on the structure and activity of the two series above, we replaced the alcohol with –CH2. As shown in Table 3, the inhibitory activity of compounds 11a–d containing the ethyl linker were similar to better than that of compounds 9a–c with 2-ethanol linker. Compounds 11b, 11c, and 11d were the most active compounds with an IC50 value of 14, 14 and 47 nM, respectively. Compound 11c is metabolically stable in buffer as well as in plasma (Table 5), but compound 11b and 11d were metabolically unstable (11b = 11.7% and 17.3%, 11d = 0.50% and 5.33% in Rat and Human respectively) after 30 min incubation in the liver microsome. Additionally, we attempted to synthesize compound 11c derivatives without a substituent (11e) and with a methyl (21) substituent on the tricyclic core instead of chloro. Compounds 11e and 21 showed less potency than compound 11c. Moreover, we separated two enantiomers (22 f and 22 g) of compound 11c by using the reported synthesis procedure23. Both enantiomers (+)-22f (IC50 = 10.6 nM) and (-)-22g (IC50 = 11.08 nM) showed similar activity and drug metabolism and pharmacokinetics (DMPK) profiles therefore, we chose racemic compound 11c for further biological evaluation.
Table 3.
SAR Exploration of compounds 11a-e, 21 and 22f-g
| Compound | Structure | % Inhibition at 1 μM (SD) | IC50 (nM) |
|---|---|---|---|
| 9c | 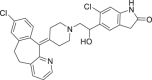 |
93.85 ± 3.13 | 22.19 |
| 11a | 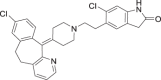 |
94.47 ± 1.53 | ND |
| 11b | 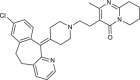 |
92.63 ± 0.60 | 14 |
| 11c | 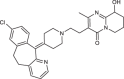 |
92.56 ± 0.77 | 14 |
| 11d | 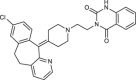 |
102 ± 0.03 | 47 |
| 11e | 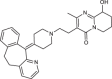 |
89.22 ± 10.98 | 61.01 |
| 21 | 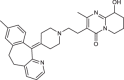 |
28.80 ± 3.68 | 67.74 |
| 22 f | 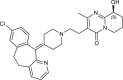 |
96.07 ± 1.87 | 10.6 |
| 22 g |  |
97.17 ± 7.11 | 11.08 |
SD Standard Deviation, ND Not determined.
Table 5.
Stability, cytotoxicity, CYP, AMES, solubility, acute toxicity, and selectivity results of compound 11c
| Assay | Result |
|---|---|
| Chemical stability |
99.8% (250C, 3weeks), 99.6% (600C, 3weeks) |
| Hepatocyte stability (% remaining after 3 h) |
94%(human), 88.5% (dog), 84.6% (monkey), 68.9% (rat), 75% (mouse) |
| Plasma stability (% remaining after 4 h) | >99.9% (m), >99.9% (r), >98.6 + 11.7% (h), |
| CYP inhibition (10 µM) |
1A2: < 1%, 2C9:3.17% 2C19:6.83% 2D6: 26.3% 3A4:17.6% |
| Cytotoxicity (IC50) |
VERO > 100 µM, HFL-1 > 100 µM, L929 > 100 µM, NIH3T3 > 100 µM, CHO-K1 > 100 µM |
| AMES | Negative |
| Solubility | 1.3 mg/mL pH = 6.8 |
| Acute toxicity | LD50 > 1000 mpk |
| Selectivity |
1 A: 9 % inhibition 1B: 16 % inhibition 1D: 3 % inhibition 2 A: 102 % inhibition, IC50 = 14 nM 2B: 94 % inhibition, IC50 = 290 nM 2 C: 103 % inhibition, IC50 = 170 nM 3: -1 % inhibition 4E: -3 % inhibition 6: 7 % inhibition 7: 91 % inhibition, IC50 = 100 nM |
Molecular docking
To better optimize compound 2 (hit), molecular docking was performed using the crystal structure of human 5HT2A (Protein Data Bank 7WC4). First, docking of compound 2 was performed (Fig. 1a, left panel) and seven interactions were determined (Fig. 1a, right panel). Based on these interactions, we introduced diverse moieties but found that the nitrogen group derivatives were critical for efficacy. The modeling was analyzed, and the nitrogen of compound 2 showed pocket-binding interaction with serine 242, SER-242. Docking of compound 11c (Fig. 2b, left panel) had an increased number of interactions to ten for compound 11c (Fig. 2b, right panel). Compound 11c bound to all the same side chains as compound 2 (ILE205, PRO-209, ILE-210, PHE-234, ILE-237, VAL-241, SER-242) with the addition of three more interactions (TRP-164, SER-203, ILE-202). In addition, we performed docking of 5HT2A antagonists, Risperidone, and Zotepine that was previously reported24. We compared binding affinities and it was shown that compound 11c had a better binding affinity than Compound 2 and commercially available Resperidone and Zoptepine, (−9.2, −8.0, −8.4, and −6.3 kcal/mol respectively. Risperidone showed better binding than Zotepine in our molecular docking, which could correlate to better efficacy that was previously reported25. This analysis further confirms that compound 11c ability as an antagonist for 5HT2A.
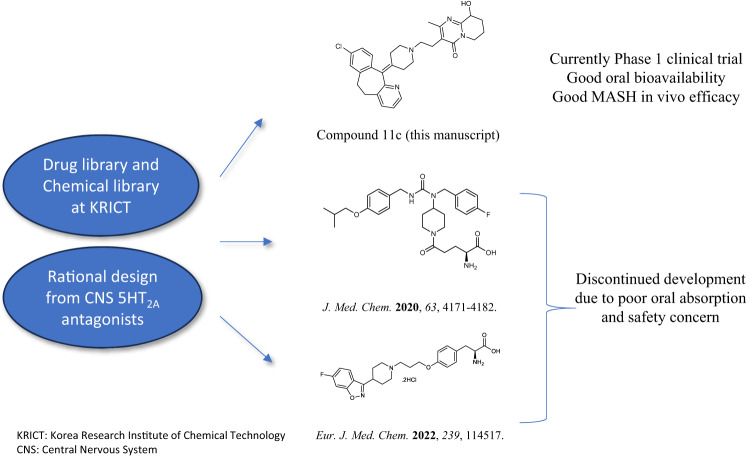
Fig. 1. Strategy and history of 5HT2A antagonists.
