Abstract
Background and Aims
CAM photosynthesis is hypothesized to have evolved in atmospheres of low CO2 concentration in recent geological time because of its ability to concentrate CO2 around Rubisco and boost water use efficiency relative to C3 photosynthesis. We assess this hypothesis by compiling estimates of when CAM clades arose using phylogenetic chronograms for 73 CAM clades. We further consider evidence of how atmospheric CO2 affects CAM relative to C3 photosynthesis.
Results
Where CAM origins can be inferred, strong CAM is estimated to have appeared in the past 30 million years in 46 of 48 examined clades, after atmospheric CO2 had declined from high (near 800 ppm) to lower (<450 ppm) values. In turn, 21 of 25 clades containing CAM species (but where CAM origins are less certain) also arose in the past 30 million years. In these clades, CAM is probably younger than the clade origin. We found evidence for repeated weak CAM evolution during the higher CO2 conditions before 30 million years ago, and possible strong CAM origins in the Crassulaceae during the Cretaceous period prior to atmospheric CO2 decline. Most CAM-specific clades arose in the past 15 million years, in a similar pattern observed for origins of C4 clades.
Conclusions
The evidence indicates strong CAM repeatedly evolved in reduced CO2 conditions of the past 30 million years. Weaker CAM can pre-date low CO2 and, in the Crassulaceae, strong CAM may also have arisen in water-limited microsites under relatively high CO2. Experimental evidence from extant CAM species demonstrates that elevated CO2 reduces the importance of nocturnal CO2 fixation by increasing the contribution of C3 photosynthesis to daily carbon gain. Thus, the advantage of strong CAM would be reduced in high CO2, such that its evolution appears less likely and restricted to more extreme environments than possible in low CO2.
Keywords: CAM photosynthesis, drought adaptation, hydraulic luxury consumption, low atmospheric CO2, Miocene, Oligocene, photosynthetic evolution, succulence
INTRODUCTION
Four photosynthetic carbon-concentrating mechanisms (CCMs) have been described in the Earth’s flora – C4 photosynthesis, dissolved inorganic carbon (DIC) concentration into pyrenoids or carboxysomes of algae and hornworts, C2 photosynthesis, and crassulacean acid metabolism (CAM photosynthesis). Each of these physiologies serves as an auxiliary to the ubiquitous C3 photosynthetic metabolism by transporting and concentrating CO2 around Rubisco, thereby suppressing photorespiration and enhancing carboxylation rate, which enables the enzyme to operate with greater efficiency (Sage and Stata, 2015). By functioning as a CCM, these mechanisms offset the reduction in atmospheric CO2 concentrations from >800 to < 500 ppm estimated to have occurred during the Oligocene epoch 23–34 million years ago (Ma) (Foster et al., 2017; Mills et al., 2019; Rae et al., 2021). Because of the advantages of CCMs at reduced CO2, it has been hypothesized that each originated when CO2 supply was restricted, either by low atmospheric values or high diffusion limitations around photosynthetic tissue (Ehleringer et al., 1991, 1997; Raven et al., 2008; Arakaki et al., 2011; Sage et al., 2018). In the case of C4 photosynthesis, time-calibrated phylogenies and fossilized proxy records indicate most if not all C4 clades appeared after a CO2 decline in Earth’s atmosphere in the Oligocene (Christin et al., 2008, 2011; Vicentini et al., 2008; Sage, 2016). Models of C4 evolution also describe how low CO2 would cause high rates of photorespiration that in turn would promote intermediate steps in the evolution of C4 photosynthesis (Sage et al., 2012; Heckmann et al., 2013; Williams et al., 2013; Mallmann et al., 2014). Inorganic carbon concentration by algae is similarly hypothesized to have arisen during low CO2 episodes or in situations where photorespiration can be elevated, such as within dense algal mats in warm ponds or the interiors of stromatolites (Badger and Price, 2003; Griffiths et al., 2017; Raven et al., 2017). In the case of CAM photosynthesis, a few early phylogenetic studies addressing CAM origins and diversification support possible origins during low CO2 conditions of the post-Oligocene (Crayn et al., 2004 for bromeliads; Arakaki et al., 2011 for agaves and core cacti; see also Edwards and Ogburn, 2012; Heyduk, 2022). However, CAM evolution in non-flowering plant lineages, notably the lycophyte Isoëtes, the fern genus Pyrrosia and the gymnosperm Welwitschia mirabilis, has been hypothesized to be ancient (Cretaceous or earlier) given the antiquity of these lineages (Griffiths, 1989; Raven and Spicer, 1996; Winter and Smith, 1996; Keeley, 1998).
If low CO2 is an environmental enabler of CAM evolution, how strong is the evidence for its role, and how might it have promoted the evolutionary rise of CAM? This question can be addressed in greater depth today than a decade ago, due to advances in phylogenetic understanding in CAM-evolving lineages (e.g. Horn et al., 2014 for Euphorbia; Crayn et al., 2015 for bromeliads; and for orchids, Bone et al., 2015; Li et al., 2019; Gamisch et al., 2021). Large-scale carbon isotope surveys of CAM lineages have been forthcoming in the past two decades, often coupled with species-rich phylogenies that allow strong CAM to be mapped onto the phylogenies in greater detail (Silvera et al., 2005, 2010a, b; Bone et al., 2015; Li et al., 2019; Gamisch et al., 2021). Increasing numbers of time-calibrated phylogenies enable better estimates of when CAM may have originated within specific clades; these phylogenies often examine greater numbers of species using genomic data that can allow for greater precision in the dating of stem and crown nodes (Li et al., 2019; Gamisch et al., 2021; Wang et al., 2023). [Stem node refers to the phylogenetic node where a clade diverges from its sister clade, and crown node refers to the node that represents the most recent common ancestor of all extant species in the clade.]
In this review, we integrate the CAM phylogenetic, physiological and isotopic literature to develop a more comprehensive picture of the timing of CAM origins. In some cases, the resolution achieved in phylogenetic reconstruction and molecular dating allows for fairly precise estimates of CAM origins, at least in the case of species termed strong CAM, which are delineated by carbon isotope ratios (δ13C) less negative than −20 ‰ and in which the majority (>50 %) of daily C enters the plant at night via phosphoenolpyruvate (PEP) carboxylation (Winter and Holtum, 2002; Edwards, 2019). In numerous clades, where CAM is known to occur but its presence has not been systematically surveyed using δ13C or physiological assessments, we use node divergence dates to bracket the origin of a CAM clade, and by doing so establish a maximum age for CAM in that clade. We also address evidence for the origins of CAM in plants that typically acquire a majority of their carbon via C3 photosynthesis, and therefore exhibit δ13C values more negative than −20 ‰ that overlap with C3 plants. These weaker CAM plants have been termed ‘C3+CAM’ (Edwards, 2019). Here, we use this term to encompass all forms of weak, low-level CAM activity (whether constitutive or facultative), including the CAM idling and CAM cycling functional types (Winter, 2019). Weak CAM functional types presumably pre-date strong CAM functional types during CAM evolution, possibly as far back as the higher CO2 epochs before the Oligocene (Griffiths, 1989; Arakaki et al., 2011; Edwards, 2019). We next consider the radiation of CAM species after CAM acquisition. The CAM flora is estimated to comprise some 17 000–20 000 species, or nearly 7 % of all vascular plants (Gilman et al., 2023). This diversity evolved after CAM origins, and often appears to have been rapid as clades exploited the novel CAM physiology and changing environments.
We next address how low CO2 may have enabled CAM evolution, through consideration of low and high CO2 effects on C3 and CAM photosynthesis in extant species. We hypothesize that low CO2 may have enabled C3 ancestors to evolve traits such as leaf succulence that predisposed subsequent CAM evolution. We acknowledge that numerous other environmental factors such as heat, drought, elevated salinity and high evapotranspiration probably also played important roles in CAM evolution, possibly in tandem with low CO2. However, because our main objective is to evaluate whether reduced CO2 could have been a contributing factor for CAM evolution, we discuss these other potential drivers in less detail. A comprehensive treatment that fully addresses the multiple factors contributing to CAM origins is clearly needed, but this will probably require a robust model of how CAM evolved, clearer delineation of the early steps in CAM evolution and a more comprehensive phenotyping of CAM trait acquisition across multiple phylogenies (Edwards, 2023). We close with some thoughts on how the CAM flora may fare in a world of elevated atmospheric CO2, when the relative importance of these hypothetical drivers for CAM evolution and diversification may be strongly modified.
ATMOSPHERIC CO2 AND THE ORIGIN OF CAM – THE EVIDENCE
A role for reduced atmospheric CO2 in promoting CCM evolution was first proposed by Ehleringer et al. (1991) for C4 origins and hypothesized for CAM origins by Raven and Spicer (1996), Keeley and Rundel (2003), Crayn et al. (2004) and Arakaki et al. (2011). Atmospheric CO2 is modelled to have declined from elevated (>800 ppm) to near current values (300–400 ppm) during the Oligocene (34–23 Ma; Fig. 1A; Tipple and Pagani, 2007). Consistently, nearly all C4 origins are predicted by phylogenetic dating to have followed the late Oligocene decline in atmospheric CO2, and a surge in C4 evolution is evident in the late Miocene, 5–10 Ma (Fig. 1A; Christin et al., 2008, 2011; Vicentini et al., 2008; Sage et al., 2018). The Oligocene and later Miocene are also recognized as times when climates deteriorated, with many lower latitude regions drying, causing forests to retreat and a commensurate expansion in savanna grasslands and semi-arid, open landscapes (Tipple and Pagani, 2007; Osborne and Freckleton, 2009; Edwards et al., 2010). In response to this low- to mid-latitude aridification during the Oligocene and then later in the Miocene, xerophytic traits appeared in a wide range of plant taxa, and from these clades, many of the existing C4 and CAM lineages evolved, indicating that adaptations to drought in ancestral C3 populations also enabled CCM evolution and diversification (Sage, 2001, 2002; Arakaki et al., 2011; Christin et al., 2013; Griffiths et al., 2013; Sage et al., 2018). Because CCM taxa largely appear in regions with warm and dry climates, or in locally saline conditions, it is generally recognized that discussions of low CO2 influences on CAM and C4 evolution should be considered in the context of environmental stressors such as heat, drought, salinity, fire disturbance, or soil-less environments such as rock outcrops and epiphytic niches (Raven and Spicer, 1996; Sage, 2001; Crayn et al., 2004; Tipple and Pagani, 2007; Edwards and Smith, 2010; Edwards et al., 2010).
Fig. 1.
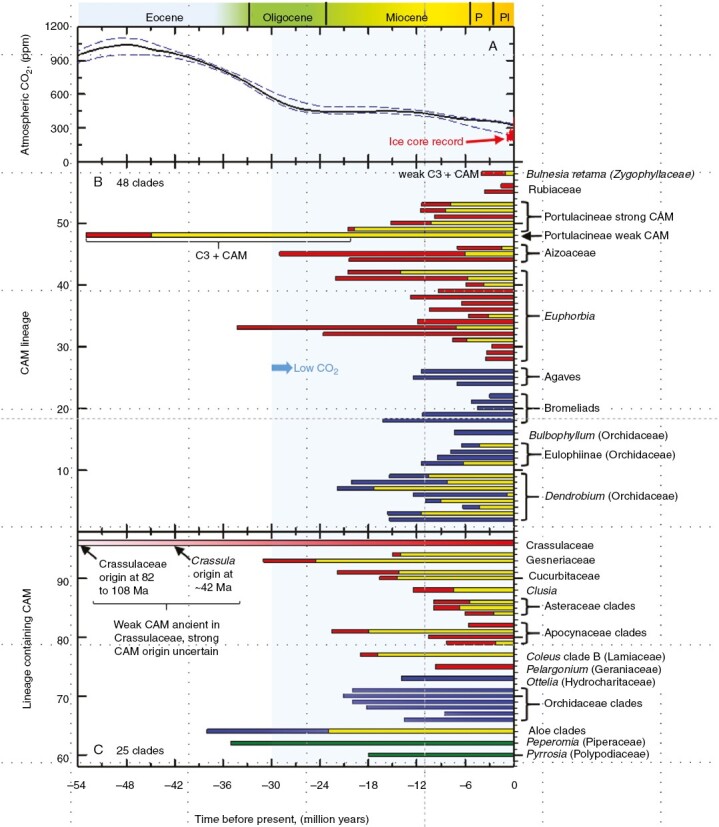
(A) Proxy-based estimates of atmospheric CO2 for the past 54 million years, from fig. 4b in Foster et al. (2017). Solid lines show the 50 % quantile estimates, and dashed lines show the 2.5 and 97.5 % quantile estimates. (B) Age estimates for CAM acquisition in lineages containing CAM species. A full bar length represents stem divergence dates from the source chronograms or stem dates stated within the referenced source. Blue-toned bars indicate stem ages for monocot lineages, and red-toned bars indicate stem dates for eudicot lineages. Where crown dates are available, they are also shown in all cases as yellow bars within the longer stem-date bars. (C) Age estimates for clades that contain C3 and CAM taxa, where the time of CAM acquisition cannot be inferred. The dates thus estimate the earliest possible origin of CAM in the clade, not necessarily when CAM did arise. In most clades, CAM origins would occur later than the bars indicate. Stem node dates of monocots are indicated with blue-toned bars, and for eudicots, as red-toned bars. The green-toned bars indicate the stem node dates for Pyrrosia ferns and Peperomia, a genus in the early angiosperm family Piperaceae. See Supplementary Data Appendix S1 for methods and literature sources. Abbreviations: P, Pliocene; PL, Pleistocene. Legend scales and tick marks in B and C correspond to specific clades as follows. In B, tick marks 2–9 correspond to Dendrobium CAM clades 1–8 (Li et al., 2019), while ticks 11–14 indicate Eulophiinae CAM clades 1–4 from Bone et al. (2015). The remaining clades in B are as follows (tick numbers in parentheses): Bulbophyllum (16); the bromeliad clades Hechtioideae (18), Dyckia (19), Puya/core bromeliads (20), core Tillandsia (21) and Tillandsia utriculata (22); Agave clades Hesperaloe (24), Yucca crown (25) and Agave (26); Euphorbia clades E. mauritanica (28), E. schimperi (29), E. laterifolia (30), section Anthacanthae stem 8 (31), E. platyclada (32) and section Articulofruticosae (33); E. ceroderma (34), subsection Tirucalli (35); E. weberbaueri (36); E. lomelii (37); E. sipolisii (38); E. alluaudii (39), section Goniostema (40), section Monadenium (41) and section Euphorbia (42); Aizoaceae clades Tetragonia (44), Mesembryanthemoideae (45) and core Ruschioideae (46); Portulacineae (48); core Cactoideae (49), Tephrocactus–Opuntia (50), Grusonia (51), Didiereaceae (52) and Anacampseros (53); Rubiaceae clades Myrmecodia (55) and Squamellaria (56); and Bulnesia retama in Zygophyllaceae (58). In C, the order of lineages are (tick position in parentheses): Pyrrosia (60); Peperomia (62); aloids (64); Orchidaceae clades Maxillariinae (66), Stanhopeinae (67), Oncidiinae (68), Laeliinae (69), Vandeae (70) and Pleurothallidinae (71); Ottelia (73); Pelargonium (75); Coleus clade B in the Lamiaceae (77); Apocynaceae clades Ceropegia (79), Cynanchum (80), Hoya–Dischidia (81) and Pachypodium (82); Asteraceae clades Caputia (84), Crassothonna (85) and the Gynurids (86); and Clusia (88). The Cucurbitaceae clades are Seyrigia (90) and Xerosicyos (91); and Gesneriaceae are clades Ramondinae (93) and Codonanthe–Codonanthiopsis–Nematanthus (94). The family Crassulaceae (96) is shown to represent probable ancient origins of C3 + CAM that extend to near the origin of the family, estimated to occur between 82 Ma (crown node age) and 108 Ma (stem node).
