Abstract
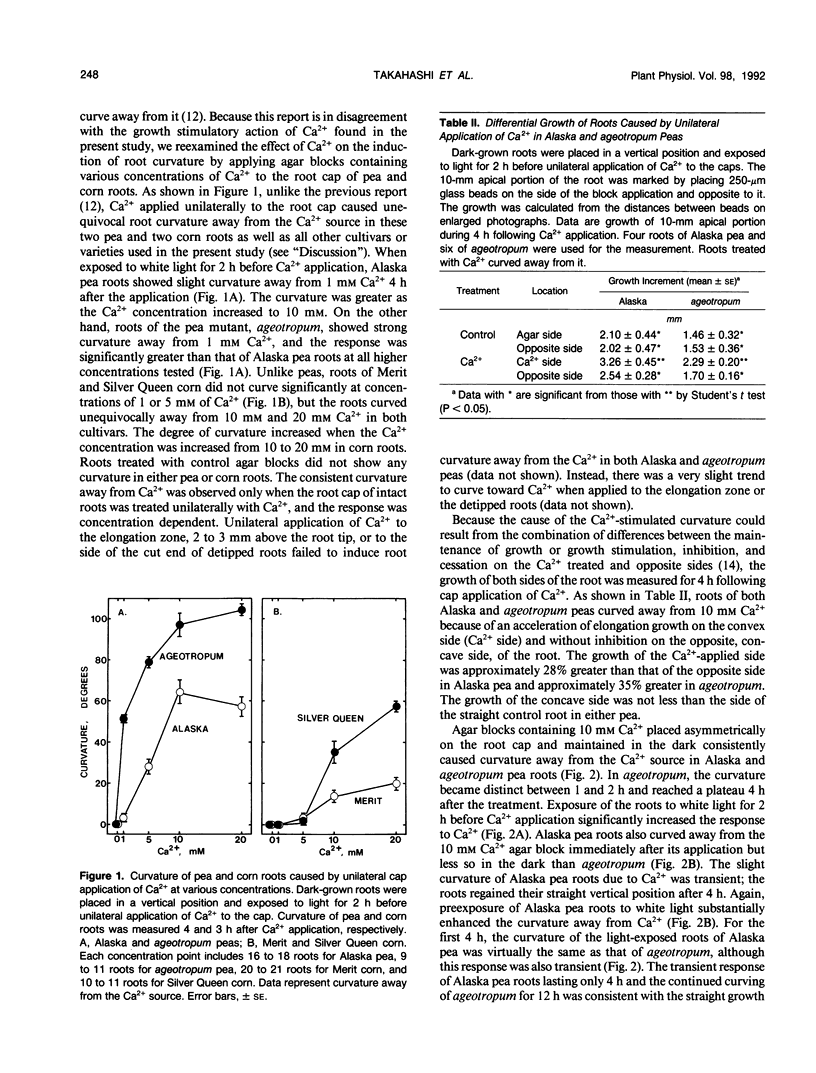
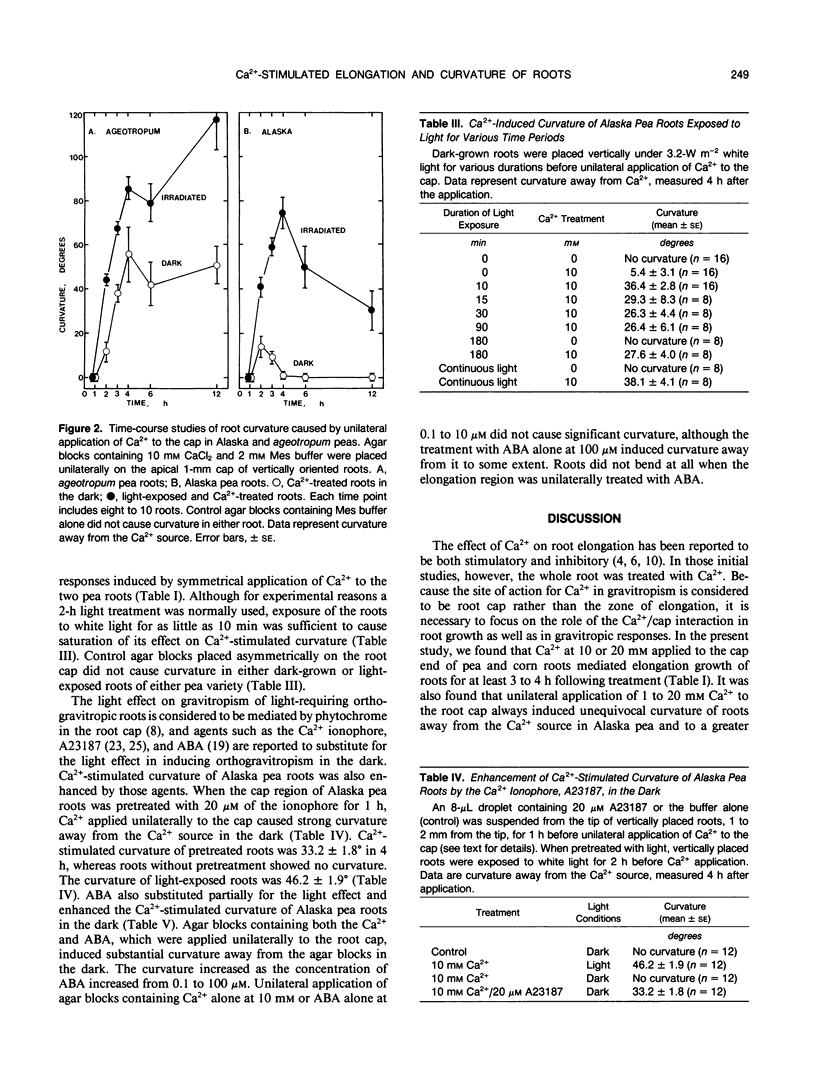
Ca2+ has been proposed to mediate inhibition of root elongation. However, exogenous Ca2+ at 10 or 20 millimolar, applied directly to the root cap, significantly stimulated root elongation in pea (Pisum sativum L.) and corn (Zea mays L.) seedlings. Furthermore, Ca2+ at 1 to 20 millimolar, applied unilaterally to the caps of Alaska pea roots, caused root curvature away from the Ca2+ source, which was caused by an acceleration of elongation growth on the convex side (Ca2+ side) of the roots. Roots of an agravitropic pea mutant, ageotropum, responded to a greater extent. Roots of Merit and Silver Queen corn also responded to Ca2+ in similar ways but required a higher Ca2+ concentration than that of pea roots. Roots of all other cultivars tested (additional four cultivars of pea and one of corn) curved away from the unilateral Ca2+ source as well. The Ca2+-stimulated curvature was substantially enhanced by light. A Ca2+ ionophore, A23187, at 20 micromolar or abscisic acid at 0.1 to 100 micromolar partially substituted for the light effect and enhanced the Ca2+-stimulated curvature in the dark. Unilateral application of Ca2+ to the elongation zone of intact roots or to the cut end of detipped roots caused either no curvature or very slight curvature toward the Ca2+. Thus, Ca2+ action on root elongation differs depending on its site of application. The stimulatory action of Ca2+ may involve an elevation of cytoplasmic Ca2+ in root cap cells and may participate in root tropisms.
Full text
PDF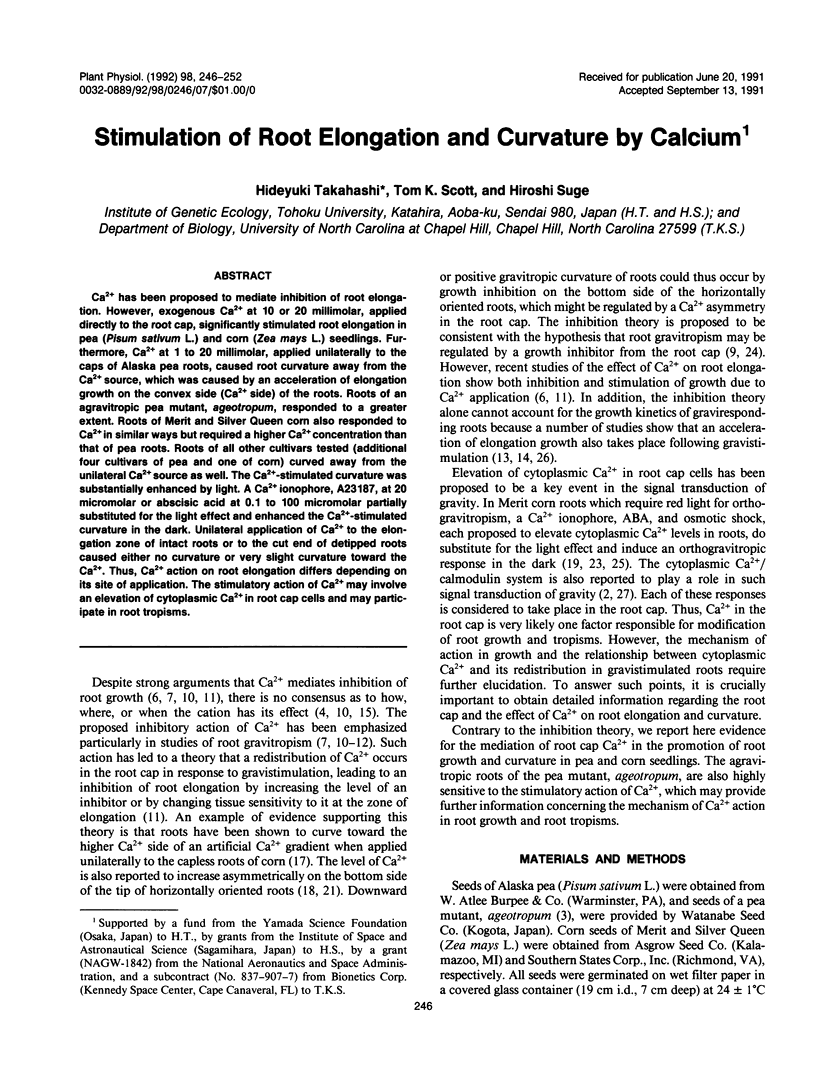