Library and rational design for the development of compound 11c as a potent 5HT2A antagonist. Previous research efforts were discontinued due to limited oral absorption and safety.
Fig. 2. Molecular docking of compounds to Human 5HT2A.
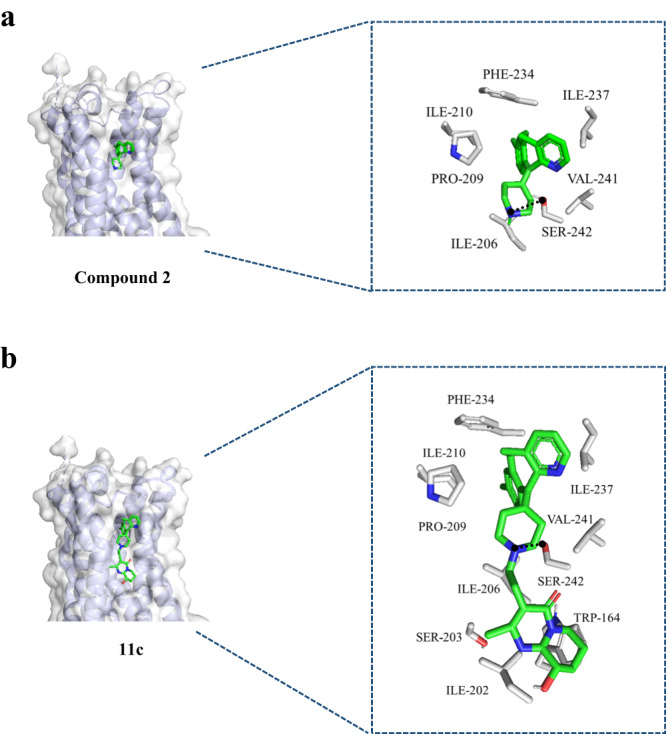
a The predicted binding model of compound 2 binding to 5HT2A (left panel) and interactions with 5HT2A side chains (right panel). b The predicted binding model of compound 11c binding to 5HT2A (left panel) and interactions with 5HT2A side chains. The three-dimensional structure of human 5HT2A is shown with a ribbon expression. 5HT2A side chains and compounds 2 and 11c are represented as sticks. PHD phenylalanine, ILE Isoleucine, PRO Proline, VAL valine, SER Serine, TRP Tryptophan.
DMPK, selectivity and toxicity
Pharmacokinetic parameters of compound 11c were determined and summarized in Table 4. Compound 11c demonstrated good PK profiles with the half-life was determined to be 4.14 h and 8.6 h in rats and dogs respectively for intravenous administration, and 10.2 h in dogs for oral administration while its bioavailability was 63% (5 mpk, rat), 92.5% (10 mpk, rat) and 73% (5 mpk) in dogs respectively. Compound 11c also exhibited a reasonable area under the curve (AUC, Table 4).
Compound 11c showed good chemical, hepatocyte, and plasma stability, no significant cytotoxicity in five representative mammalian cell lines (VERO, HFL-1, L929, NIH3T3, CHO-K1) and low CYP inhibition in five subtypes (Table 5). Compound 11c didn’t show mutagenic potential (AMES test) and exhibited LD50 value of over 1000 mpk in acute tox study. For further evaluation, the inhibition percentages of other subtypes of serotonin receptors were screened and compound 11c showed selective inhibition of 5HT2A among the subtypes. Although compound 11c also inhibited 5HT2c and 5HT7, they are mainly expressed in brain26,27 therefore peripheral compound 11c may not inhibit them.
Using radio labeled [14C]-compound 11c, we conducted the tissue distribution study and the results are summarized in Table 6. [14C]-compound 11c was below the lower limit of quantitation in the brain, and it remained mainly in liver, intestine, and pancreas.
Table 6.
Pharmacokinetic parameters of compound 11c
| Tissue | Cmax (ng equiv./g) | Tmax (h) | T1/2 (h) | AUC0-t (ng equiv./g) | AUCinf (ng equiv./g) | Tissue: plasma** |
|---|---|---|---|---|---|---|
| Plasma | 225 | 0.5 | NC | 783 | NC | 1.00 |
| Whole Blood | 158 | 0.5 | NC | 589 | NC | 0.75 |
| Adrenal gland | 1510 | 6 | NC | 7210 | NC | 9.21 |
| Brain | BQL | NC | NC | NR | NC | NA |
| Heart | 369 | 6 | NC | 1800 | NC | 2.30 |
| Kidney | 1840 | 6 | NC | 7860 | NC | 10.0 |
| Large Intestine | 9800 | 6 | NC | 127000 | NC | 162 |
| Liver | 2920 | 6 | NC | 15400 | NC | 19.7 |
| Lung | 941 | 6 | NC | 4290 | NC | 5.48 |
| Pancreas | 4330 | 6 | NC | 15400 | NC | 19.7 |
| Small Intestine | 45000 | 0.25 | NC | 16600 | NC | 21.2 |
| Spleen | 2290 | 6 | NC | 9520 | NC | 12.2 |
| Stomach | 3240 | 0.25 | NC | 5130 | NC | 6.55 |
Equivalents in Plasma and Tissue of Male Sprague Dawley Rats following an Oral Dose of [14C]-11c at 5 mg/kg. (NC= Not calculated due to insufficient terminal elimination phase, NA Not applicable, NR Not reportable due to <3 consecutive quantifiable concentrations, BQL Below the lower limit of quantitation 148 ng equiv./g, **Tissue: plasma ratio based on AUC0-t).