The timing of CAM origins
Does a similar timing exist for CAM origins as observed for C4 origins? To address this, we compiled age estimates for 48 strong CAM clades delineated by δ13C and one weaker C3 + CAM clade identified using gas exchange and titratable acidity (Fig. 1B). We also show divergence time for clades known to contain CAM species, but in which specific CAM nodes are difficult to discern given current levels of phylogenetic resolution (Fig. 1C). In the latter case, the CAM origin(s) within a clade are not known precisely, but would have corresponded to or post-dated the clade origin. Sources and assumptions used in preparing Fig. 1 are presented in online Supplementary Data Appendix S1.
In the data presented in Fig. 1B, strong CAM is emphasized because it can be readily delineated with δ13C values less negative than −20 ‰ obtained from herbarium specimens and living plants (Winter and Holtum, 2002; Winter et al., 2015; Winter, 2019; Messerschmid et al., 2021). Strong CAM plants also exhibit high degrees of succulence in photosynthetic tissues, reflecting the contribution of succulence to CAM function (Kluge and Ting, 1978; Nelson and Sage, 2008; Edwards, 2019; Luján et al., 2022; Leverett et al., 2023); however, succulence also occurs in C3, C4 and weak CAM plants, so by itself is an uncertain indicator of strong CAM (Griffiths and Males, 2017; Borland et al., 2018; Leverett et al., 2023). We thus had to treat claims of CAM based on succulence alone with caution. The origins of C3 + CAM physiology are more difficult to delineate because of the overlap between more negative CAM values and typical C3 values of δ13C, so they require physiological assessments of CAM activity, notably nocturnal acid accumulation, diel patterns of CO2 fixation and/or malate accumulation (Winter, 2019; Winter and Smith, 2022). Because physiological assessments require living plant material, and CAM activity in facultative species may only be revealed when plants are subject to the appropriate degree of water deficit, surveys for C3 + CAM function are often incomplete for a given clade; in such cases, mapping C3 + CAM onto a phylogenetic tree may not clearly identify where CAM originates within the phylogeny. Hence, the inferred age of the entire clade containing C3 + CAM species is often the only possibility for estimating the earliest possible CAM origins, as presented for numerous clades in Fig. 1C.
Altogether, origin estimates for 73 CAM clades or lineages containing CAM are presented in Fig. 1, whilst recognizing that the true number of independent origins could be greater or less depending on alternative interpretations of certain phylogenies (Gilman et al., 2023). In Fig. 1B, 46 lineages of strong CAM are estimated to have originated during the past 30 Ma when atmospheric CO2 concentration had declined to current values of 424 ppm or less (June 2023 CO2 values, www.co2earth). Over 90 % of the CAM lineages in Fig. 1B are estimated to be younger than 25 Ma, which corresponds to a period after Oligocene CO2 concentration is modelled to have declined to below 400 ppm (Fig. 1A). Estimates for two CAM clades – the Euphorbia clade Articulofruticosae and subfamily Mesembryanthemoideae of Aizoaceae – extend back to the Oligocene when atmospheric CO2 was in decline. One large clade – the Portulacineae – appears to have a relatively ancient origin of weak C3 + CAM extending to near 50 Ma, when atmospheric CO2 concentrations were high, being two to three times those of Miocene to present-day values. The Portulacineae is a large suborder of eight mostly succulent families in the Caryophyllales containing multiple clades of CAM species, including the Cactaceae, Didiereaceae and the C4 + CAM species in the Portulacaceae (Nyffeler and Eggli, 2010; Wang et al., 2019; Gilman et al., 2023). The appearance of strong CAM in some individual Portulacineae clades such as Anacampseros, the Cactaceae and the Didiereaceae corresponds to the Miocene, when atmospheric CO2 was reduced (Fig. 1B). Most of the speciose CAM clades, notably Euphorbia and Aizoaceae of the Palaeotropics, bromeliads and agaves of the Neotropics, and both Palaeo- and Neotropical orchids form CAM clades in post-Oligocene time. Numerous small clades of CAM species, such as two clades in Rubiaceae subtribe Hydnophytinae, are relatively young, evolving CAM in the Pliocene or later (Fig. 1B). A newly discovered C3 + CAM xerophyte in the Zygophyllaceae with photosynthetic stems, Bulnesia retama (Mok et al., 2023), is estimated to have diverged from non-CAM sisters some 1–4 Ma (Böhnert et al., 2020).
We were also able to use age estimates for 25 clades containing CAM that have sufficient precision to allow us to conclude CAM evolved after cladogenesis (Fig. 1C). The genus Peperomia (Piperaceae) originated in the high CO2 conditions of the Eocene at 40–50 Ma (Naumann et al., 2013; Massoni et al., 2015), but phylogenetic, isotopic and physiological details are currently too sparse to indicate when the CAM clades within this large genus (>1400 spp.) appeared. As this information is acquired, CAM in Peperomia might turn out to occur in multiple, disparate clades of relatively young age that date to epochs of reduced CO2, or it could be more widespread, indicating fewer, more ancient origins extending to the Eocene. Six large orchid clades containing CAM species are estimated to have Miocene origins, allowing us to conclude the CAM origins are Miocene or younger in these lineages (Fig. 1C). As an example of how much younger a specific CAM origin may be relative to a clade age, we consider the case of the Dendrobiinae, a tropical clade of orchids estimated to have a stem age of 32 Ma (Gustaffson et al., 2010; Givnish et al., 2015). A detailed study of CAM occurrence within Dendrobium indicates that CAM clades within the Dendrobiinae subtribe emerged during the late to early Miocene, a period characterized by reduced atmospheric CO2 levels (Li et al., 2019). As a result, these CAM clades are hypothesized to be considerably younger than the estimated node age for this subtribe (Fig. 1B; Li et al., 2019). Similarly, the orchid clade Pleurothallidinae stem age is 14.2 Ma (Givnish et al., 2015), yet three CAM clades diverged within this subtribe in the undated phylogeny of Silvera et al. (2009), indicating younger ages for CAM. In the Vandeae orchids, CAM is present in Campylocentrum (Silvera et al., 2009), whose crown node is subtended by a long branch to the stem node, indicating a much younger age than the Vandeae age shown in Fig. 1C (Givnish et al., 2015). We envisage this pattern of younger ages for CAM nodes than the clade age will be commonly observed in other clades once CAM phenotyping is complete (i.e. testing for CAM activity in living material) and the precise origins of CAM can be mapped onto the phylogenies. For now, we emphasize that the estimates in Fig. 1 are based on surveys involving a minority of species in what are often species-rich clades. Changes to these estimates are likely as taxonomic and physiological sampling improve and allow for more precise dating and species placement with the respective phylogenies.
The large monocot clade of CAM species in Asphodelaceae subfamily Alooideae includes the notable CAM genus Aloe (>500 spp.), plus 11 other mainly succulent CAM genera, including a number recently segregated from Aloe (Grace et al., 2015). Members of the other subfamily Asphodeloideae generally lack pronounced succulence, but weak CAM activity has been reported in the genus Bulbine, which has been resolved in a sister-group relationship to the alooids (Grace et al., 2015; Gilman et al., 2023). The crown node date for the separation of the Alooideae and Asphodeloideae is near 23 Ma, when CO2 was reduced (Fig. 1C). However, the stem age for the family is considerably older, near 38 Ma, when CO2 was elevated. The genus Aloe and related alooids represent a large clade of CAM species that has yet to be fully characterized, but focused work on the early diverging alooids and their relationship to the asphodeloids could clarify the timing and nature of the CAM origin in this major CAM clade.
Figure 1C also shows age estimates for three clades that contain weaker C3 + CAM species. Ottelia from the Hydrocharitaceae contains aquatic CAM species that are probably younger than its 13.9 Ma node age (Li et al., 2020), while Pelargonium in the Geraniaceae is estimated to have diverged 9.7 Ma (van der Kerke, 2019). The genus Clusia (Clusiaceae) exhibits a wide range of CAM phenotypes (Lüttge, 2007a; Luján et al., 2023), from weaker C3 + CAM to strong CAM species, and is estimated to have stem and crown dates of 12.5 and 7.5 Ma, respectively, so the multiple origins of strong CAM in this well-studied clade correspond to the late Miocene or later (Luján et al., 2022). Strong CAM probably originated in Clusia three to five times, all probably in the past 5 million years from C3 + CAM ancestors that branch near the base of the Clusia phylogeny (Ruhfel et al., 2016; Luján et al., 2022). Because of the extensive physiological and ecological research already on Clusia and its high number of diverse CAM character states (Lüttge, 2007a; Borland et al., 2018; Leverett et al., 2023; Luján et al., 2023), the genus could become an excellent model for CAM evolution with further phylogenetic resolution.
Most clades containing CAM species have received some level of phylogenetic investigation, but limited physiological assessments prevent a clear picture of CAM origins. In these cases, estimates of clade origins allow us to establish a putative oldest boundary on CAM age. In the Apocynaceae, CAM is estimated to have arisen four times in distinct clades. The CAM Hoya–Dischidia clade is sister to the non-CAM genus Oreosparte, implying a CAM origin at or later than this split, which is dated to the early Miocene (Fig. 1C; Liede-Schumann et al., 2022). Ceropegia is part of a large clade containing the stem-succulent stapeliads and its origin can be estimated at 2–8 Ma (Fig. 1C; Bruyns et al., 2015). Similarly, the clade containing the CAM genera Cynanchum and Pachypodium are each nested in presumably non-CAM clades with divergence estimates at 10.6 and 5.6 Ma, respectively, although the CAM assignation for Pachypodium currently rests upon a single species, reported to be weak C3 + CAM (von Willert et al., 1980, 1992). In the Asteraceae, each of the three clades in which CAM has been documented (Caputia, Crassothonna and the Gynurids) are nested within distinct non-CAM clades with divergence dates estimated at 5–10 Ma (Fig. 1C; Pelser et al., 2010). The Gynurid clade is one of the prominent southern African and Madagascar CAM clades, containing species in Senecio, Curio and Kleinia (Gilman et al., 2023). In the Cucurbitaceae, CAM occurs in two distantly related genera nested in C3 clades – Seyrigia and Xerosicyos, both endemic to Madagascar – which are dated to 14–22 Ma (Guo et al., 2020). The two genera are relatively species-poor (six species each), and thus may be relatively simple and tractable systems in which to study CAM evolution. In the Gesneriaceae (African violet family), two CAM lineages are identified, one in subtribe Ramondinae (the Haberlea–Ramonda clade), and the other nested within subtribe Columneinae (the Codonanthe–Codonanthopsis–Nematanthus clade). Petrova et al. (2015) date the Ramondinae spilt from a non-CAM sister clade at a crown age of 24.5 Ma, with a stem node of 30.5 Ma, whereas in the dating analysis of Roalson and Roberts (2016) the equivalent stem node is at 41.5 Ma. The other CAM-inclusive lineage, the Codonanthe–Codonanthopsis–Nematanthus clade, is dated by Roalson and Roberts (2016) to a stem node at 14–15 Ma, but the only CAM taxon so far identified within this clade, Codonanthopsis crassifolia (formerly Codonanthe crassifolia: Guralnick et al., 1986), appears younger, diverging 3–6 Ma and showing weak C3 + CAM. Relative to their large size (~3800 spp.), the Gesneriaceae have so far been severely undersampled for CAM activity and would merit further study, especially as the family contains many tropical epiphytes and climbers.
A more prominent family of CAM is the Crassulaceae. In addition to providing the name for the photosynthetic pathway, the Crassulaceae has many important clades of strong and weak CAM, in genera such as Aeonium, Cotyledon, Crassula, Kalanchoë, Sedum and Sempervivum (Smith and Winter, 1996; Gilman et al., 2023). A wide range of CAM character states are reported in most major subclades of the family, based on gas exchange, nocturnal acidity accumulation and carbon isotope ratios; succulence is also widespread (Kluge and Ting, 1978; Lösch, 1984; Pilon-Smits et al., 1996; Smith and Winter, 1996; Messerschmid et al., 2020). When mapped onto the Crassulaceae phylogeny, strong CAM is evident in clades with nodes deep within the Crassulaceae, supporting a possibility that CAM is ancient, evolving in some lineages during episodes of high atmospheric CO2. Recent phylogenetic investigations, however, estimate widely varying divergence times for the family and major subclades. Bruyns et al. (2019) infer a Crassulaceae crown age of 44.9–65.0 Ma, while Messerschmid et al. (2020) estimate the Crassulaceae diverged 81.7 Ma (crown node) to 107.5 Ma (stem node). While these dates vary substantially, both suggest a pre-Oligocene origin of at least weak CAM in Crassulaceae, when atmospheric CO2 was 750–1000 ppm. In addition to the broad age estimates, pinpointing the origins of CAM in the Crassulaceae becomes particularly difficult due to uncertainty over the phylogenetic distribution of the strong and weak CAM clades. This uncertainty is represented in Fig. 1C by a bar with faded shading that extends beyond the Fig. 1C panel. For example, Crassula probably pre-dates the Oligocene based on crown nodes of 36 Ma (Lu et al., 2022), 40 Ma (Messerschmid et al., 2020) and 46 Ma (Bruyns et al., 2019). Strong CAM has been demonstrated in a handful of Crassula species of the highly succulent clades B and C (Kluge and Ting, 1978; Bruyns et al., 2019), leading Bruyns et al. (2019) to hypothesize that CAM (presumably strong CAM) arose in these clades with the acquisition of pronounced succulence. If true, age estimates for clades B and C of 4–16 Ma would indicate a strong CAM origin in the late Miocene to early Pliocene (Bruyns et al., 2019; Lu et al., 2022). Even the older dates of Messerschmid et al. (2020) suggest that most extant Crassulaceae subclades containing strong CAM diversified after the early Oligocene (e.g. the strongly succulent subfamily Kalanchoideae has a crown age of 32.9 Ma). Moreover, in Aeonium, Messerschmid et al. (2023) using phylogenomic-scale data estimated a crown age of just 1.7‒8.1 Ma, roughly 40 million years younger than predicted by Messerschmid et al. (2020) using ITS and three plastid markers. While broader isotopic surveys and phylogenetic taxon sampling are clearly needed to firmly delineate the strong CAM clades in Crassulaceae, the currently known diversity of CAM and pronounced succulence in each subclade suggests that, even if the earliest CAM taxa may have originated in the Cretaceous, many lineages were primed for rapid evolution of strong CAM in more recent epochs.