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkman T., Leopold A. C. Effect of inhibitors of auxin transport and of calmodulin on a gravisensing-dependent current in maize roots. Plant Physiol. 1987;84:847–850. doi: 10.1104/pp.84.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L. J. Light-enhanced protein synthesis in gravitropically stimulated root caps of corn. Plant Physiol. 1983 Jul;72(3):833–836. doi: 10.1104/pp.72.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons G. S., Wilkins M. B. Growth inhibitor production by root caps in relation to geotropic responses. Nature. 1970 May 9;226(5245):558–559. doi: 10.1038/226558a0. [DOI] [PubMed] [Google Scholar]
- Hasenstein K. H., Evans M. L. Calcium dependence of rapid auxin action in maize roots. Plant Physiol. 1986;81:439–443. doi: 10.1104/pp.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstein K. H., Evans M. L. Effects of cations on hormone transport in primary roots of Zea mays. Plant Physiol. 1988;86:890–894. doi: 10.1104/pp.86.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstein K. H., Evans M. L., Stinemetz C. L., Moore R., Fondren W. M., Koon E. C., Higby M. A., Smucker A. J. Comparative effectiveness of metal ions in inducing curvature of primary roots of Zea mays. Plant Physiol. 1988;86:885–889. doi: 10.1104/pp.86.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Hasenstein K. H., Evans M. L. Computer-based video digitizer analysis of surface extension in maize roots: kinetics of growth rate changes during gravitropism. Planta. 1991 Feb;183(3):381–390. doi: 10.1007/BF00197737. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Mulkey T. J., Evans M. L. Gravity-Induced Polar Transport of Calcium across Root Tips of Maize. Plant Physiol. 1983 Dec;73(4):874–876. doi: 10.1104/pp.73.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Mulkey T. J., Evans M. L. Reversible loss of gravitropic sensitivity in maize roots after tip application of calcium chelators. Science. 1983 Jun 24;220(4604):1375–1376. doi: 10.1126/science.220.4604.1375. [DOI] [PubMed] [Google Scholar]
- Leopold A. C., LaFavre A. K. Interactions between red light, abscisic acid, and calcium in gravitropism. Plant Physiol. 1989;89:875–878. doi: 10.1104/pp.89.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet B., Pickard B. G. Early wrong-way response occurs in orthogravitropism of maize roots treated with lithium. Physiol Plant. 1988;72:555–559. doi: 10.1111/j.1399-3054.1988.tb09165.x. [DOI] [PubMed] [Google Scholar]
- Perdue D. O., LaFavre A. K., Leopold A. C. Calcium in the regulation of gravitropism by light. Plant Physiol. 1988;86:1276–1280. doi: 10.1104/pp.86.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Reddy A. S. Calcium messenger system in plants. CRC Crit Rev Plant Sci. 1987;6(1):47–103. doi: 10.1080/07352688709382247. [DOI] [PubMed] [Google Scholar]
- Stinemetz C. L., Kuzmanoff K. M., Evans M. L., Jarrett H. W. Correlation between calmodulin activity and gravitropic sensitivity in primary roots of maize. Plant Physiol. 1987;84:1337–1342. doi: 10.1104/pp.84.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Scott T. K. Hydrotropism and its interaction with gravitropism in maize roots. Plant Physiol. 1991;96:558–564. doi: 10.1104/pp.96.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]