In vivo efficacy of compound 11c in DIO mice
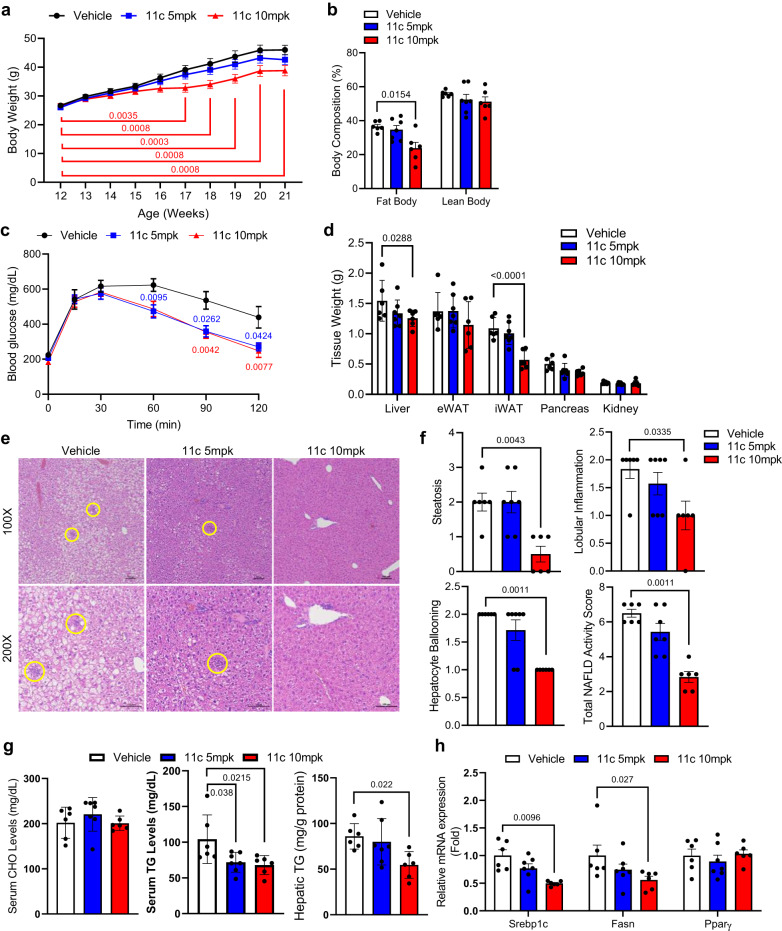
It has been reported that genetic deletion or inhibition of 5HT2A has an anti-MASLD through the reduction of lipid accumulation or inhibition of de novo lipogenesis in the liver15,28. Accordingly, we evaluated the potential in vivo efficacy of compound 11c for MASLD. C57BL6/J mice were fed a HFD for eight weeks and were administered 5 or 10 mpk of compound 11c daily oral gavage. The body weight gain upon HFD was attenuated and fat mass was reduced in mice treated with compound 11c (10 mpk) compared to vehicle-treated mice (Fig. 3a, b). Glucose tolerance was improved by compound 11c (5 or 10 mpk) treatment (Fig. 3c). Tissue weight of liver and inguinal white adipose tissue (iWAT) significantly decreased in compound 11c-treated mice (Fig. 3d). In the histological analysis using H&E staining, steatosis, lobular inflammation, and hepatocyte ballooning in liver tissue was markedly reduced in compound 11c-treated mice in a dose-dependent manner (Fig. 3e, f). Furthermore, compound 11c-treated mice showed decreased hepatic TG concentration (Fig. 3g), and the expression of lipogenesis-related genes were reduced in compound 11c-treated mice (Fig. 3h). These data indicated that compound 11c could protect against diet-induced MASLD.
Fig. 3. 5HT2A antagonist, compound 11c, attenuates HFD-induced obesity, MASLD, and metabolic syndrome.
a–h Twelve-week-old mice were treated with vehicle or compound 11c (5 and 10 mpk) daily oral gavage with a high-fat diet for eight weeks, n = 6 for vehicle, n = 7 for compound 11c 5mpk, and n = 6 for 10mpk. a body weight trend of vehicle- or compound 11c-treated mice, two-way ANOVA with post hoc Dunnett’s test. b Percent fat and lean mass (body composition) of vehicle- or compound 11c-treated mice, two-way ANOVA with post hoc Dunnett’s test. c Intraperitoneal glucose tolerance test (IPGTT) after 16 h fasting, two-way ANOVA with post hoc Dunnett’s test. d Tissue weight of vehicle-treated or compound 11c-treated mice, two-way ANOVA with post hoc Dunnett’s test. e Representative liver histology by H&E staining in vehicle- or compound 11c-treated mice. Scale bars, 100 μm. f Total NAFLD activity score (NAS) of vehicle- or compound 11c-treated mice. Scale bars, 100 μm, 2-sided Mann-Whitney’s U-test. g Serum cholesterol (CHO), Serum triglyceride (TG), and hepatic TG levels of vehicle- or compound 11c-treated mice, one-way ANOVA with post hoc Dunnett’s test. h Relative mRNA expression of lipogenesis-related genes in vehicle- or compound 11c-treated mice, two-way ANOVA with post hoc Tukey’s Honestly Significant Difference (HSD) test. Data are expressed as the means Data are expressed as the means ± SEM.
In vivo efficacy of Compound 11c for liver fibrosis and inflammation
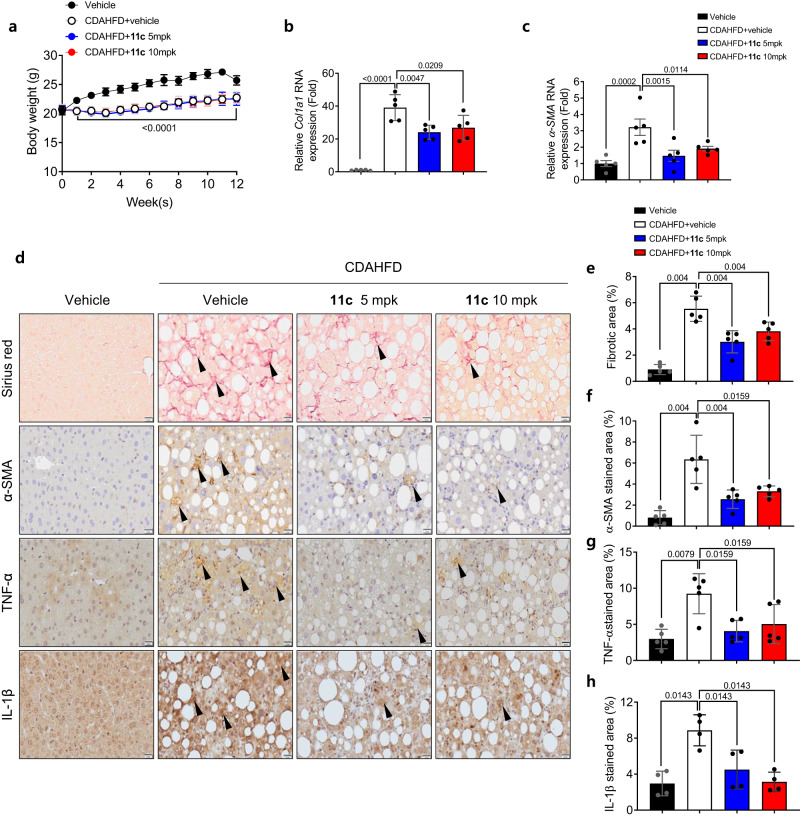
Our previous report revealed that genetic deletion of 5HT2A resulted in the downregulation of genes related to hepatic fibrosis and inflammation15. To determine the in vivo efficacy of compound 11c for liver fibrosis and inflammation, the mice were subjected to a choline-deficient, L-amino acid-defined high-fat diet (CDAHFD) for 12 weeks and administered compound 11c daily by oral gavage. During the study period, no abnormal clinical signs were observed, and no significant difference in food intake was noted amongst CDAHFD-fed groups (Supplementary Fig. 4). Body weights of mice were significantly decreased in CDAHFD-fed groups compared to the normal diet-fed group (vehicle) but the body weight changes among the CDAHFD groups were not significantly different (Fig. 4a). Collagen accumulation and fibrosis were assessed by examining the levels of Col1a1 and α-SMA expressions in liver tissue. The mRNA expressions of both ol1a1 and α-SMA in liver tissue were significantly increased in CDAHFD-fed vehicle group compared to control and were significantly decreased in CDAHFD-fed 11c and compared to CDAHFD-fed vehicle group (Figs. 4b, 3c). Similarly, collagen accumulation assessed in the liver tissue using Sirius red staining showed a substantial increase in the CDAHFD-fed vehicle group comparing normal-diet group and a significant decrease in CDAHFD-fed compound 11c and compared to CDAHFD-fed vehicle group (Fig. 4d, e). Immunohistochemical staining of liver tissue was conducted to assess the expression of α-SMA, a marker of fibrosis, TNF-α, and IL-1β, a maker of inflammation. It was shown that α-SMA, TNF-α, and IL-1β expressions were significantly increased in the CDAHFD-fed