Many families with CAM species were not considered in Fig. 1C due to uncertainties in clade age and CAM occurrence within the phylogeny, which made estimations of CAM origin difficult. The Vitaceae provide a case in point. Here, the speciose yet distinct genera Cissus (~290 spp.) and Cyphostemma (~240 spp.) contain at least a few CAM species (Virzo De Santo et al., 1983; Virzo De Santo and Bartoli, 1996). In Cissus, six CAM species occur in three disparate clades, with four species including C. quadrangularis occurring in a succulent African clade of Miocene age (Liu et al., 2013). A survey of online images of Cissus by the authors suggest typical C3 morphology is common in the genus, yet C3 determinations are not reported; hence, where and when CAM might have originated in the phylogeny is unknown, but is probably later than the estimated genus age of 62 Ma (crown) to 72 Ma (stem) (Liu et al., 2013). Similarly, in Cyphostemma, CAM is documented in three species from southern Africa, all of which occur in a clade of caudiciform stem succulents, suggesting this entire succulent clade is CAM. Hearn et al. (2018) date this clade to 16.3 Ma, much younger than their estimated age for Cyphostemma of 44 Ma (crown) to 63 Ma (stem). Again, because the taxonomic distribution of C3 species is unknown, it is not possible to clearly identify where and when the CAM lineages have originated in Cyphostemma. The Vitaceae example highlights the need to report not just positive CAM identifications, but also C3 identifications in surveys of CAM photosynthesis.
With respect to when CAM evolved in non-flowering plant lineages, we consider possibilities for the lycophyte Isoëtes, the fern genus Pyrrosia, and the gymnosperms Welwitschia and Dioon where phylogenetics and physiology allow for limited inferences. CAM is believed to be ancestral in Isoëtes, which is estimated to have a crown age of 45‒65 Ma and a stem potentially extending to ~370 Ma (Wood et al., 2020). However, the evolution of CAM in Isoëtes is probably an adaptation to the low diffusion potential of CO2 in aqueous environments, rather than in response to declining atmospheric CO2, as evidenced by a phenotypic transition to exclusively C3 photosynthesis when growing terrestrially (Keeley, 1998). In the epiphytic fern genus Pyrrosia, C3 + CAM has been reported in a clade of plants with deeply sunken stomata that is specific to Southeast Asia, Indonesia and northeastern Australia, with an estimated mean divergence time of 18 Ma (Fig. 1C; Wei et al., 2017). CAM has not been reported for other Pyrrosia clades from Africa and Asia, suggesting CAM originated in the clade with sunken stomata (Wei et al., 2017). The sister genus to Pyrrosia, Platycerium, is reported to contain very weak CAM (Holtum and Winter, 1999), but the C3Pyrrosia costata clade is sister to both the Pyrrosia clade with CAM and Platycerium, indicating distinct CAM origins (Wei et al., 2017). Weak C3 + CAM has been reported in the monotypic gnetophyte Welwitschia mirabilis (reviewed in von Willert et al., 2005). The family Welwitschiaceae is considered ancient, dated by fossils to at least the lower Cretaceous; however, these fossils lack xeromorphic characters and some even appear aquatic (Jürgens et al., 2021). These characters suggest CAM was unlikely in ancestral Welwitschia, and it may have arisen more recently as the ancestors of the surviving W. mirabilis plants became adapted to arid African landscapes. Similarly in the cycads, another ancient lineage of gymnosperms, diversification into more arid habitats appears to have occurred in the Miocene, although weak C3 + CAM activity (CAM cycling) has so far been reported only in the single taxon Dioon edule (Vovides et al., 2002; Gutiérrez-Ortega et al., 2018).
Using the age estimates in Fig. 1B, we present a frequency diagram of strong CAM origins and compare it with origin estimates of C4 clades presented by Sage (2016; Fig. 2). For both CAM and C4, the majority of lineages are estimated to have arisen in the Miocene or later, with a peak occurring in the late Miocene at 5–10 Ma. The late Miocene also corresponds to the interval when C4-dominated grass biomes expanded across low to mid-latitudes (Cerling et al., 1997; Edwards et al., 2010). This synchronous spike in C4 origins and expansion of C4 grasslands during the Miocene is viewed as being enabled by the establishment of low CO2 conditions, but other factors such as increasing aridity, seasonality, fire frequency and the radiation of large herbivore guilds may have played a triggering role (Osborne and Freckleton, 2009; Edwards et al., 2010; Charles-Dominique et al., 2016). A recent review of the atmospheric CO2 literature also estimates CO2 continued to drift downward from the mid-Miocene Climatic Optimum (~16.9‒14.7 Ma) during the late Miocene, from near 500 to 400 ppm (Rae et al., 2021; Steinhorsdottir et al., 2021). A combination of stresses, notably low CO2, heat, salinity and drought, are now thought to have promoted the initial origins of C4 photosynthesis, because together they cause high rates of photorespiration in C3 plants, leading to more glycine production and the release of photorespiratory CO2 and ammonia at the glycine decarboxylation step (Sage et al., 2018). Drought and salinity reduce stomatal conductance, which acts in concert with low atmospheric CO2 to further reduce chloroplast CO2 concentration, further enhancing photorespiration (Sage, 2013). To compensate for high rates of photorespiration, certain plants evolved mechanisms to trap and refix photorespired CO2, one of which is C2 photosynthesis wherein photorespiratory glycine is shuttled from the mesophyll to bundle sheath cells, at which point it is decarboxylated to yield CO2 and ammonia (Monson and Rawsthorne, 2000; Sage et al., 2012). The C4 biochemical cycle is hypothesized to have initially appeared to assist in the recovery of photorespired ammonia liberated in the bundle sheath cells by glycine decarboxylation, after which it was upregulated to form a strong CCM (Mallmann et al., 2014; Adachi et al., 2023). This incipient C4 metabolism became an exaptation for C4 pathway evolution, because its original role was to facilitate the function of the C2 pathway, but in doing so it enabled the subsequent rise of CO2 concentration into the bundle sheath. In considering CAM evolution, and how declining CO2 may have influenced it, it will also be worth considering how aspects of the C3 physiology became exaptations for subsequent CAM evolution (Edwards, 2023). For example, succulence of chlorenchyma cells to enhance hydraulic capacitance and stomatal conductance in C3 ancestors could have been one exaptation that facilitated nocturnal malate accumulation (Griffiths, 1989; Edwards, 2019).
Fig. 2.
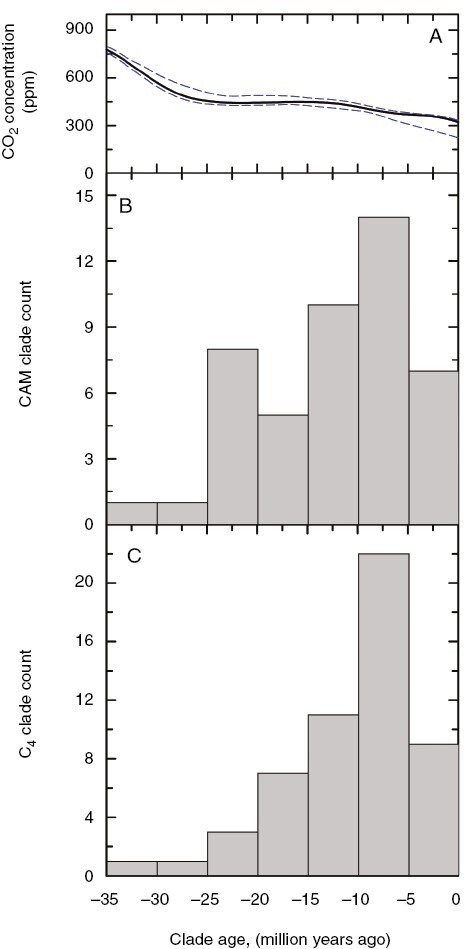
Frequency diagram showing when the estimated strong CAM and C4 lineages originated over the past 35 million years. (A) CO2 history shown in Fig. 1A. (B) Origin estimates of strong CAM clades from Fig. 1B, using divergence dates (usually stem node estimates) given in the cited papers. C4 clade values are from Sage (2016).
The timing of CAM diversification
If low CO2 favoured CAM evolution, then this would inevitably also have promoted species diversification within CAM lineages as they exploited the new physiology and radiated into a wide range of environments and growth forms (Crayn et al., 2004; Arakaki et al., 2011; Horn et al., 2014). Such a possibility is supported in C4 clades such as the Andropogoneae grasses and the Euphorbia subgenus Chamaesyce, where a diversity of C4 species arose as lineages expanded into new niches following acquisition of the C4 pathway (Spriggs et al., 2014; Horn et al., 2014; Lundgren et al., 2015). Rapid species radiation following CAM evolution is documented for at least five of the Euphorbia CAM clades from the mid-Miocene to the Pliocene, roughly coincident with C4 grassland expansion across the globe and the peak of both C4 and CAM origins (Fig. 2). Together with the C4 species expansion within the subgenus Chamaesyce, these CAM radiations produced half of the species in the large genus Euphorbia (Horn et al., 2014). In the bromeliads, a doubling of the speciation rate was modelled to follow CAM evolution in subfamily Bromelioideae (Silvestro et al., 2014; but see Givnish et al., 2014, who did not detect this pattern across the family as a whole). Indeed, in the bromeliad lineages consisting wholly of CAM species, such as the Dyckia and Hechtia clades of xeric terrestrial succulents, and the extreme epiphytic ‘atmospheric’ forms in Tillandsia, it could be argued that acquisition of CAM was a prerequisite for colonization of these respective habitats (Crayn et al., 2004, 2015). An increased diversification rate following CAM acquisition is also documented in numerous but not all CAM orchid clades (Givnish et al., 2015; Bone et al., 2015; Li et al., 2019; Gamisch et al., 2021). In the Eulophiinae orchids, a trend towards increased diversification rate is inferred in the late Miocene following CAM acquisition (Bone et al., 2015). Similarly, a late Miocene to Pliocene diversification is reported for Crassula clades B and C, which contain known strong CAM species and well-developed succulent perennials (Bruyns et al., 2019; Lu et al., 2022). Rapid diversification of the most speciose clades of the Cactaceae and Agavoideae is also predicted to have occurred in the late Miocene to Pleistocene (Good-Avila et al., 2006; Arakaki et al., 2011; Hernández-Hernández et al., 2014; Jiménez-Barron et al., 2020). One of the most spectacular species radiations occurred in southern Africa in the core Ruschioideae of family Aizoaceae, in which lineage diversification rates 3–10 times greater than typical angiosperm rates are estimated for the late Miocene to mid-Pleistocene interval (about 8–1 Ma), when aridification was intensifying in the region (Klak et al., 2004, 2017).
Numerous hypotheses are proposed for the increased diversification of CAM lineages, with climate deterioration and regional drying being the common mechanism. In southern Africa, a seasonal shift to cool, somewhat moist winters is suggested to be a particularly strong cause of CAM species diversification because the physiology of CAM functions well where nights are relatively cool and humid (Lüttge, 2004; Holtum et al., 2016). We argue here that low CO2 should also be considered as a driver for diversification of CAM species, probably in concert with these other environmental changes. One interesting observation raised by Horn et al. (2014) is that the increased diversification rate in the CCM-clades of Euphorbia in the later Miocene could be due to increased establishment enabled by CAM or C4 photosynthesis (although CAM activity may take several months to be manifested in newly germinated seedlings of terrestrial succulents). Greater degrees of seedling establishment and plant survival could have reduced extinction rates and, by doing so, increased diversification rates. Low CO2 has recently been linked to reduced establishment of C3 seedlings, particularly in warm, dry environments (McCann and Sage, 2022). By enhancing early carbon gain and water savings, CAM could offset the deleterious effects of low CO2 on mortality of establishing plants and thus enhance species fitness and diversification.
PHYSIOLOGICAL EFFECTS OF CO2 VARIATION ON C3 AND CAM PHOTOSYNTHESIS
Low atmospheric CO2 challenges C3 plants in three fundamental ways (Sage, 2013). First, below 1000 ppm, CO2 becomes limiting for the Rubisco carboxylation reaction of photosynthesis in its role as a substrate, with recent atmospheric values of CO2 falling below the KM of Rubisco for CO2. At 25 °C, Rubisco KM for CO2 is 8–21 µm in C3 plants, whilst CO2 concentration in the chloroplast solution is about 8 µm when atmospheric CO2 is 400 ppm, assuming the equilibrium gas phase ratio between chloroplast stromal CO2 and atmospheric CO2 is 0.6 (von Caemmerer and Quick, 2000). Second, Rubisco also oxygenates its substrate RuBP in the first step of photorespiration, with CO2 acting as a competitive inhibitor of the oxygenase reaction. With less CO2, particularly below 500 ppm, the suppression of Rubisco oxygenation is reduced and the O2 inhibition of Rubisco carboxylation increases (Ehleringer et al., 1991). Together, reduced CO2 supply as a substrate and greater photorespiration markedly reduce photosynthetic capacity of C3 plants during the post-Oligocene period of depleted atmospheric CO2 (Sage and Stata, 2015). Elevated temperature directly aggravates CO2 limitations by reducing the solubility of CO2 relative to O2, increasing the KM of Rubisco for CO2, and enhancing oxygenase activity and photorespiratory inhibition (Sharkey, 1988). Thus, low CO2 effects on Rubisco activity are greatest in warm conditions where C4 and CAM species richness can be high (Kluge and Ting, 1978; Sage et al., 2018).