vehicle group compared to normal diet-fed group, and a significant decrease was observed in the group treated with compound 11c compared to the CDAHFD-fed vehicle group (Fig. 4f–h). Overall, the efficacy of compound 11c was evaluated in an in vivo mouse model of liver fibrosis and inflammation following a CDAHFD for 12 weeks. Compound 11c administration at 5 and 10 mpk dose for 12 weeks had significant reduction in the RNA expressions of Col1a1 and α-SMA were observed in the liver of the mice treated with compound 11c administration at both doses compared to the CDAHFD-fed vehicle control animals. These findings clearly indicate that compound 11c has a therapeutic benefit for the treatment of liver fibrosis and inflammation with liver disease. These beneficial effects of compound 11c on hepatic inflammation and fibrosis could be attributed to the reduction in hepatic lipid accumulation. However, considering that elevated ROS production and mitochondrial stress are important in the development of hepatic inflammation and fibrosis in CDAHFD mouse model, our data also suggested that anti-inflammation and fibrosis effects of compound 11c could be attributed to the inhibition of 5HT2A signaling in other target cells such as macrophage and hepatic stellate cells.
Fig. 4. In vivo efficacy of Compound 11c for CDAHFD-induced liver fibrosis and inflammation C57BL/6 J mice (6 weeks old) underwent 12 weeks of CDAHFD feeding and for the same period oral injections of compound 11c.
a Body weight of CDAHFD-fed control and compound 11c-treated mice, one-way ANOVA with post hoc Tukey’s Honestly Significant Difference (HSD) test. The data are presented as mean ± SD (n = 5 per group). b, c Relative liver Col1a1 and α-SMA RNA expressions in mice, measured using quantitative RT-PCR, one-way ANOVA with post hoc Tukey’s Honestly Significant Difference (HSD) test. The data are presented as mean ± SEM (n = 5 per group). d Representative sirius red and immunohistochemical staining images (α-SMA, TNF-α, and IL-1β) from CDAHFD-subjected vehicle and compound 11c-treated mice (scale bars, 20 μm, arrows: positive areas). e–h Percentage of the sections positive for fibrotic area (sirius red), α-SMA, TNF-α, and IL-1β, 2-sided Mann-Whitney’s U-test. The data are presented as mean ± SD (n = 4–5 per group).
Discussion
In this study, we developed compound 11c as a novel peripheral 5HT2A antagonist for MASH and steatosis. Currently there is no approved treatment for MASH, although diverse drug candidates are undergoing clinical trials. Our goal is to identify oral and non-BBB permeable 5HT2A antagonists for chronic disease including MASLD and MASH. We discovered new hits by drug library screening and employing rational design. Fortunately, Desloratadine, which is known as a peripheral agent, was identified as a hit with IC50 value of 232 nM. Molecular docking supported the optimization process to identify compound 11c, which showed good in vitro activity with an IC50 value of 14 nM, using the crystal structure of human 5HT2A. Compound 11c is a racemic mixture, therefore, both chiral isomers were synthesized and evaluated for their in vitro activities. Both isomers showed the same activities, so racemic 11c was used for further evaluation. Compound 11c exhibited a good chemical profile by having hepatocyte and plasma stability with no significant cytotoxicity and low CYP inhibition. Compound 11c didn’t show mutagenic potential (AMES test) and exhibited LD50 value of over 1000 mpk in the acute tox study. In PK profiles, it had over 60% oral bioavailability in rats and dogs. According to the tissue distribution study with [14C]-labeled compound 11c, it was determined to be a peripheral 5HT2A antagonist. Compound 11c demonstrated significant efficacy in reducing fatty liver and fibrosis in both DIO and CDAHFD mice models.
We also showed the possibility of the direct anti-inflammatory and anti-fibrotic effects of compound 11c using CDAHFD mouse model. Although we did not show any direct evidence of 5-HT’s role in inflammation and fibrosis, 5-HT is known to regulate the activation and proliferation of hepatic stellate cells (HSCs) and macrophages29–31. Thus, further studies are being conducted to elucidate the detailed mechanisms of how compound 11c can reduce hepatic inflammation and fibrosis.
In conclusion, we suggest that compound 11c is a promising drug candidate, which functions as a peripheral 5HT2A antagonist aiming for MASLD and liver fibrosis, which is in phase 1 clinical trial (NCT05517564)32.