The third fundamental limitation imposed by reduced CO2, and the one perhaps most relevant to CAM evolution, is the aggravation of water deficiency. C3 plants respond to low atmospheric CO2 by opening stomata, thus increasing stomatal conductance (gs) to water-vapour diffusion (Fig. 3A; Sage and Coleman, 2001). As gs increases with declining CO2, transpiration rates rise and water use efficiency (WUE) of photosynthesis declines (Fig. 3B). This creates two potential threats to plants, which are most acute in hot, dry environments. In the short term, rapid rates of transpiration can reduce leaf water status (declining water potential and cell turgor), potentially to the point of tissue injury unless water supply is sufficient and the hydraulic transport capacity of the plant can deliver water to the leaves as fast as it evaporates (Schulze and Hall, 1982; Kocacinar and Sage, 2004; Osborne and Sack, 2012). High vapour pressure difference (VPD) between leaf and air directly enhance transpiration, which may bring the benefit of transpirational cooling if water supply is adequate, but which increases the probability of leaf wilting and injury when water availability is restricted. High VPD occurs in warmer, drier climates of low humidity, particularly in hot midday conditions (Schulze and Hall, 1982). Over the long term, enhanced gs at reduced atmospheric CO2, particularly at high VPD, will promote elevated transpiration rates that more rapidly consume soil water and raise the risk of crippling water deficit. Consistent with this reasoning, soil water deficits are widely documented to be more common in low relative to high atmospheric CO2 treatments (Polley et al., 1997; Morgan et al., 2004, 2011). As drought intensifies, plants close stomata to conserve water, but this aggravates CO2 deficiency in low atmospheric CO2 (Sage and Coleman, 2001; Gerhart and Ward, 2010; Franks et al., 2013). For this reason, selection for CCMs is considered to have been particularly strong in warm, dry climates of reduced atmospheric CO2 (Sage and Stata, 2015).
Fig. 3.
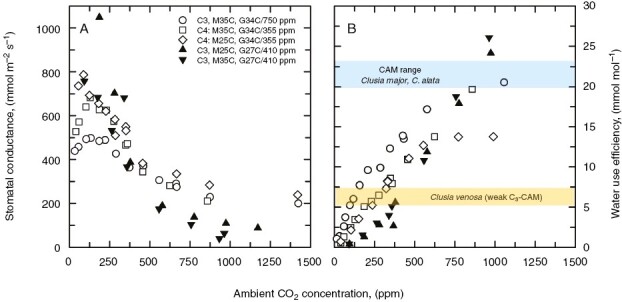
(A) The response of stomatal conductance to ambient measurement CO2 for a C3 plant (Chenopodium album) and a C4 plant (Amaranthus retroflexus) grown (G) at either 27 or 34 °C, and 355, 410 or 750 ppm CO2 as indicated, and measured (M) at 25 or 35 °C. (B) The photosynthetic water use efficiency for the responses shown in A, where water use efficiency is determined as the ratio of photosynthesis to transpiration determined using leaves in a gas exchange chamber. Reprinted from Sage and Stata (2021), with results from Sage et al. (1995), where growth conditions and gas exchange measurement procedures are described. Clusia values are from Lüttge (2007b).
Plants have a number of mechanisms to compensate for the combination of low CO2, drought and heat (which elevates VPD). To sustain high transpiration rates without turgor loss and potential leaf injury, plants can invest in proportionally greater fractions of root mass and hydraulic conductivity in stems and leaves than they might when water is abundant (Sage, 2001; Osborne and Sack, 2012; Griffiths et al., 2013). At the leaf level, this could mean a denser network of veins and larger bundle sheath cells, facilitating the rise of photorespiratory glycine shuttling in hot environments (Sage, 2001; Osborne and Sack, 2012; Christin et al., 2013; Griffiths et al., 2013). An alternative compensation mechanism is to form water storage tissue, increasing tissue succulence and resilience to episodic water deficiency (Gessner, 1956; Ogburn and Edwards, 2010; Griffiths and Males, 2017). In many species, succulence occurs in heterotrophic tissues of roots and stems, or non-photosynthetic hydrenchyma of leaves, leaving the photosynthetic tissue little changed and C3 in structure and function (Barrera Zambrano et al., 2014; Borland et al., 2018). However, if the photosynthetic chlorenchyma becomes succulent, the possibilities for altered photosynthetic function may arise because a larger cell and vacuolar volume allows greater metabolite storage per unit surface area of leaf tissue (Smith et al., 1996). In this manner, succulence of photosynthetic cells for drought resistance may become an exaptation for the initiation of CAM function. As high vein density may predispose C4 evolution in response to the combination of drought and low CO2, succulence of photosynthetic tissues may predispose C3 plants to evolve CAM (Sage, 2002; Edwards and Ogburn, 2012; Edwards, 2019; Heyduk et al., 2016; Luján et al., 2022). Moreover, as succulence increases, the large photosynthetic cells become more densely packed, which could impede diffusion of CO2 into the tissue (Maxwell et al., 1997; Nelson et al; 2005). In this manner, succulence itself could aggravate CO2 deficiencies and become a contributing factor for CAM evolution.
One of the important findings from research on the CO2-response of C3 plants is a marked reduction in root growth in low CO2 treatments (<400 ppm), due to both slower growth and reduced allocation to root relative to shoot biomass (Sage, 1995; Campbell and Sage, 2002; Gerhart and Ward, 2010; Bond and Midgley, 2012). Reduced root allocation aggravates drought limitations by reducing a plant’s ability to acquire water, which in turn restricts gs. Because roots are expensive to make and maintain, the relative cost of water acquisition should rise in low-CO2 atmospheres, potentially leading to stronger selection for other mechanisms that reduce water costs. C3 plants can reduce relative water costs through two important strategies – reducing the cost of water acquisition and reducing the cost of water consumption (Fig. 4). Reducing water acquisition costs can be achieved through luxury consumption of water, where water is absorbed when it is abundant and cheap, and stored for later use when external supplies are scarce (Fig. 4A; Chapin et al., 1990). The concept of luxury consumption generally refers to nutrient acquisition and storage (Lambers et al., 2008), but can equally well describe acquisition and storage of water when it is available at low cost. With luxury consumption comes a need for water storage tissue, which can be achieved in a number of ways involving roots, woody tissue or the mesophyll cells of leaves. When water storage arises in non-photosynthetic tissues, it is unlikely to enhance CAM possibilities and may even act as an impediment. When it occurs in photosynthetic tissues, then it can readily become an exaptation for CAM.
Fig. 4.
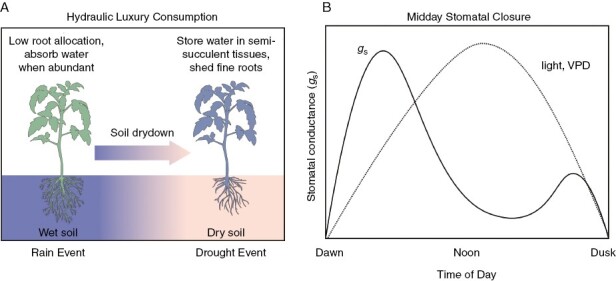
A schematic outlining two key drought strategies in C3 plants that may facilitate the initiation of CAM evolution. (A) Outline of the function of hydraulic luxury consumption, where water is absorbed by a plant when abundant and maintained in storage tissues when the soil dried, thereby extending the growing season and/or enhancing survival during severe drought. (B) Strong midday stomatal closure is shown (solid line), where stomatal conductance (gs) serves as an index of stomatal aperture. Typical patterns of light intensity and vapour pressure difference (VPD) between leaf and air are shown over the course of the day. Midday stomatal closure profiles are idealized patterns based on responses in Schulze and Hall (1982).
A second mechanism to reduce water costs is to minimize water use when the cost of transpiration is high. This may mean specialization for growth in the cooler, wetter seasons of the year, as seen in desert ephemerals and drought-deciduous trees and shrubs (Smith et al., 1997). For plants active in hot, dry locations, or in warm climates with limited soil, water costs can be reduced by greater degrees of midday stomata closure (Schulze and Hall, 1982; Lüttge et al., 1986). As outlined in Fig. 4B, midday stomata closure is the phenomenon where gs is greater early and late in the day when VPD is low and transpiration potential is reduced, and then reduced at midday when VPD is high. In plants with strong midday stomatal closure, the stomatal rhythm approximates stomatal rhythms in CAM during daytime phases (compare Figs 4B and 5). We hypothesize that if such a pattern were to become constitutive in a C3 plant, perhaps by linkage to a circadian regulator or via increased sensitivity to abscisic acid (ABA), calcium signals and VPD, then midday stomatal closure could also become a precursor for CAM by facilitating eventual establishment of the CAM stomatal rhythm (Hotta et al., 2007; Males and Griffiths, 2017a, b; Kamrani et al., 2022). Control of circadian oscillators over stomatal rhythms and WUE has been established in Arabidopsis (Simon et al., 2020; Kamrani et al., 2022), suggesting alteration of midday stomatal depression by circadian control is a possibility worth examination.
Fig. 5.
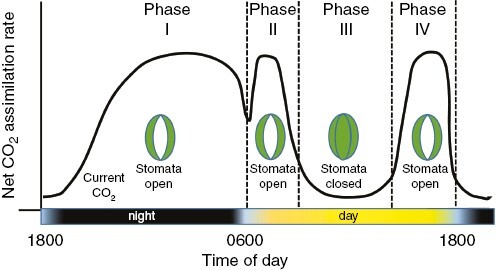
Idealized phases of CO2 assimilation exhibited by a strong CAM plant during a 24-h diurnal cycle, as described by Osmond (1978). Note that obligate CAM plants often exhibit an essentially biphasic cycle, with only phases I and III being apparent. The diagram closely follows Fig. 10.2 in Borland et al. (2018).
Experimental responses of CAM photosynthesis to CO2 variation
While numerous studies have investigated CAM plant responses to elevated atmospheric CO2 concentrations predicted for the next century (Winter et al., 1997; Drennan and Nobel, 2000; Ceusters and Borland, 2010; Pereira et al., 2021; Sage and Stata, 2021; Hultine et al., 2023), only a few have examined responses of CAM plants to CO2 atmospheres below recent historical concentrations (Winter et al., 1992, 2014). This does not appear to be a major impediment to evaluation of interactions between declining CO2 and CAM evolution, because most CAM origins appear to be Miocene to Pliocene in age, when CO2 was similar to current atmospheric levels near 400 ppm. The research with CAM plants in very low CO2 (170–280 ppm) is notable in that it shows the relative contribution of nocturnal CO2 uptake by CAM to 24-h CO2 fixation is markedly enhanced relative to what is observed at higher CO2 levels (Fig. 6D; Winter et al., 1992, 2014).
Fig. 6.
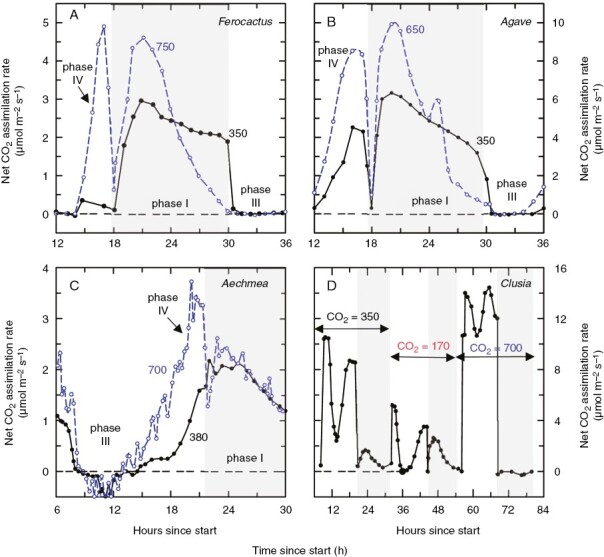
The diurnal cycle of net CO2 assimilation rate in select CAM plants measured at recent and elevated levels of atmospheric CO2. In A–C, plants were grown and measured at either recent (black lines and filled symbols) or elevated CO2 (blue lines and open symbols), with measurement CO2 in ppm given above each curve. Grey shading indicates nocturnal periods. In D, plants were grown at 350 ppm CO2 and measured at the CO2 values indicated in the panel above the double-headed arrows. (A) Ferocactus viridescens (originally published under the synonym Ferocactus acanthodes) grown and measured at 350 or 650 ppm (Nobel and Hartsock, 1986); (B) Agave deserti grown at 350 or 650 ppm CO2 (Nobel and Hartsock, 1986); (C) the CAM bromeliad Aechmea ‘Maya’ grown and measured at 380 or 700 ppm (Ceusters et al., 2008); (D) the flexible CAM hemiepiphyte Clusia uvitana grown at 350 ppm CO2 and measured at either 170, 350 or 700 ppm as indicated in the panel (Winter et al., 1992). Note in A–C that phase IV is enhanced by high CO2 relative to the lower CO2 response; while in A–C, phase I is enhanced early in the dark period but not later. In D, note how elevated CO2 eliminates dark CO2 fixation, after enhancing daytime CO2 fixation, while at 170 ppm CO2 the daytime fixation rate is much less than at higher levels of CO2, whereas the nighttime fixation rate is higher.
CAM plants are widely noted to perform better at elevated CO2, with improvements in carbon gain and growth that often exceed that of C3 species (Cui et al., 1993; Cui and Nobel, 1994; Graham and Nobel, 1996; Winter et al., 1997; Zhu et al., 1999; Drennan and Nobel, 2000; Zotz et al., 2010, 2023; Wagner and Zotz, 2018). This observation could lead to a conclusion that high CO2 enhances CAM photosynthesis such that low CO2 would not be necessary for CAM evolution. However, the improvement of CAM plant performance in elevated CO2 is due more to increased C assimilation during the C3 phases of the diurnal CAM cycle and, for facultative CAM plants, increased C gain when the plants are operating in the C3 mode (Fig. 6; Winter et al., 1997, 2014; Zhu et al., 1999; Ceusters and Borland, 2010; Zotz et al., 2023). Responses of four representative CAM plants are shown in Fig. 6. In each, there is a pronounced stimulation of daytime CO2 assimilation, mostly in phase IV. Notably, phase IV can appear in obligate CAM plants that do not normally exhibit it when they are exposed to reduced CO2 (Fig. 6A; Graham and Nobel, 1996). Phase I usually, but not always, exhibits enhanced C gain in elevated CO2 (Drennan and Nobel, 2000). Often, phase I CO2 uptake in elevated CO2 is enhanced early in the dark period relative to rates observed in plants at lower CO2 concentration, but then drops below CO2 assimilation rates of plants in low CO2 later at night (Fig. 6A, B). This response is interpreted to result from earlier depletion of carbohydrate reserves needed for glycolytic production of PEP, and/or the vacuole being filled to capacity with malic acid earlier in the dark period, such that CO2 assimilation becomes restricted earlier in the dark period in plants at elevated CO2 (Drennan and Nobel, 2000). In bromeliads with smaller cell vacuoles, stimulation of phase I CO2 uptake by elevated CO2 is negligible (Drennan and Nobel, 2000; Ceusters et al., 2008). Based on these observations, we hypothesize that mildly succulent C3 and incipient C3 + CAM species would be limited in their ability to accumulate malic acid at night, such that any CAM contribution at high CO2 would be a small fraction of the daily C gain. By contrast, in low CO2, the relative CAM contribution could be much higher, allowing for a stronger signal that selection could act upon, and thus favour the strengthening of CAM activity at night.
On the water side, a major fitness attribute that CAM confers is very high WUE, typically 3–10 times that of C3 photosynthesis in recent atmospheres (Kluge and Ting, 1978; Osmond 1978; Nobel, 1988; Lüttge, 2004, 2007a, b; Borland et al., 2009). Elevated CO2 enhances WUE in C3 plants through the combination of enhanced C assimilation and reduction in stomatal conductance (Sage, 1994; Ainsworth and Long, 2005; Venter et al., 2022). Figure 3B shows the effect of rising CO2 on WUE for a widely distributed C3 weed, Chenopodium album, grown at 380 ppm and elevated CO2 levels of 750 ppm. As the measurement CO2 in the leaf cuvette increases to double historical values, the WUE of C. album grown in either current CO2 or enhanced CO2 rises 3- to 4-fold, to approach values exhibited by CAM Clusia species (Fig. 3B). C3Clusia species exhibit similar WUE values to C. album grown and measured at recent historical values of atmospheric CO2 (Lüttge, 2007b). This evidence shows that in elevated CO2, CAM-like WUE values are possible in the C3 flora such that there may be a reduced need for CAM to improve the water economy. Hence, the selection pressure for CAM based on water conservation alone should have been much less in a high CO2 atmosphere.
C3 + CAM VERSUS STRONG CAM EVOLUTION IN HIGH CO2
If elevated CO2 reduces the need for strong CAM to enhance plant fitness, then why, and how, might weak C3 + CAM physiologies evolve in a high CO2 world as indicated by the pre-Miocene appearance of C3 + CAM in a number of lineages such as the Crassulaceae and Portulacineae? While the possibilities require focused research using sister C3 and C3 + CAM species, for now we provide a hypothesis. In elevated atmospheric CO2, drought and other relevant stresses would still have occurred although with less frequency and intensity than at reduced CO2, assuming identical moisture regimes. As defined by Winter (2019), weak CAM cycling contributes little to plant C balance and thus is not an important component of growth, reproduction and competitive ability. Instead, by recycling respired carbon, CAM cycling serves as a maintenance mechanism during extremes of drought or salinity, which occurs in dry climates and dry microsites such as rock outcrops and tree branches, even at high CO2 (Griffiths, 1989; Martin, 1996; Pilon-Smits et al., 1996; Holtum et al., 2016). The ability to use CAM to recycle respiratory CO2 at night can substantially delay C depletion and allow a plant to survive dry episodes with completely closed stomata. In this regard, a weak CAM cycle and any associated succulence could serve as a survival mechanism, conferring fitness regardless of the prevailing CO2. Moreover, the costs of weak C3 + CAM physiology and structure are probably low compared to strong CAM, both in an absolute sense and in terms of how they might interfere with C3 photosynthesis (Edwards, 2019). Compared to strong CAM plants, succulence of weak CAM plants tends to be low, mesophyll cells are not tightly packed, investment into the C4 enzymes is small and the energy costs of CAM function are minimal (Edwards, 2019; Luján et al., 2022; Leverett et al., 2023). As these costs rise with the evolution of stronger CAM phenotypes, the benefits should also increase, but in high CO2, C3 plants already experience the benefits of high WUE and carbon gain without the cost.
Given these considerations, how might strong CAM evolve in elevated CO2 atmospheres as suggested by certain Crassulaceae clades? For strong CAM to evolve in elevated CO2, we hypothesize that it would occur when plants experience chronically low internal CO2 concentrations and C deficiency brought about by persistently low conductance to CO2 diffusion. Persistently low conductance would be most evident in warm environments with high VPD and restricted water supply (Cernusak et al., 2013; Mok et al., 2023), such as the soil-free lithophyte habit on rock outcrops and cliff-faces. Consistent with this hypothesis, many modern Crassulaceae species are lithophytes specializing in survival on rock surfaces with minimal soil (Fig. 7), leading us to hypothesize that the lithophyte habit in Crassulaceae is also ancient, pre-dating low atmospheric CO2. If so, they may have relied on extreme water conservation measures that facilitated the transition from weak to strong CAM in elevated atmospheric CO2 (see Males and Griffiths, 2017b, for a discussion of stomatal control in lithophytic bromeliads). These considerations highlight the probability that low CO2 is not an absolute requirement for strong CAM evolution, but rather, a condition that increases probabilities for CAM origin, particularly in combination with other stresses such as heat and drought.
Fig. 7.

Two Crassulaceae lithophytes growing on rock faces. (A) Sempervivum tectorum growing on rock outcrops near Gravenhurst, Ontario, Canada; and (B) Dudleya farinosa growing on a cliff face along the Mendocino coast of California, USA (lat. 40.28, long. −124.36). The photos demonstrate examples of where strong CAM may have arisen in high-CO2 environments of the pre-Miocene. We hypothesize strong CAM evolution may have occurred in elevated CO2 on rocky substrates such as these shown, where water deficiency could have been so frequent and intense as to select for persistent stomatal conservatism, low internal CO2 and, possibly, CAM intensification.
SYNTHESIS – DID LOW CO2 PROMOTE CAM ORIGINS?
The evidence presented here supports a role for low CO2 in facilitating the evolution of strong CAM photosynthesis and, for many lineages, C3 + CAM physiologies as well. Weak CAM appears to have arisen in some lineages during elevated CO2 episodes prior to the Oligocene, which could be consistent with its role in enabling plant survival during episodic drought extremes. Such use of weak CAM is widely considered to facilitate evolution of stronger CAM modes, by establishing the biochemical and regulatory capacity for limited CAM activity, which could then be upregulated (Pilon-Smits et al., 1996; Sage, 2002; Bräutigam et al., 2017; Winter and Smith, 2022). However, this presents a conundrum. If it were a simple matter of upregulation of weak CAM to produce strong CAM, then why is there not widespread evidence for strong CAM physiology dating well into the Oligocene or Eocene when CO2 was elevated? One possibility is that weak CAM was uncommon at elevated CO2, and hence there was little opportunity for strong CAM to evolve. The relatively few C3 + CAM clades postulated for the Eocene support this case. Another possibility is there were significant constraints to evolving strong CAM, such that strong and consistent selection pressure was needed to surmount these constraints. Edwards (2019) argues that acquisition of pronounced succulence may have been the evolutionary barrier to strong CAM evolution. If so, then surmounting this barrier may have required the strong selection pressure created by low atmospheric CO2, or a particularly harsh set of circumstances as may have occurred on dry rock outcrops in a warm, high-CO2 world.
A second consideration is that CAM is widely regarded as a drought-tolerance mechanism, and in many regions where CAM species arose and diversified, intensification of aridity in the past 35 million years corresponds with the appearance of the CAM clades (Keeley and Rundel, 2003; Bone et al., 2015; Li et al., 2019; Heyduk, 2022). Aridification is generally considered a primary driver for CAM evolution, with low CO2 generally being ignored or mentioned in passing (but see Arakaki et al., 2011; Heyduk, 2022). If drought were the predominant driver, then why did strong CAM not commonly arise in older arid habitats that pre-date the Oligocene? The most common dry environment of the pre-Miocene world would have been the epiphytic habits of the widespread tropical and subtropical forests of Eocene and Oligocene landscapes; yet, as indicated in Fig. 1, there is little evidence for ancient origins of strong CAM epiphytes prior to 30 Ma. [Tropical forests have existed since pre-Cretaceous time, although dominated by gymnosperms until the mid- to late Cretaceous when angiosperms asserted their dominance (Jaramillo, 2023). The epiphytic niche probably pre-dated the angiosperms, with ferns occupying this habitat as they do now in temperate rainforests. The modern type of angiosperm-dominated rainforest became widespread by the Eocene (Jaramillo, 2023), and hence the epiphytic niche in its modern form should have been present before epiphytic CAM lineages appeared. A critical event in development of the rainforest in its modern form was the establishment of habitat where many lineages that later evolved CAM would arise, thus meeting a precondition (compatable taxa) for later CAM evolution.]
Strong CAM origins in the tropical epiphytes such as the bromeliads, orchids and Hoya–Dischidia clades are clearly Miocene or later, when reduced CO2 is evident. Extensive radiation of terrestrial CAM plants in southern Africa is also evident in the later Miocene and Pliocene, despite semi-arid to arid landscapes being present since the Cretaceous in Namibia (Ward et al., 1983). Aridity in the Atacama region of South America is also ancient, extending back 150 Ma (Hartley et al., 2005). We argue that low CO2 may have made the difference in the evolutionary emergence of CAM, and should therefore be considered a primary environmental driver, along with drought.
As we move forward, rising CO2 should remove an important facilitating agent for CAM success, and it is possible that CAM species will become restricted and outcompeted by C3 plants. In epiphytic floras, for example, more aggressive growth of C3 species in elevated CO2 might be expected to crowd out CAM species, particularly since many of the CAM plants use obligate CAM with weak C3 phases; however, the evidence for this possibility is not strong (Zotz et al., 2023). Elevated CO2 is unlikely to harm CAM species directly, given their flexibility and propensity to rely increasingly on C3 metabolism in higher CO2, and one might hypothesize CAM being able to move to more extreme epiphytic microsites as their WUE improves. Before any of this happens, however, there are far more serious threats to the CAM flora that must be addressed, namely from habitat destruction, run-away fire cycles, over-harvesting of CAM plants for horticultural uses and invasive species outbreaks (Grace, 2019; Sage and Stata, 2021; Zotz et al., 2023; Hultine et al., 2023). CAM photosynthesis is not unique in feeling the heat of anthropogenic global change, as the C4 flora and much of the C3 flora are under similar threats (Sage, 2020). Because so much of the world’s biota is currently threatened, there may be too many critical needs to prioritize conservation of CAM plants; however, the CAM flora has many human friends in the numerous orchid, bromeliad and succulent societies around the world. Conservation biologists should collaborate with these enthusiasts to ensure CAM diversity is protected as global change intensifies.
To close, we are encouraged by the extensive phylogenetic progress of the past 10–20 years which made the age estimates presented here possible. These estimates will undoubtedly be refined as comprehensive studies add new CAM taxa and character states to increasingly detailed phylogenies. While age estimates of CAM will improve, an important additional benefit will be a greater understanding of how CAM was assembled from C3 ancestors, allowing CAM to become one of the powerful models for understanding complex trait evolution in general. One of the key challenges to developing a more comprehensive understanding of CAM evolution will be the phenotyping of CAM character states. As a first pass, we recommend continued work to survey δ13C values of bulk leaf tissue to distinguish strong CAM phenotypes from C3 and C3 + CAM phenotypes. Surveys of δ13C can exploit large collections of plants in the world’s herbaria, enabling comprehensive representation across a phylogeny. To better resolve potential CAM species, δ13C values could be determined on starch and sugars extracted from herbarium specimens. Leaf starch reflects C gain over the prior day or two, so it would be less prone to dilution of the δ13C signal by C3 photosynthesis that predominated early in development or before CAM is induced in facultative CAM plants. Within the group of species with C3-like δ13C values, follow-up physiological assessments such as of diurnal acid cycling could be used to further screen for CAM, using phylogenies of C3 to CAM lineages to strategically target transitional species. Physiology studies will require living plants, which may turn out to be the greatest challenge, given the cost, regulatory restrictions and in some places danger of collecting species. Where the challenge is great, so can be the opportunity. An excellent way to gain access to plant species is to build relationships between diverse groups of colleagues from across the globe. As Klaus Winter has demonstrated throughout his career (Holtum, 2023), such relationships should not simply be a means to acquire plants, but also a way to build networks of expertise by forming collaborations with colleagues and their students in regions rich with CAM diversity. While CAM research immediately profits through such networks, the ultimate benefit may be the establishment of local experts who will lead efforts to preserve Earth’s CAM flora. The investment by Klaus Winter into this human side of the CAM adventure may turn out to be one of his greatest legacies.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Appendix S1: Sources and assumptions for dating the CAM lineages presented in Fig. 1B and C.
ACKNOWLEDGEMENTS
We thank Professor Dana Royer and colleagues for sharing their CO2-history data set and Ms Charlie Olson for assistance in manuscript preparation. We also thank Daniel Mok for assistance in preparing the manuscript.
Contributor Information
Rowan F Sage, Department of Ecology and Evolutionary Biology, University of Toronto, 25 Willcocks Street, Toronto, ON, M5S 3B2, Canada.
Ian S Gilman, Department of Ecology and Evolutionary Biology, Yale University, New Haven, CT 06511, USA.
J Andrew C Smith, Department of Biology, University of Oxford, South Parks Road, Oxford, OX1 3RB, UK.
Katia Silvera, Department of Botany and Plant Sciences, University of California, Riverside, CA 92521, USA.
Erika J Edwards, Department of Ecology and Evolutionary Biology, Yale University, New Haven, CT 06511, USA.
FUNDING
This research was supported by Natural Sciences and Engineering Research Council of Canada Discovery grant RGPIN-2017-06476 to RFS, the Oxford Martin School Dryland Bioenergy programme, University of Oxford, to JACS, and National Science Foundation (USA) grant IOS-1754662 to EJE.