Methods
Animal experiments
For in vivo efficacy in DIO models and CDAHFD models, the experimental protocol for this study was approved by the Institutional Animal Care and Use Committee (IACUC) at the Korea Advanced Institute of Science and Technology (No. KA2023-027-v2) or Kyungpook National University (No. KNU-20200134). All relevant ethical guidelines were followed. C57BL/6 J male mice (11 weeks old) were purchased from the Charles River Japan and were housed in a specific pathogen-free barrier facility under a regular light-dark cycle (12-hour light/12-hour dark) at 24°C with 40–60% humidity. Food (standard chow diet, Envigo, 2018S) and water were provided ad libitum. At 12 weeks, mice were fed a high-fat diet (HFD, D12492, Research Diet) with vehicle or compound 11c treatment daily by oral gavage. Mice were euthanized by the method of cervical dislocation. The measurement of lean and fat mass in live mice was performed by a body composition analyzer (LF50 BCA-analyzer, Bruker). After 6 h of fasting, mice were sacrificed, and tissue samples were obtained.
Pharmacokinetic study in rats and dogs
The experimental protocol for this study was approved by the IACUC of KPC Co., Ltd. (No. KPC IACUC-P203046; Beagle dogs) or QuBEST BIO (No.QBIACUC-A20131). All relevant ethical guidelines were followed. Beagle male dogs (11 ~ 20 months old) were purchased from ORIENT BIO Inc. and were housed in the barrier animal facility of KPC Co., Ltd. under a regular light-dark cycle (12-hour light/12-hour dark) at 21.1–23.4°C with 55.3–72.2% humidity. Food (standard irradiated pelleted commercial laboratory diet, Cargill Agri Purina, 300 g/dog.day) and water were provided ad libitum. All study dogs were returned to the laboratory colony after the study without any euthanasia. Sprague-Dawley male rats (7 weeks old) were purchased from SAMTAKOBIOKOREA, Inc. and were housed in the barrier animal facility of Nonclinical Research Center, QuBEST BIO under a regular light-dark cycle (12-hour light/12-hour dark) at 19.7–22.7°C with 54.5–65.6% humidity. Food (standard irradiated pelleted commercial laboratory diet, Purina Rodent Chow, 38057) and water were provided ad libitum. All study rats were euthanized by CO2 gas upon study completion.
Chemistry
Detailed synthesis protocols and characterization of compounds can be found in the Supplementary Methods section of the Supplementary Information.
Human 5HT2A inhibition assay
The derivatives were evaluated at a concentration of 1 μM for their antagonist activity on the 5HT2A receptor (Tables 1–3)33,34. Human 5HT2A stably transfected HEK293 cells (a type of cell derived from kidney embryos)20 were seeded in a 96-well plate (Costar; 3603) at a density of 9 × 104 in 100 μL of minimum essential medium (Welgene; LM007-86) containing 10% dialyzed fetal bovine serum (Gibco; 30067–334) and 1% penicillin/streptomycin (Gibco; 15140-122) and kept in a 37 °C incubator for 24 h. The cells were stained with 100 μL of cytosolic calcium dye using the FLIPR calcium 6 assay solution (Molecular devices, #R8190) supplemented with 2.5 mM probenecid (Sigma; P8761) and incubated for 1 h at 37 °C. After 1 h, the designated amount of the compound was treated in each well and incubated for another 1 h at 37 °C. Then, the cells were loaded in a Flexstation 3 microplate reader (Molecular Devices; FlexStaion) and 50 μL of 5 μM 5HT (serotonin hydrochloride, Sigma; H9523) was injected from a source plate (Nunc 96-well polystyrene conical bottom MicroWell Plate, Thermo; 249662) and the fluorescence intensity (Ex/Em-485nm/ 525 nm) was measured. (Supplementary Fig. 5) For determining the IC50 values of other 5HT subtypes, an Inhibition assay was performed by Eurofins Cerep (Study ID: FR095-0014987, FR095-0016438). Compound 11c was tested at several concentrations ranging from 3.0E-09 M to 1.0E-05 M, and the IC50 value was calculated from concentration-response curves. Experimental conditions for the 5HT subtypes can be found in the Supporting Information (Supplementary Table 1).
Hepatocyte stability test
Hepatocyte incubations for the test compound (10 μM final concentration, n = 2 per each time point) were carried out in mouse, rat, dog, monkey and human cryopreserved hepatocytes, resuspended at a density of 0.5 million cells/ml of modified Krebs-Henseleit buffer (KHB). The information regarding the hepatocytes used in the experiment is as follows: liverPool® 10-donor mixed gender human (BioIVT, Cat.No. X008001, lot EAV), male cynomolgus monkey (BioIVT, Cat.No. M00305, lot COJ), male Beagle dog (BioIVT, Cat.No. M00205, lot USY), male Sprague Dawley rat (BioIVT, Cat.No. M00005, lot TQE) and male CD1 mouse (BioIVT, Cat.No.005052, lot LFF). In addition, the compound was also incubated with blank KHB. The final concentration of solvent (DMSO) was 0.1% of the final volume. The incubations were performed for 3 hours at 37°C and pH 7.4. Reactions were terminated at 0 and 180 minutes by the addition of 3 volumes of stop solution (acetonitrile/methanol (2:1, v/v) containing internal standard–diclofenac at 100 ng/ml). Samples (compound spiked buffer and compound spiked hepatocytes, as well as blank samples, i.e. hepatocytes with the same DMSO%) were then centrifuged and aliquots were transferred to 96-deep micronic tubes and frozen at -80 °C until LC-MS/MS analysis. The metabolic activity of hepatocytes was verified by simultaneous incubation of the following assaycontrols: testosterone (substrate for Phase I enzymes from CYP3A enzyme family, high clearance in all species), umbelliferone (substrate for Phase II conjugation enzymes: UDP glucuronyl transferases (UGT) and sulfotransferases (SULT); high clearance in all speciess) and caffeine (stable compound). All controls were within acceptable range in all tested species. The Quantification of compound 11c was done on a Q-TOF X500R high resolution mass spectrometer (Sciex) equipped with a TurbolonSpray source and coupled to Shimadzu Nexera Series 30 HPLC pumps and autosampler.