LITERATURE CITED
- Adachi S, Stata M, Martin DG, et al. 2023. The evolution of C4 photosynthesis in Flaveria (Asteraceae): insights from the Flaveria linearis complex. Plant Physiology 191: 233–251. doi: 10.1093/plphys/kiac467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth EA, Long SP.. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165: 351–371. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- Arakaki M, Christin P-A, Nyffeler R, et al. 2011. Contemporaneous and recent radiations of the world’s major succulent plant lineages. Proceedings of the National Academy of Sciences of the United States of America 108: 8379–8384. doi: 10.1073/pnas.1100628108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Price GD.. 2003. CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. Journal of Experimental Botany 54: 609–622. doi: 10.1093/jxb/erg076. [DOI] [PubMed] [Google Scholar]
- Barrera Zambrano VA, Lawson T, Olmos E, Fernández-Garcia N, Borland AM.. 2014. Leaf anatomical traits which accommodate the facultative engagement of crassulacean acid metabolism in tropical trees of the genus Clusia. Journal of Experimental Botany 65: 3513–3523. doi: 10.1093/jxb/eru022. [DOI] [PubMed] [Google Scholar]
- Böhnert T, Weigend M, Merklinger FF, Quandt D, Luebert F.. 2020. Historical assembly of Zygophyllaceae in the Atacama Desert. Frontiers of Biogeography 12: e45197. [Google Scholar]
- Bond WJ, Midgley GF.. 2012. Carbon dioxide and the uneasy interactions of trees and savannah grasses. Philosophical Transactions of the Royal Society B-Biological Sciences 367: 601–612. doi: 10.1098/rstb.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone RE, Smith JAC, Arrigo N, Buerki S.. 2015. A macro-ecological perspective on crassulacean acid metabolism (cam) photosynthesis evolution in Afro-Madagascan drylands: Eulophiinae orchids as a case study. New Phytologist 208: 469–481. doi: 10.1111/nph.13572. [DOI] [PubMed] [Google Scholar]
- Borland AM, Griffiths H, Hartwell J, Smith JAC.. 2009. Exploiting the potential of plants with crassulacean acid metabolism for bioenergy production on marginal lands. Journal of Experimental Botany 60: 2879–2896. doi: 10.1093/jxb/erp118. [DOI] [PubMed] [Google Scholar]
- Borland AM, Leverett A, Hurtado-Castano N, Hu R, Yang X.. 2018. Functional anatomical traits of the photosynthetic organs of plants with crassulacean acid metabolism. In: Adams WW III, Terashima I, eds. The leaf: a platform for performing photosynthesis. Advances in photosynthesis and respiration, vol. 44. Berlin: Springer, 281–305. [Google Scholar]
- Bräutigam A, Schlüter U, Eisenhut M, Gowik U.. 2017. On the evolutionary origin of CAM photosynthesis. Plant Physiology 174: 473–477. doi: 10.1104/pp.17.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyns PV, Hanáček P, Klak C.. 2019. Crassula, insights into an old, arid-adapted group of southern African leaf-succulents. Molecular Phylogenetics and Evolution 131: 35–47. doi: 10.1016/j.ympev.2018.10.045. [DOI] [PubMed] [Google Scholar]
- Bruyns PV, Klak C, Hanáček P.. 2015. Recent radiation of Brachystelma and Ceropegia (Apocynaceae) across the Old World against a background of climatic change. Molecular Phylogenetics and Evolution 90: 49–66. doi: 10.1016/j.ympev.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Campbell CD, Sage RF.. 2002. Interactions between atmospheric CO2 concentration and phosphorus nutrition on the formation of proteoid roots in white lupin (Lupinus albus L.): effects of [CO2] and P on proteoid root formation in L. albus. Plant, Cell and Environment 25: 1051–1059. [Google Scholar]
- Cerling TE, Harris JM, MacFadden BJ, et al. 1997. Global vegetation change through the Miocene/Pliocene boundary. Nature 389: 153–158. doi: 10.1038/38229. [DOI] [Google Scholar]
- Cernusak LA, Ubierna N, Winter K, Holtum JAM, Marshall JD, Farquhar GD.. 2013. Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytologist 200: 950–965. doi: 10.1111/nph.12423. [DOI] [PubMed] [Google Scholar]
- Ceusters J, Borland AM.. 2010. Impacts of elevated CO2 on the growth and physiology of plants with crassulacean acid metabolism. In: Lüttge UE, Beyschlag W, Büdel B, Francis D, eds. Progress in botany 72. Berlin: Springer, 163–181. [Google Scholar]
- Ceusters J, Borland AM, Londers E, Verdoodt V, Godts C, De Proft MP.. 2008. Diel shifts in carboxylation pathway and metabolite dynamics in the CAM Bromeliad Aechmea ‘Maya’ in response to elevated CO2. Annals of Botany 102: 389–397. doi: 10.1093/aob/mcn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin FS, Schulze E, Mooney HA.. 1990. The ecology and economics of storage in plants. Annual Review of Ecology and Systematics 21: 423–447. doi: 10.1146/annurev.es.21.110190.002231. [DOI] [Google Scholar]
- Charles-Dominique T, Davies TJ, Hempson GP, et al. 2016. Spiny plants, mammal browsers, and the origin of African savannas. Proceedings of the National Academy of Sciences of the United States of America 113: E5572–E5579. doi: 10.1073/pnas.1607493113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin P-A, Besnard G, Samaritani E, et al. 2008. Oligocene CO2 decline promoted C4 photosynthesis in grasses. Current Biology 18: 37–43. doi: 10.1016/j.cub.2007.11.058. [DOI] [PubMed] [Google Scholar]
- Christin P-A, Osborne CP, Chatelet DS, et al. 2013. Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proceedings of the National Academy of Sciences of the United States of America 110: 1381–1386. doi: 10.1073/pnas.1216777110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin P-A, Osborne CP, Sage RF, Arakaki M, Edwards EJ.. 2011. C4 eudicots are not younger than C4 monocots. Journal of Experimental Botany 62: 3171–3181. doi: 10.1093/jxb/err041. [DOI] [PubMed] [Google Scholar]
- Crayn DM, Winter K, Schulte K, Smith JAC.. 2015. Photosynthetic pathways in Bromeliaceae: phylogenetic and ecological significance of CAM and C3 based on carbon isotope ratios for 1893 species. Botanical Journal of the Linnean Society 178: 169–221. doi: 10.1111/boj.12275. [DOI] [Google Scholar]
- Crayn DM, Winter K, Smith JAC.. 2004. Multiple origins of crassulacean acid metabolism and the epiphytic habit in the Neotropical family Bromeliaceae. Proceedings of the National Academy of Sciences of the United States of America 101: 3703–3708. doi: 10.1073/pnas.0400366101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Miller PM, Nobel PS.. 1993. CO2 exchange and growth of the crassulacean acid metabolism plant Opuntia ficus-indica under elevated CO2 in open-top chambers. Plant Physiology 103: 519–524. doi: 10.1104/pp.103.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Nobel PS.. 1994. Gas exchange and growth responses to elevated CO2 and light levels in the CAM species Opuntia ficus-indica. Plant, Cell and Environment 17: 935–944. doi: 10.1111/j.1365-3040.1994.tb00322.x. [DOI] [Google Scholar]
- Drennan PM, Nobel PS.. 2000. Responses of CAM species to increasing atmospheric CO2 concentrations. Plant, Cell & Environment 23: 767–781. [Google Scholar]
- Edwards EJ. 2019. Evolutionary trajectories, accessibility and other metaphors: the case of C4 and cam photosynthesis. New Phytologist 223: 1742–1755. doi: 10.1111/nph.15851. [DOI] [PubMed] [Google Scholar]
- Edwards EJ. 2023. Reconciling continuous and discrete models of C4 and CAM evolution. Annals of Botany, this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EJ, Ogburn RM.. 2012. Angiosperm responses to a low-CO2 world: CAM and C4 photosynthesis as parallel evolutionary trajectories. International Journal of Plant Sciences 173: 724–733. doi: 10.1086/666098. [DOI] [Google Scholar]
- Edwards EJ, Osborne CP, Stromberg CAE, et al. ; C4 Grasses Consortium. 2010. The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328: 587–591. doi: 10.1126/science.1177216. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Smith SA.. 2010. Phylogenetic analyses reveal the shady history of C4 grasses. Proceedings of the National Academy of Sciences of the United States of America 107: 2532–2537. doi: 10.1073/pnas.0909672107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer JR, Cerling TE, Helliker BR.. 1997. C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112: 285–299. doi: 10.1007/s004420050311. [DOI] [PubMed] [Google Scholar]
- Ehleringer J, Sage R, Flanagan L, Pearcy R.. 1991. Climate change and the evolution of C4 photosynthesis. Trends in Ecology & Evolution 6: 95–99. [DOI] [PubMed] [Google Scholar]
- Foster GL, Royer DL, Lunt DJ.. 2017. Future climate forcing potentially without precedent in the last 420 million years. Nature Communications 8: 14845. doi: 10.1038/ncomms14845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Adams MA, Amthor JS, et al. 2013. Sensitivity of plants to changing atmospheric CO2 concentration: from the geological past to the next century. New Phytologist 197: 1077–1094. doi: 10.1111/nph.12104. [DOI] [PubMed] [Google Scholar]
- Gamisch A, Winter K, Fischer GA, Comes HP.. 2021. Evolution of crassulacean acid metabolism (CAM) as an escape from ecological niche conservatism in Malagasy Bulbophyllum (Orchidaceae). New Phytologist 231: 1236–1248. doi: 10.1111/nph.17437. [DOI] [PubMed] [Google Scholar]
- Gerhart LM, Ward JK.. 2010. Plant responses to low [CO2] of the past. New Phytologist 188: 674–695. doi: 10.1111/j.1469-8137.2010.03441.x. [DOI] [PubMed] [Google Scholar]
- Gessner F. 1956. Wasserspeicherung und Wasserverschiebung. In: Ruhland W, ed. Handbuch der Pflanzenphysiologie 3. Berlin: Springer, 247–256. [Google Scholar]
- Gilman IS, Smith JAC, Holtum JAM, et al. 2023. The CAM lineages of planet Earth. Annals of Botany (this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givnish TJ, Barfuss MHJ, Ee BV, et al. 2014. Adaptive radiation, correlated and contingent evolution, and net species diversification in Bromeliaceae. Molecular Phylogenetics and Evolution 71: 55–78. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Spalink D, Ames M, et al. 2015. Orchid phylogenomics and multiple drivers of their extraordinary diversification. Proceedings of the Royal Society B: Biological Sciences 282: 20151553. doi: 10.1098/rspb.2015.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good-Avila SV, Souza V, Gaut BS, Eguiarte LE.. 2006. Timing and rate of speciation in Agave (Agavaceae). Proceedings of the National Academy of Sciences of the United States of America 103: 9124–9129. doi: 10.1073/pnas.0603312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace OM. 2019. Succulent plant diversity as natural capital. Plants, People, Planet, 1: 336–345. doi: 10.1002/ppp3.25. [DOI] [Google Scholar]
- Grace OM, Buerki S, Symonds MR, et al. 2015. Evolutionary history and leaf succulence as explanations for medicinal use in aloes and the global popularity of Aloe vera. BMC Evolutionary Biology 15: 29. doi: 10.1186/s12862-015-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham EA, Nobel PS.. 1996. Long-term effects of a doubled atmospheric CO2 concentration on the CAM species Agave deserti. Journal of Experimental Botany 47: 61–69. doi: 10.1093/jxb/47.1.61. [DOI] [Google Scholar]
- Griffiths H. 1989. Carbon dioxide concentrating mechanisms and the evolution of CAM in vascular epiphytes. In: Lüttge U, ed. Vascular plants as epiphytes: evolution and ecophysiology. Berlin: Springer, 42–86. [Google Scholar]
- Griffiths H, Males J.. 2017. Succulent plants. Current Biology 27: R890–R896. doi: 10.1016/j.cub.2017.03.021. [DOI] [PubMed] [Google Scholar]
- Griffiths H, Meyer MT, Rickaby REM.. 2017. Overcoming adversity through diversity: aquatic carbon concentrating mechanisms. Journal of Experimental Botany 68: 3689–3695. doi: 10.1093/jxb/erx278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths H, Weller G, Toy LFM, Dennis RJ.. 2013. You’re so vein: bundle sheath physiology, phylogeny and evolution in C3 and C4 plants: origins and function of bundle sheath in C3 plants. Plant, Cell and Environment 36: 249–261. doi: 10.1111/j.1365-3040.2012.02585.x. [DOI] [PubMed] [Google Scholar]
- Guo J, Xu W, Hu Y, et al. 2020. Phylotranscriptomics in Cucurbitaceae reveal multiple whole-genome duplications and key morphological and molecular innovations. Molecular Plant 13: 1117–1133. doi: 10.1016/j.molp.2020.05.011. [DOI] [PubMed] [Google Scholar]
- Guralnick LJ, Ting IP, Lord EM.. 1986. Crassulacean acid metabolism in the Gesneriaceae. American Journal of Botany 73: 336–345. doi: 10.1002/j.1537-2197.1986.tb12046.x. [DOI] [Google Scholar]
- Gustafsson AL, Verola CF, Antonelli A.. 2010. Reassessing the temporal evolution of orchids with new fossils and a Bayesian relaxed clock, with implications for the diversification of the rare South American genus Hoffmannseggela (Orchidaceae: Epidendroideae). BMC Evolutionary Biology 10: 177. doi: 10.1186/1471-2148-10-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Ortega JS, Yamamoto T, Vovides AP, et al. 2018. Aridification as a driver of biodiversity: a case study for the cycad genus Dioon (Zamiaceae). Annals of Botany 121: 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley AJ, Chong G, Houston J, Mather AE.. 2005. 150 million years of climate stability: evidence from the Atacama Desert, northern Chile. Journal of the Geological Society, London 162: 421–424. [Google Scholar]
- Hearn DJ, Evans M, Wolf B, McGinty M, Wen J.. 2018. Dispersal is associated with morphological innovation, but not increased diversification, in Cyphostemma (Vitaceae): dispersal and morphological evolution. Journal of Systematics and Evolution 56: 340–359. doi: 10.1111/jse.12417. [DOI] [Google Scholar]
- Heckmann D, Schulze S, Denton A, et al. 2013. Predicting C4 photosynthesis evolution: modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell 153: 1579–1588. doi: 10.1016/j.cell.2013.04.058. [DOI] [PubMed] [Google Scholar]
- Hernández-Hernández T, Brown JW, Schlumpberger BO, Eguiarte LE, Magallón S.. 2014. Beyond aridification: multiple explanations for the elevated diversification of cacti in the New World succulent biome. New Phytologist 202: 1382–1397. doi: 10.1111/nph.12752. [DOI] [PubMed] [Google Scholar]
- Heyduk K. 2022. Evolution of crassulacean acid metabolism in response to the environment: past, present, and future. Plant Physiology 190: 19–30. doi: 10.1093/plphys/kiac303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyduk K, McKain MR, Lalani F, Leebens-Mack J.. 2016. Evolution of a CAM anatomy predates the origins of crassulacean acid metabolism in the Agavoideae (Asparagaceae). Molecular Phylogenetics and Evolution 105: 102–113. doi: 10.1016/j.ympev.2016.08.018. [DOI] [PubMed] [Google Scholar]
- Holtum JAM. 2023. Klaus Winter – the indefatigable CAM experimentalist. Annals of Botany (this issue): mcad067. doi: 10.1093/aob/mcad067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtum JAM, Hancock LP, Edwards EJ, et al. 2016. Australia lacks stem succulents but is it depauperate in plants with crassulacean acid metabolism (CAM)? Current Opinion in Plant Biology 31: 109–117. doi: 10.1016/j.pbi.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Holtum JAM, Winter K.. 1999. Degrees of crassulacean acid metabolism in tropical epiphytic and lithophytic ferns. Australian Journal of Plant Physiology 26: 749–757. [Google Scholar]
- Horn JW, Xi Z, Riina R, et al. 2014. Evolutionary bursts in Euphorbia (Euphorbiaceae) are linked with photosynthetic pathway: evolutionary bursts in Euphorbia. Evolution 68: 3485–3504. doi: 10.1111/evo.12534. [DOI] [PubMed] [Google Scholar]
- Hotta CT, Gardner MJ, Hubbard KE, et al. 2007. Modulation of environmental responses of plants by circadian clocks. Plant, Cell and Environment 30: 333–349. doi: 10.1111/j.1365-3040.2006.01627.x. [DOI] [PubMed] [Google Scholar]
- Hultine KR, Hernández-Hernández T, Williams DG, et al. 2023. Global change impacts on cacti (Cactaceae): current threats, challenges and conservation solutions. Annals of Botany (this issue): mcad040. doi: 10.1093/aob/mcad040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo C. 2023. The evolution of extant South American tropical biomes. New Phytologist 239: 477–493. doi: 10.1111/nph.18931. [DOI] [PubMed] [Google Scholar]
- Jiménez-Barron O, García-Sandoval R, Magallón S, et al. 2020. Phylogeny, diversification rate, and divergence time of Agave sensu lato (Asparagaceae), a group of recent origin in the process of diversification. Frontiers in Plant Science 11: 536135. doi: 10.3389/fpls.2020.536135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens N, Oncken I, Oldeland J, Gunter F, Rudolh B.. 2021. Welwitschia: phylogeography of a living fossil, diversified within a desert refuge. Scientific Reports 11: 2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamrani YY, Shomali A, Aliniaeifard S, et al. 2022. Regulatory role of circadian clocks on ABA production and signaling, stomatal responses, and water-use efficiency under water-deficit conditions. Cells 11: 1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley JE. 1998. CAM photosynthesis in submerged aquatic plants. The Botanical Review 64: 121–175. doi: 10.1007/bf02856581. [DOI] [Google Scholar]
- Keeley JE, Rundel PW.. 2003. Evolution of CAM and C4 carbon-concentrating mechanisms. International Journal of Plant Sciences 164: S55–S77. doi: 10.1086/374192. [DOI] [Google Scholar]
- Klak C, Hanáček P, Bruyns PV.. 2017. Out of southern Africa: origin, biogeography and age of the Aizooideae (Aizoaceae). Molecular Phylogenetics and Evolution 109: 203–216. doi: 10.1016/j.ympev.2016.12.016. [DOI] [PubMed] [Google Scholar]
- Klak C, Reeves G, Hedderson T.. 2004. Unmatched tempo of evolution in Southern African semi-desert ice plants. Nature 427: 63–65. doi: 10.1038/nature02243. [DOI] [PubMed] [Google Scholar]
- Kluge M, Ting IP.. 1978. Crassulacean acid metabolism. Analysis of an ecological adaptation. Berlin: Springer. [Google Scholar]
- Kocacinar F, Sage RF.. 2004. Photosynthetic pathway alters hydraulic structure and function in woody plants. Oecologia 139: 214–223. doi: 10.1007/s00442-004-1517-3. [DOI] [PubMed] [Google Scholar]
- Lambers H, Chapin FS III, Pons TL.. 2008. Plant physiological ecology, 2nd edn. Berlin: Springer. [Google Scholar]
- Leverett A, Hartzell S, Winter K, et al. 2023. Dissecting succulence: crassulacean acid metabolism and hydraulic capacitance are independent adaptations in Clusia leaves. Plant, Cell and Environment 46: 1472–1488. doi: 10.1111/pce.14539. [DOI] [PubMed] [Google Scholar]
- Li Z-Z, Lehtonen S, Martins K, et al. 2020. Phylogenomics of the aquatic plant genus Ottelia (Hydrocharitaceae): implications for historical biogeography. Molecular Phylogenetics and Evolution 152: 106939. doi: 10.1016/j.ympev.2020.106939. [DOI] [PubMed] [Google Scholar]
- Li M-H, Liu D-K, Zhang G-Q, et al. 2019. A perspective on crassulacean acid metabolism photosynthesis evolution of orchids on different continents: Dendrobium as a case study. Journal of Experimental Botany 70: 6611–6619. doi: 10.1093/jxb/erz461. [DOI] [PubMed] [Google Scholar]
- Liede-Schumann S, Reuss SJ, Meve U, et al. 2022. Phylogeny of Marsdenieae (Apocynaceae, Asclepiadoideae) based on chloroplast and nuclear loci, with a conspectus of the genera. Taxon 71: 833–875. doi: 10.1002/tax.12713. [DOI] [Google Scholar]
- Liu X-Q, Ickert-Bond SM, Chen L-Q, Wen J.. 2013. Molecular phylogeny of Cissus L. of Vitaceae (the grape family) and evolution of its pantropical intercontinental disjunctions. Molecular Phylogenetics and Evolution 66: 43–53. doi: 10.1016/j.ympev.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Lösch R. 1984. Species-specific responses to temperature in acid metabolism and gas exchange performance of Macronesian Sempervivoideae. In: Margalis NS, Arianoustou-Farragitaki M, Oechel WC, eds. Tasks for vegetation science, vol. 13. The Hague: W. Junk, 117–127. [Google Scholar]
- Lu M, Fradera-Soler M, Forest F, Barraclough TG, Grace OM.. 2022. Evidence linking life-form to a major shift in diversification rate in Crassula. American Journal of Botany 109: 272–290. doi: 10.1002/ajb2.1797. [DOI] [PubMed] [Google Scholar]
- Luján M, Leverett A, Winter K.. 2023. Forty years of research into crassulacean acid metabolism in the genus Clusia: anatomy, ecophysiology and evolution. Annals of Botany (this issue) doi: 10.1093/aob/mcad039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luján M, Oleas NH, Winter K.. 2022. Evolutionary history of CAM photosynthesis in neotropical Clusia: insights from genomics, anatomy, physiology and climate. Botanical Journal of the Linnean Society 199: 538–556. doi: 10.1093/botlinnean/boab075. [DOI] [Google Scholar]
- Lundgren MR, Besnard G, Ripley BS, et al. 2015. Photosynthetic innovation broadens the niche within a single species. Ecology Letters 18: 1021–1029. doi: 10.1111/ele.12484. [DOI] [PubMed] [Google Scholar]
- Lüttge U. 2004. Ecophysiology of Crassulacean Acid Metabolism (CAM). Annals of Botany 93: 629–652. doi: 10.1093/aob/mch087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U, ed.2007a. Clusia: a woody neotropical genus of remarkable plasticity and diversity. Berlin: Springer. [Google Scholar]
- Lüttge U. 2007b. Photosynthesis. In: Lüttge U, ed. Clusia: a woody neotropical genus of remarkable plasticity and diversity. Berlin: Springer, 135–186. [Google Scholar]
- Lüttge U, Stimmel K-H, Smith JAC, Griffiths H.. 1986. Comparative ecophysiology of CAM and C3 bromeliads. II. Field measurements of gas exchange of CAM bromeliads in the humid tropics. Plant, Cell and Environment 9: 377–383. [Google Scholar]
- Males J, Griffiths H.. 2017a. Stomatal biology of CAM plants. Plant Physiology 174: 550–560. doi: 10.1104/pp.17.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Males J, Griffiths H.. 2017b. Specialized stomatal humidity responses underpin ecological diversity in C3 bromeliads. Plant, Cell and Environment 40: 2931–2945. doi: 10.1111/pce.13024. [DOI] [PubMed] [Google Scholar]
- Mallmann J, Heckmann D, Bräutigam A, et al. 2014. The role of photorespiration during the evolution of C4 photosynthesis in the genus Flaveria. eLife 3: e02478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CE. 1996. Putative causes and consequences of recycling CO2 via crassulacean acid metabolism. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer, 192–203. [Google Scholar]
- Massoni J, Couveur TLP, Saquet H.. 2015. Five major shifts of diversification through the long evolutionary history of Magnoliidae (angiosperms). BMC Evolutionary Biology 15: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, von Caemmerer S, Evans JR.. 1997. Is a Low Internal Conductance to CO2 Diffusion a Consequence of Succulence in Plants with Crassulacean Acid Metabolism? Australian Journal of Plant Physiology 24: 777–786. [Google Scholar]
- McCann HC, Sage RF.. 2022. Seed size effects on plant establishment under low atmospheric CO2, with implications for seed size evolution. Annals of Botany 130: 825–834. doi: 10.1093/aob/mcac112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmid TFE, Abrahamczyk S, Bañares-Baudet A, et al. 2023. Inter- and intra-island speciation and their morphological and ecological correlates in Aeonium (Crassulaceae), a species-rich Macaronesian radiation. Annals of Botany 131: 697–721. doi: 10.1093/aob/mcad033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmid TFE, Klein JT, Kadereit G, Kadereit JW.. 2020. Linnaeus’s folly – phylogeny, evolution and classification of Sedum (Crassulaceae) and Crassulaceae subfamily Sempervivoideae. Taxon 69: 892–926. [Google Scholar]
- Messerschmid TFE, Wehling J, Bobon N, et al. 2021. Carbon isotope composition of plant photosynthetic tissues reflects a crassulacean acid metabolism (CAM) continuum in the majority of CAM lineages. Perspectives in Plant Ecology, Evolution and Systematics 51: 125619. [Google Scholar]
- Mills BJW, Krause AJ, Scotese CR, Hill DJ, Shields GA, Lenton TM.. 2019. Modelling the long-term carbon cycle, atmospheric CO2, and Earth surface temperature from late Neoproterozoic to present day. Gondwana Research 67: 172–186. [Google Scholar]
- Mok D, Leung A, Searles P, Sage TL, Sage RF.. 2023. CAM photosynthesis in Bulnesia retama (Zygophyllaceae), a non-succulent desert shrub from South America. Annals of Botany (this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson RK, Rawsthorne S.. 2000. Carbon dioxide assimilation in C3-C4 intermediate plants. In: Leegood RC, Sharkey TD, von Caemmerer S, eds. Photosynthesis: physiology and metabolism. Advances in photosynthesis, vol. 9. Dordrecht: Kluwer Academic, 533–550. [Google Scholar]
- Morgan JA, LeCain DR, Pendall E, et al. 2011. C4 grasses prosper as carbon dioxide eliminates desiccation in warmed semi-arid grassland. Nature 476: 202–205. doi: 10.1038/nature10274. [DOI] [PubMed] [Google Scholar]
- Morgan JA, Pataki DE, Körner C, et al. 2004. Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia 140: 11–25. doi: 10.1007/s00442-004-1550-2. [DOI] [PubMed] [Google Scholar]
- Naumann J, Salomo K, Der JP, et al. 2013. Single-copy nuclear genes place haustorial Hydnoraceae within Piperales and reveal a Cretaceous origin of multiple parasitic angiosperm lineages. PLoS One 8: e79204. doi: 10.1371/journal.pone.0079204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EA, Sage RF.. 2008. Functional constraints of CAM leaf anatomy: tight cell packing is associated with increased CAM function across a gradient of CAM expression. Journal of Experimental Botany 59: 1841–1850. doi: 10.1093/jxb/erm346. [DOI] [PubMed] [Google Scholar]
- Nelson EA, Sage TL, Sage RF.. 2005. Functional leaf anatomy of plants with crassulacean acid metabolism. Functional Plant Biology 32: 409–419. doi: 10.1071/FP04195. [DOI] [PubMed] [Google Scholar]
- Nobel PS. 1988. Environmental biology of agaves and cacti. Cambridge: Cambridge University Press. [Google Scholar]
- Nobel PS, Hartsock TL.. 1986. Short-term and long-term responses of crassulacean acid metabolism plants to elevated CO2. Plant Physiology 82: 604–606. doi: 10.1104/pp.82.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyffeler R, Eggli U.. 2010. Disintegrating Portulacaceae: a new familial classification of the suborder Portulacineae (Caryophyllales) based on molecular and morphological data. Taxon 59: 227–240. doi: 10.1002/tax.591021. [DOI] [Google Scholar]
- Ogburn R, Edwards E.. 2010. The ecological water-use strategies of succulent plants. Advances in Botanical Research 55: 180–223. [Google Scholar]
- Osborne CP, Freckleton RP.. 2009. Ecological selection pressures for C4 photosynthesis in the grasses. Proceedings of the Royal Society B: Biological Sciences 276: 1753–1760. doi: 10.1098/rspb.2008.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CP, Sack L.. 2012. Evolution of C4 plants: a new hypothesis for an interaction of CO2 and water relations mediated by plant hydraulics. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 583–600. doi: 10.1098/rstb.2011.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond CB. 1978. Crassulacean acid metabolism: a curiosity in context. Annual Review of Plant Physiology 29: 379–414. doi: 10.1146/annurev.pp.29.060178.002115. [DOI] [Google Scholar]
- Pelser PB, Kennedy AH, Tepe EJ, et al. 2010. Patterns and causes of incongruence between plastid and nuclear Senecioneae (Asteraceae) phylogenies. American Journal of Botany 97: 856–873. doi: 10.3732/ajb.0900287. [DOI] [PubMed] [Google Scholar]
- Pereira PN, Niechayev NA, Blair BB, Cushman JC.. 2021. Climate change responses and adaptations in crassulacean acid metabolism (CAM) plants. In: Becklin KM, Ward JK, Way DA, eds. Photosynthesis, respiration and climate change. Advances in photosynthesis and respiration, vol. 48. Berlin: Springer, 283–329. [Google Scholar]
- Petrova G, Moyankova D, Nishii K, et al. 2015. The European paleoendemic Haberlea rhodopensis (Gesneriaceae) has an Oligocene origin and a Pleistocene diversification and occurs in a long-persisting refugial area in southeastern Europe. International Journal of Plant Sciences 176: 499–514. doi: 10.1086/681990. [DOI] [Google Scholar]