Molecular docking
The compounds were designed with Chemdraw 3D Ultra and converted to PDB files for docking and were designated as ligands. Human 5HT2A crystal structure was obtained from Protein Data Bank (7WC4)35 was used as a designated receptor. AutoDock Vina36 was used to prepare the ligand and receptors for docking. Docking was performed using AutodockTools with grid box center coordinates -27.980, -10.325, 113.215 and grid dimensions 80 x 100 x 126 with an exhaustiveness of eight. The interactions of the side chains of 5HT2A within four Angstroms was visualized with PyMOL2.537.
In vivo efficacy for choline-deficient, L-amino acid-defined high-fat diet (CDAHFD)-induced liver fibrosis and inflammation
C57BL/6 J male mice were purchased from the Japan SLC. Inc (Hamamatsu, Japan). Mice (5 weeks old) were fed Teklad Certitied Irradiated Global 18% protein rodent diet purchased from Dooyeol Biotech Co. Ltd (Seoul, South Korea) during the acclimation period. The liver fibrosis/inflammation-induced groups were CDAHFD-fed (A06071302, Research diet). After the acclimation period, mice (6 weeks old) were divided into four groups (Vehicle, CDAHFD, CDAHFD treated with 5 and 10 mpk of compound 11c, n = 5/group) for each experimental regime. Compound 11c and vehicle (40 mM citrate buffer, pH 3.0) groups were orally administrated once daily for 12 weeks. All animal experiments complied with the relevant ethical regulations.
Collagen I and α-SMA RNA expression in liver tissue
The frozen liver tissue was homogenized and put into QiAzol (Qiagen, Hilden) to isolate RNA, and 4 μg of RNA was synthesized into cDNA using the RevertAid First Strand cDNA synthesis kit (Thermo Fischer, Waltham). The expressions of mouse collagen I (Forward: 5’-TGT GTT CCC TAC TCA GCC GTG-3’. Reverse: 5’-CAT CGG TCA TGC TCT CTC CAA-3’), mouse α-SMA (Forward:5’-TCC TGA CGC TGA AGT ATC CGA T-3’, Reverse: 5’-GGC CAC ACA AAG CTC GTT ATA G-3’) and mouse 36B4 (Forward: 5’-ACC TCC TTC TTC CAG GCT TT-3’, Reverse: 5’-CTC CAG TCT TTA TCA GCT GC-3’) as house-keeping gene were measured using ViiA 7 Real-time PCR system (Thermo Fischer scientific).
Sirius red and immunohistochemical staining
Mouse liver tissues were fixed in 4% formalin solution (Sigma–Aldrich, St. Louis, MO, USA) and embedded in paraffin. 4-μm-thick liver sections were deparaffinized in xylene, and rehydrated through descending grades of ethanol. To evaluate liver fibrosis, sections were stained with Sirius red solution and subjected to immunohistochemical staining38. Tissue sections, after deparaffinization, underwent a 16-hour incubation in a solution containing 0.1% Direct Red 80 (365548, Sigma-Aldrich) dissolved in Picric acid (P-201, Spectrum Chemical, CA, USA) for Sirius red staining. The sections were briefly immersed in a 0.01 N HCl solution (1129, Ducksan, Republic of Korea) for 2 min. and then rinsed with distilled water for destaining. Subsequently, the sections underwent dehydration through descending grades of ethanol. Immunohistochemical staining was performed using the Polyview Plus HRP-DAB (anti-Rabbit) Kit (ENZ-KIT159-0150, ENZO Life Science, NY, USA) following the manufacturers’ instructions. Deparaffinized sections were boiled in IHC-TekTM Epitope Retrieval solution (IW-1100, IHCWORLD, MD, USA) for 45 min., cooled for 1 h, incubated in a 3% H2O2 solution (69355-0350, JUNSEI, Japan) for 15 min., washed, and then incubated overnight with primary antibodies (α-SMA, ab5694, Abcam, 1:500 dilution; TNF-α, ab6671, Abcam, 1:100 dilution and IL-1β, ab9722, Abcam, 1:100 dilution). Subsequently, the sections were treated with Polyview Plus HRP for 2 h and developed with DAB chromogen/substrate. Stained kidney sections were confirmed by light microscopy (Olympus BX53 upright microscope, Tokyo, Japan). The degree of hepatic fibrosis and immunohistochemical staining was quantified by morphometric analysis of stained liver sections using NIH ImageJ software.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8 (MA, USA). All data are presented as mean ± standard error of the mean (SEM) of at least three independent experiments as indicated in the figure legends. Statistical analyses were performed using one-way analysis of variance (ANOVA) or two-way ANOVA to compare more than three groups, followed by post hoc Dunnett’s test or Tukey’s Honestly Significant Difference (HSD) test, and Mann–Whitney’s U-test used for two groups nonparametric comparison as appropriate. A value of P < 0.05 was considered to be statistically significant.
Histological analysis
Epididymal, inguinal, and liver were harvested, fixed in 10% (w/v) neutral buffered formalin solution (Sigma-Aldrich), and embedded in paraffin. Five-micron-thick tissue sections were deparaffinized, rehydrated, and stained with hematoxylin and eosin (H&E)10. The total NAFLD activity score (NAS) is a widely used measure of grading for steatosis, lobular inflammation, and ballooning. The total NAS ranges from 0-8 and is the sum of unweighted scores for steatosis (0: <5%, 1: 5 ~ 33%, 2: 33 ~ 66%, 3: >66% fat accumulation in the liver), lobular inflammation (0: no foci inflammation in x200 field, 1: <2 foci inflammation, 2: 2 ~ 4 foci inflammation, 3: >4 foci inflammation), and hepatocellular ballooning (0: none, 1: few ballooning cells, 2: prominent ballooning cells). MASH was diagnosed when NAS was >5, while non-MASH was diagnosed when NAS was <4. According to the MASH CRN scoring system, a certified pathologist determined the NAS39. The histological scoring was performed blindly, without knowledge of any other information.
Glucose tolerance tests
For the glucose tolerance test, overnight fasted mice were intraperitoneally injected with 2 g/kg D-glucose (Sigma-Aldrich). Glucose concentrations were measured using a Gluco DR. TOP glucometer (Allmedicus). The AUC (area under the curve) during the GTT was calculated using GraphPad Prism 8.0 software (La Jolla, CA, USA) by the trapezoidal method.
Blood profiling
Blood samples were obtained by cardiac puncture, and plasma was isolated. Blood profiling was performed by GC Pharma (Green Cross Corporation). Plasma TG and cholesterol were detected with an in vitro enzymatic colorimetric assay (CHOL2 and TRIGL; Roche, Germany) using a Cobas 8000 clinical analyzer with a c702 module (Roche).