- Pilon-Smits EAH, ‘t Hart H, van Brederode J.. 1996. Evolutionary aspects of crassulacean acid metabolism in the Crassulaceae. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer, 349–359. [Google Scholar]
- Polley HW, Mayeux HS, Johnson HB, Tischler CR.. 1997. Viewpoint: atmospheric CO2, soil water, and shrub/grass ratios on rangelands. Rangeland Ecology & Management/Journal of Range Management Archives 50: 278–284. [Google Scholar]
- Rae JWB, Zhang YG, Liu X, Foster GL, Stoll HM, Whiteford RDM.. 2021. Atmospheric CO2 over the past 66 million years from marine archives. Annual Review of Earth and Planetary Sciences 49: 609–641. [Google Scholar]
- Raven JA, Cockell CS, De La Rocha CL.. 2008. The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 2641–2650. doi: 10.1098/rstb.2008.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA, Beardall J, Sánchez-Baracaldo P.. 2017. The possible evolution and future of CO2-concentrating mechanisms. Journal of Experimental Botany 68: 3701–3716. doi: 10.1093/jxb/erx110. [DOI] [PubMed] [Google Scholar]
- Raven JA, Spicer RA.. 1996. The evolution of crassulacean acid metabolism. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer, 360–385. [Google Scholar]
- Roalson EH, Roberts WR.. 2016. Distinct processes drive diversification in different clades of Gesneriaceae. Systematic Biology 65: 662–684. doi: 10.1093/sysbio/syw012. [DOI] [PubMed] [Google Scholar]
- Ruhfel BR, Bove CP, Philbrick CT, Davis CC.. 2016. Dispersal largely explains the Gondwanan distribution of the ancient tropical clusioid plant clade. American Journal of Botany 103: 1117–1128. doi: 10.3732/ajb.1500537. [DOI] [PubMed] [Google Scholar]
- Sage RF. 1994. Acclimation of photosynthesis to increasing atmospheric CO2: the gas-exchange perspective. Photosynthesis Research 39: 351–368. doi: 10.1007/BF00014591. [DOI] [PubMed] [Google Scholar]
- Sage RF. 1995. Was low atmospheric CO2 during the Pleistocene a limiting factor for the origin of agriculture? Global Change Biology 1: 93–106. doi: 10.1111/j.1365-2486.1995.tb00009.x. [DOI] [Google Scholar]
- Sage RF. 2001. Environmental and evolutionary preconditions for the origin and diversification of the C4 photosynthetic syndrome. Plant Biology 3: 202–213. doi: 10.1055/s-2001-15206. [DOI] [Google Scholar]
- Sage RF. 2002. Are crassulacean acid metabolism and C4 photosynthesis incompatible? Functional Plant Biology 29: 775–785. doi: 10.1071/PP01217. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2013. Photorespiratory compensation: a driver for biological diversity. Plant Biology 15: 624–638. doi: 10.1111/plb.12024. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2016. A portrait of the C4 photosynthetic family on the 50th anniversary of its discovery: species number, evolutionary lineages, and Hall of Fame. Journal of Experimental Botany 67: 4039–4056. doi: 10.1093/jxb/erw156. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2020. Global change biology: a primer. Global Change Biology 26: 3–30. doi: 10.1111/gcb.14893. [DOI] [PubMed] [Google Scholar]
- Sage RF, Coleman JR.. 2001. Effects of low atmospheric CO2 on plants: more than a thing of the past. Trends in Plant Science 6: 18–24. doi: 10.1016/s1360-1385(00)01813-6. [DOI] [PubMed] [Google Scholar]
- Sage RF, Monson RK, Ehleringer JR, Adachi S, Pearcy RW.. 2018. Some like it hot: the physiological ecology of C4 plant evolution. Oecologia 187: 941–966. doi: 10.1007/s00442-018-4191-6. [DOI] [PubMed] [Google Scholar]
- Sage RF, Stata M.. 2015. Photosynthetic diversity meets biodiversity: the C4 plant example. Journal of Plant Physiology 172: 104–119. doi: 10.1016/j.jplph.2014.07.024. [DOI] [PubMed] [Google Scholar]
- Sage RF, Stata M.. 2021. Terrestrial CO-concentrating mechanisms in a high CO2 world. In: Becklin KM, Ward JK, Way DA, eds. Photosynthesis, respiration and climate change. Advances in photosynthesis and respiration 48. Berlin: Springer, 193–250. [Google Scholar]
- Sage RF, Santrucek J, Grise DJ.. 1995. Temperature effects on the photosynthetic response of C3 plants to long-term CO2 enrichment. Vegetatio 121: 67–77. doi: 10.1007/bf00044673. [DOI] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F.. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63: 19–47. doi: 10.1146/annurev-arplant-042811-105511. [DOI] [PubMed] [Google Scholar]
- Schulze E-D, Hall AE.. 1982. Stomatal responses, water loss and CO2 assimilation rates of plants in contrasting environments. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, eds. Physiological plant ecology II: water relations and carbon assimilation. Berlin: Springer, 181–230. [Google Scholar]
- Sharkey TD. 1988. Estimating the rate of photorespiration in leaves. Physiologia Plantarum 73: 147–152. doi: 10.1111/j.1399-3054.1988.tb09205.x. [DOI] [Google Scholar]
- Silvera K, Neubig KM, Whitten WM, Williams NH, Winter K, Cushman JC.. 2010a. Evolution along the crassulacean acid metabolism continuum. Functional Plant Biology 37: 995–1010. doi: 10.1071/fp10084. [DOI] [Google Scholar]
- Silvera K, Santiago LS, Cushman JC, Winter K.. 2009. Crassulacean acid metabolism and epiphytism linked to adaptive radiations in the Orchidaceae. Plant Physiology 149: 1838–1847. doi: 10.1104/pp.108.132555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvera K, Santiago LS, Winter K.. 2005. Distribution of crassulacean acid metabolism in orchids of Panama: evidence of selection for weak and strong modes. Functional Plant Biology 32: 397–407. doi: 10.1071/FP04179. [DOI] [PubMed] [Google Scholar]
- Silvera K, Santiago LS, Cushman JC, Winter K.. 2010b. The incidence of crassulacean acid metabolism in Orchidaceae derived from carbon isotope ratios: a checklist of the flora of Panama and Costa Rica. Botanical Journal of the Linnean Society 163: 194–222. doi: 10.1111/j.1095-8339.2010.01058.x. [DOI] [Google Scholar]
- Silvestro D, Zizka G, Schulte K.. 2014. Disentangling the effects of key innovations on the diversification of Bromelioideae (Bromeliaceae): key innovations in Bromelioideae. Evolution 68: 163–175. doi: 10.1111/evo.12236. [DOI] [PubMed] [Google Scholar]
- Simon NML, Graham CA, Comben NE, Hetherington AM, Dodd AN.. 2020. The circadian clock influences the long-term water use efficiency of Arabidopsis. Plant Physiology 183: 317–330. doi: 10.1104/pp.20.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JAC, Ingram J, Tsiantis MS, et al. 1996. Transport across the vacuolar membrane in CAM plants. In: Winter K, Smith, JAC, eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer, 53–71. [Google Scholar]
- Smith SD, Monson RK, Anderson JE.. 1997. Physiological ecology of desert plants. Berlin: Springer. [Google Scholar]
- Smith JAC, Winter K.. 1996. Taxonomic distribution of crassulacean acid metabolism. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer, 427–436. [Google Scholar]
- Spriggs EL, Christin P-A, Edwards EJ.. 2014. C4 photosynthesis promoted species diversification during the Miocene grassland expansion. PLoS One 9: e97722. doi: 10.1371/journal.pone.0097722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhorsdottir M, Jardine PE, Rember WC.. 2021. Near-future pCO2 during the hot Miocene climatic optimum. Paleoceanography and Paleoclimatology 36: e2020–PA003900. [Google Scholar]
- Tipple BJ, Pagani M.. 2007. The early origins of terrestrial C4 photosynthesis. Annual Review of Earth and Planetary Sciences 35: 435–461. doi: 10.1146/annurev.earth.35.031306.140150. [DOI] [Google Scholar]
- van der Kerke SJ, Shrestha B, Ruhlman TA, Weng M-A, Jansen RK, et al. 2019. Plastome based phylogenetics and younger crown age in Pelargonium. Molecular Phylogenetics and Evolution 137: 33–43. [DOI] [PubMed] [Google Scholar]
- Venter N, Cowie BW, Paterson ID, Witkowski ETF, Byrne MJ.. 2022. The interactive effects of CO2 and water on the growth and physiology of the invasive alien vine Pereskia aculeata (Cactaceae): implications for its future invasion and management. Environmental and Experimental Botany 194: 104737. [Google Scholar]
- Vicentini A, Barber JC, Aliscioni SS, Giussani LM, Kellogg EA.. 2008. The age of the grasses and clusters of origins of C4 photosynthesis: clustered origins of C4 photosynthesis. Global Change Biology 14: 2963–2977. doi: 10.1111/j.1365-2486.2008.01688.x. [DOI] [Google Scholar]
- Virzo De Santo A, Alfani A, Russo G, Fioretto A.. 1983. Relationship between CAM and succulence in some species of Vitaceae and Piperaceae. Botanical Gazette 144: 342–346. [Google Scholar]
- Virzo De Santo A, Bartoli G.. 1996. Crassulacean acid metabolism in leaves and stems of Cissus quadrangularis. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer, 216–229. [Google Scholar]
- von Caemmerer S, Quick WP.. 2000. Rubisco: physiology in vivo. In: Leegood RC, Sharkey TD, von Caemmerer S, eds. Photosynthesis: physiology and metabolism. Advances in photosynthesis, vol. 9. Dordrecht: Kluwer Academic, 85–113. [Google Scholar]
- von Willert DJ, Armbrüster N, Drees T, Zaborowski M.. 2005. Welwitschia mirabilis: CAM or not CAM – what is the answer? Functional Plant Biology 32: 389–395. doi: 10.1071/FP01241. [DOI] [PubMed] [Google Scholar]
- von Willert DJ, Brinckmann E, Scheitler B, Schulze E-D, Thomas DA, Treichel S.. 1980. Ökophysiologische Untersuchungen an Pflanzen der Namib-Wüste. Naturwissenschaften 67: 21–28. doi: 10.1007/bf00424499. [DOI] [Google Scholar]
- von Willert DJ, Eller BM, Werger MJA, Brinckmann E, Ihlenfeldt H-D.. 1992. Life strategies of succulents in deserts, with special reference to the Namib desert. Cambridge: Cambridge University Press. [Google Scholar]
- Vovides AP, Etherington JR, Dresser PQ, Groenhof A, Iglesias C, Ramirez JF.. 2002. CAM-cycling in the cycad Dioon edule Lindl. in its natural tropical deciduous forest habitat in central Veracruz, Mexico. Botanical Journal of the Linnean Society 138: 155–162. doi: 10.1046/j.1095-8339.2002.138002155.x. [DOI] [Google Scholar]
- Wagner K, Zotz G.. 2018. Epiphytic bromeliads in a changing world: the effect of elevated CO2 and varying water supply on growth and nutrient relations. Plant Biology 20: 636–640. doi: 10.1111/plb.12708. [DOI] [PubMed] [Google Scholar]
- Wang N, Yang Y, Moore MJ, et al. 2019. Evolution of Portulacineae marked by gene tree conflict and gene family expansion associated with adaptation to harsh environments. Molecular Biology and Evolution 36: 112–126. doi: 10.1093/molbev/msy200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang C-F, Ochieng Odago W, et al. 2023. Evolution of 101 Apocynaceae plastomes and phylogenetic implications. Molecular Phylogenetics and Evolution 180: 107688. doi: 10.1016/j.ympev.2022.107688. [DOI] [PubMed] [Google Scholar]
- Ward JD, Seely MK, Lancaster N.. 1983. On the antiquity of the Namib. South African Journal of Science 79: 175–183. [Google Scholar]
- Wei X, Qi Y, Zhang X, et al. 2017. Phylogeny, historical biogeography and characters evolution of the drought resistant fern Pyrrosia Mirabel (Polypodiaceae) inferred from plastid and nuclear markers. Scientific Reports 7: 12757. doi: 10.1038/s41598-017-12839-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BP, Johnston IG, Covshoff S, Hibberd JM.. 2013. Phenotypic landscape inference reveals multiple evolutionary paths to C4 photosynthesis. eLife 2: e00961. doi: 10.7554/eLife.00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K. 2019. Ecophysiology of constitutive and facultative CAM photosynthesis. Journal of Experimental Botany 70: 6495–6508. doi: 10.1093/jxb/erz002. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM.. 2002. How closely do the δ13C values of crassulacean acid metabolism plants reflect the proportion of CO2 fixed during day and night? Plant Physiology 129: 1843–1851. doi: 10.1104/pp.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Smith JAC.. 1996. Crassulacean acid metabolism: current status and perspectives. In: Winter K, Smith, JAC, eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer, 389–426. [Google Scholar]
- Winter K, Smith JAC.. 2022. CAM photosynthesis: the acid test. New Phytologist 233: 599–609. doi: 10.1111/nph.17790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Richter A, Engelbrecht B, Posada J, Virgo A, Popp M.. 1997. Effect of elevated CO2 on growth and crassulacean-acid-metabolism activity of Kalanchoë pinnata under tropical conditions. Planta 201: 389–396. doi: 10.1007/s004250050081. [DOI] [Google Scholar]
- Winter K, Garcia M, Holtum JAM.. 2014. Nocturnal versus diurnal CO2 uptake: how flexible is Agave angustifolia? Journal of Experimental Botany 65: 3695–3703. doi: 10.1093/jxb/eru097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Holtum JAM, Smith JAC.. 2015. Crassulacean acid metabolism: a continuous or discrete trait? New Phytologist 208: 73–78. doi: 10.1111/nph.13446. [DOI] [PubMed] [Google Scholar]
- Winter K, Zotz G, Baur B, Dietz K-J.. 1992. Light and dark CO2 fixation in Clusia uvitana and the effects of plant water status and CO2 availability. Oecologia 91: 47–51. doi: 10.1007/BF00317239. [DOI] [PubMed] [Google Scholar]
- Wood D, Besnard G, Beerling DJ, Osborne CP, Christin P-A.. 2020. Phylogenomics indicates the ‘living fossil’ Isoetes diversified in the Cenozoic. PLoS One 15: e0227525. doi: 10.1371/journal.pone.0227525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Goldstein G, Bartholomew DP.. 1999. Gas exchange and carbon isotope composition of Ananas comosus in response to elevated CO2 and temperature. Plant, Cell Environment 22: 999–1007. [Google Scholar]
- Zotz G, Andrade JL, Einzmann HJR.. 2023. CAM plants: their importance in epiphyte communities and prospects with global change. Annals of Botany (this issue) mcac158. doi: 10.1093/aob/mcac158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotz G, Bogusch W, Hietz P, Ketteler N.. 2010. Growth of epiphytic bromeliads in a changing world: the effects of CO2, water and nutrient supply. Acta Oecologica 36: 659–665. doi: 10.1016/j.actao.2010.10.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