Quantification of hepatic triglyceride
Liver tissues were homogenized in 5% NP-40 using FastPrep-24. To solubilize fat, the homogenates were heated to 95 °C for 5 min and cooled at 23 °C, and repeated. Triglyceride Reagent (Sigma-Aldrich) or DW was added and incubated at 37 °C for 30 min to hydrolyze TG into glycerol. For the colorimetric assay of hydrolyzed TG levels, samples were incubated with Free Glycerol Reagent (Sigma-Aldrich) at 37 °C for 5 min. Differences in absorbance at 540 nm between hydrolyzed or non-hydrolyzed TG were quantified using a glycerol standard (Sigma-Aldrich). TG contents were normalized by the protein concentrations of homogenates, which were measured with a BCA Protein Assay Kit (Thermo Fisher Scientific).
Quantitative RT-PCR analysis
Total RNA extractions from harvested tissues were performed using TRIzol according to the manufacturer’s protocol. After TURBO DNase (Invitrogen) treatment, 2 μg of total RNA was used to generate complementary DNA with a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Quantitative RT-PCR was performed with Fast SYBR Green Master Mix (Thermo Fisher Scientific) and a Viia 7 Real-time PCR System (Thermo Fisher Scientific) according to the manufacturer’s instructions. Gene expression was relatively quantified based on the delta-delta Ct (threshold cycle) method with the beta-actin gene as a reference gene. The sequence of primers can be found in the Supporting Information (Supplementary Table 2).
Blood−brain barrier (BBB) penetration study in mice
A single oral (gavage) dose of [14C]-compound 11c to male Sprague Dawley rats at 5 mpk and 100 μCi/kg resulted in radioactivity was broadly distributed and detected by quantitative whole-body autoradiography in most tissues analyzed. At approximately 0.25, 0.5, 1, 6, 24, 48, 96, and 168 h postdose, one Crl:CD(SD) rat/time point was anesthetized by isoflurane inhalation, and a blood sample of approximately 4 mL was collected via cardiac puncture into tubes containing K2EDTA. Following blood collection, each anesthetized animal was euthanized by CO2 inhalation and carcasses were submerged in a dry ice/hexane bath. Frozen carcasses were stored at approximately -20 °C until processed for QWBA. Duplicate aliquots of approximately 0.1 g whole blood were weighed into combustion boats for analysis by LSC. Duplicate aliquots of approximately 0.1 mL plasma were weighed into scintillation vials and mixed with an appropriate volume of scintillation cocktail for analysis by LSC. The frozen carcasses were individually set in a mold, submerged in 5% (w/v) low viscosity carboxymethylcellulose (CMC), and embedded by placing the stage (mold) in a dry ice/hexane bath. Once the blocked carcasses (sample blocks) were frozen, they were removed from the dry ice/hexane bath and placed in storage (approximately -20 °C) for at least 12 h prior to sectioning. Sagittal, 30-µm thick sections of the CMC-embedded carcasses, including QC standards, and of the CMC-embedded calibration standards were sectioned using a Vibratome 9800 Cryomicrotome (Vibratome; St. Louis, MO) with the temperature maintained at approximately -20 °C. The sections were dehydrated for at least 48 h in a frost-free freezer, and then allowed to acclimate to room temperature in a desiccant storage box. The sections were placed in direct contact with the phosphor imaging plates (IP), BAS-SR 2025 (FujiFilm; Tokyo, Japan). The IPs were exposed for approximately 4 days while stored at room temperature in a lead-shielded box to minimize background radiation. Digitized phosphor images (autoradioluminograms) were generated in a dark room with subdued lighting using a GE Healthcare Typhoon 7000 Phosphor Imager (Raytest GmbH; Berlin, Germany) and analyzed using AIDA software (Raytest GmbH).
Pharmacokinetic study in rats and dogs
Compound 11c was administered to Sprague-Dawley rats and Beagle dogs at 5 mpk via oral gavage (Vehicle: 50 mM Citrate buffer (pH 3.0)) and 5 mpk via intravenous route (Vehicle: NMP + PEG400 + 10% Solutol HS in saline (5:10:50:35, v/v/v/v)). Blood samples were collected at the scheduled times up to 24 h post-dose (IV: 0.083, 0.25, 0.5, 1, 2, 4, 8, 12 and 24 h post-dose, PO: 0.25, 0.5, 1, 2, 4, 8, 12 and 24 h post-dose). Determination of compound 11c in plasma was performed by UHPLC-MS/MS. Pharmacokinetic parameters were calculated by noncompartmental analysis using Phoenix® WinNonlin® (Ver. 8.3‘, Certara).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
This work was supported by grants from the Ministry of Science, ICT & Future Planning (MSIP)/National Research Foundation of Korea (NRF) (2016M3A9B6902868 (J.H.A), 2021R1A2C2008062 (J.H.A), 2020M3A9E4038695 (H.K)). This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government (MOTIE) (20202020800330, Development and demonstration of energy efficient reaction separation purification process for fine chemical industry) (J.H.A). This research was supported by Korea Drug Development Fund funded by Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (RS-2023-00218035, Republic of Korea) (J.H.A). This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR22C1832) (I-K.L, J.-H.J).
Author contributions
I-K.L., H.K. and J.H.A. contributed to the overall design and supervised the study together with R.L, D.K, P.C.G. and S.S. H.S.P., S.H.P., M.K., J.Y. contributed to the design and synthesis of chemicals. B.J., W-I.C., C.J.O. and C.W.L. contributed to the in vivo studies. S.S.K. and M.A.B. contributed to the DMPK studies. J-H.J., C.J.O., E.Y.L. and H.J.L. contributed to the in vitro studies. H.K. and J.H.A. were responsible for drafting the manuscript. All authors have read and revised the article and approved the submitted version.
Peer review
Peer review information
Nature Communications thanks jihua fu, Rong Qi, Nguan Soon Tan, Andrea Perra and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All the data supporting the findings of this study are available within the article and its supplementary information files and from the corresponding author. A reporting summary for this article is available as a Supplementary Information file. Source data are provided with this paper.
Competing interests
A.J.H., H.S.P., S.H.P., M.H.K. and W.M.L. have filed a patent application encompassing aspects of this work (US 2023/0002349 A1 and WO 2021/086133 A1). The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Haushabhau S. Pagire, Suvarna H. Pagire, Byung-kwan Jeong, Won-Il Choi.
Contributor Information
Hail Kim, Email: hailkim@kaist.edu.
Jin Hee Ahn, Email: jhahn@gist.ac.kr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-44874-3.
References
- 1.Loomba R, Sanyal A. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Rinella ME, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;29:101133. doi: 10.1016/j.aohep.2023.101133. [DOI] [PubMed] [Google Scholar]
- 4.Rinella ME, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797–1835. doi: 10.1097/HEP.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–2564. doi: 10.1016/j.cell.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Wong RJ, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 7.Mittal S, et al. Hepatocellular carcinoma in the absence of cirrhosis in united states veterans is associated with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2016;14:124–131. doi: 10.1016/j.cgh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ajmera V, et al. A prospective study on the prevalence of NAFLD, advanced fibrosis, cirrhosis and hepatocellular carcinoma in people with type 2 diabetes. J. Hepatol. 2023;78:471–478. doi: 10.1016/j.jhep.2022.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crane JD, et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat. Med. 2014;21:166–172. doi: 10.1038/nm.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh CM, et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat. Commun. 2015;6:6794. doi: 10.1038/ncomms7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat. Med. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadav VK, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cel. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumara G, Sumara O, Kim JK, Karsenty G. Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab. 2012;16:588–600. doi: 10.1016/j.cmet.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu. Rev. Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi W, et al. Serotonin signals through a gut-liver axis to regulate hepatic steatosis. Nat. Commun. 2018;9:4824. doi: 10.1038/s41467-018-07287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCullough J. The clinical features, diagnosis, and natural history of nonalcoholic fatty liver disease. Clin. Liver Dis. 2004;8:521–533. doi: 10.1016/j.cld.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Cotter TG, Charlton M. Nonalcoholic steatohepatitis after liver transplantation. Liver Transplant. 2020;26:141–159. doi: 10.1002/lt.25657. [DOI] [PubMed] [Google Scholar]
- 18.Oseini AM, Sanyal AJ. Therapies in non-alcoholic steatohepatitis (NASH) Liver Int. 2017;37:97–103. doi: 10.1111/liv.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison, S. et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J Hepatol.73, 26–39 (2020). [DOI] [PubMed]
- 20.Kim M, et al. design, synthesis, and biological evaluation of new peripheral 5ht2a antagonists for nonalcoholic fatty liver disease. J. Medicinal Chem. 2020;63:4171–4182. doi: 10.1021/acs.jmedchem.0c00002. [DOI] [PubMed] [Google Scholar]
- 21.Kim M, et al. Synthesis and biological evaluation of tyrosine derivatives as peripheral 5HT2A receptor antagonists for nonalcoholic fatty liver disease. Eur. J. Medicinal Chemisrty. 2022;239:114517. doi: 10.1016/j.ejmech.2022.114517. [DOI] [PubMed] [Google Scholar]
- 22.Pagire, H. S. et al. Tricyclic compound and pharmaceutical use thereof. Patent WO 2021/086133A1, (2021) and US 2023/0002349A1, (2023).
- 23.Weichu X, George EW, Milka Y, Ivan BY. Synthesis and absolute configuration assignment of 9-hydroxyrisperidone enantiomers. Lett. Org. Chem. 2014;11:470–473. doi: 10.2174/1570178611666140219004433. [DOI] [Google Scholar]
- 24.Lucia S, et al. A new serotonin 2A receptor antagonist with potential benefits in Non-Alcoholic Fatty Liver Disease. Life Sci. 2023;314:121315. doi: 10.1016/j.lfs.2022.121315. [DOI] [PubMed] [Google Scholar]
- 25.Leucht S, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 26.Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5HT2A and 5HT2C receptors. Mol. Brain Res. 1994;23:163–178. doi: 10.1016/0169-328X(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 27.Varnäs K, Thomas DR, Tupala E, Tiihonen J, Hall H. Distribution of 5HT7 receptors in the human brain: a preliminary autoradiographic study using [3H]SB-269970. Neurosci. Lett. 2004;367:313–316. doi: 10.1016/j.neulet.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 28.Chung JY, Jeong JH, Song JH. Resveratrol modulates the gut-brain axis: focus on glucogon-like peptide-1, 5-ht, and gut microbiota. Front. Aging Neurosci. 2020;12:588044. doi: 10.3389/fnagi.2020.588044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mederacke I, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebrahimkhani MR, et al. Stimulating healthy tissue regeneration by targeting the 5-HT2B receptor in chronic liver disease. Nat. Med. 2011;17:1668–1673. doi: 10.1038/nm.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christine Tartaro C, Ruddell R. Trouble in mayberry: a national analysis of suicides and attempts in small jails. Am. J. Crim. Justice. 2006;31:81–101. doi: 10.1007/BF02885686. [DOI] [Google Scholar]
- 32.ClinicalTrials.gov. First-in-Human Study of GM-60106 in Healthy Adults and Otherwise Healthy Adults With an Increased Body Mass Index and Markers of Non-Alcoholic Fatty Liver Disease. https://www.clinicaltrials.gov/study/NCT05517564.
- 33.Seitz PK, Bremer NM, McGinnis AG, Cunningham KA, Watson CS. Quantitative changes in intracellular calcium and extracellular-regulated kinase activation measured in parallel in cho cells stably expressing serotonin (5-HT) 5-HT2A or 5-HT2C receptors. BMC Neurosci. 2012;13:25. doi: 10.1186/1471-2202-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding L, et al. Discovery and structure-based optimization of 6- bromotryptamine derivatives as potential 5-ht2a receptor antagonists. Molecules. 2015;20:17675–17683. doi: 10.3390/molecules200917675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao D, et al. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science. 2022;375:403–411. doi: 10.1126/science.abl8615. [DOI] [PubMed] [Google Scholar]
- 36.Trott O, Olson AJ. AutoDock vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schrödinger, L.; DeLano, W. PyMOL, (2020). http://www.pymol.org/pymol.
- 38.Oh CJ, et al. Sulforaphane attenuates hepatic fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-β/Smad signaling. Free Radic. Biol. Med. 2012;52:671–682. doi: 10.1016/j.freeradbiomed.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Kleiner DE, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data supporting the findings of this study are available within the article and its supplementary information files and from the corresponding author. A reporting summary for this article is available as a Supplementary Information file. Source data are provided with this paper.