Abstract
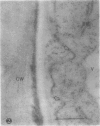
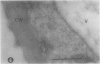
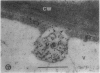
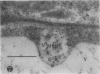
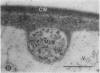
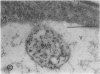
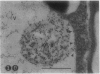
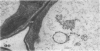
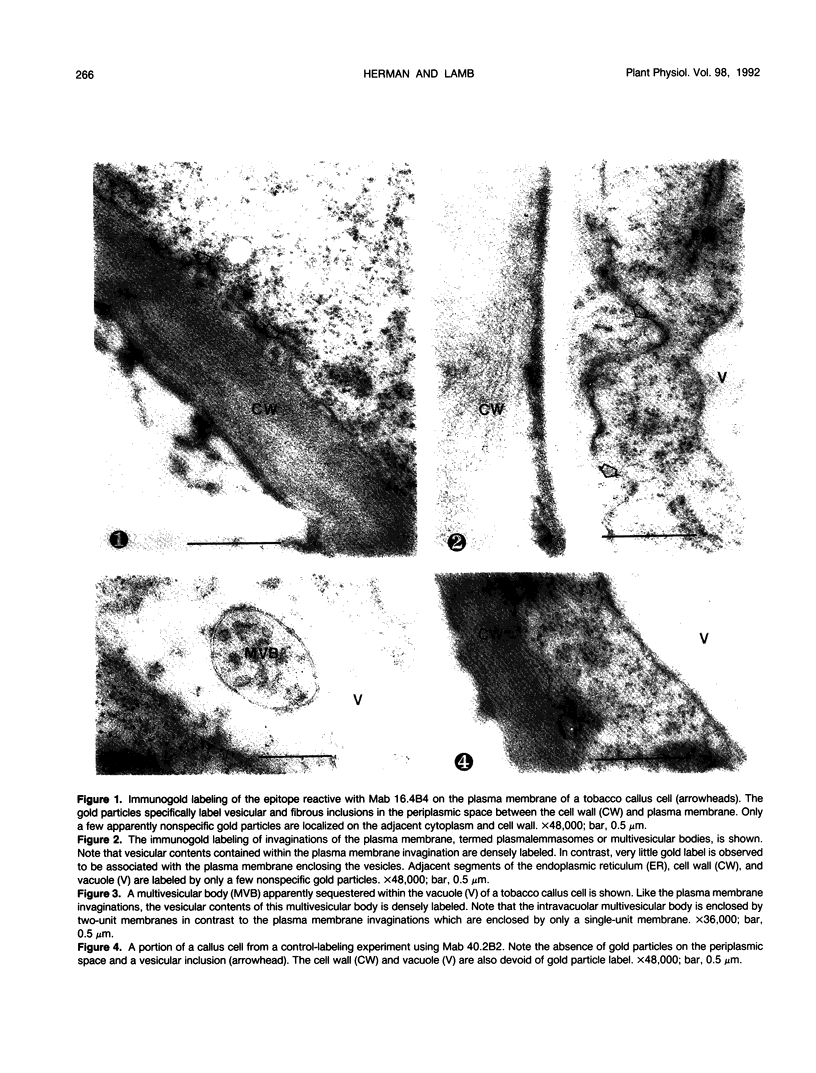
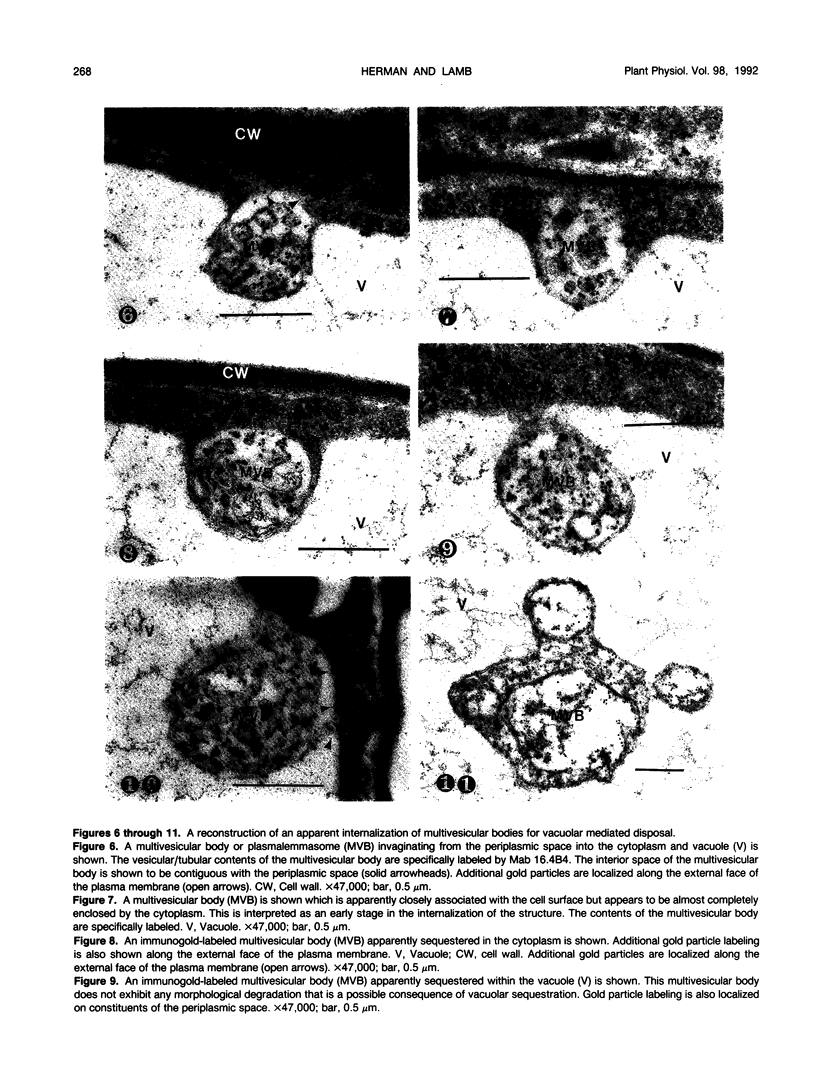
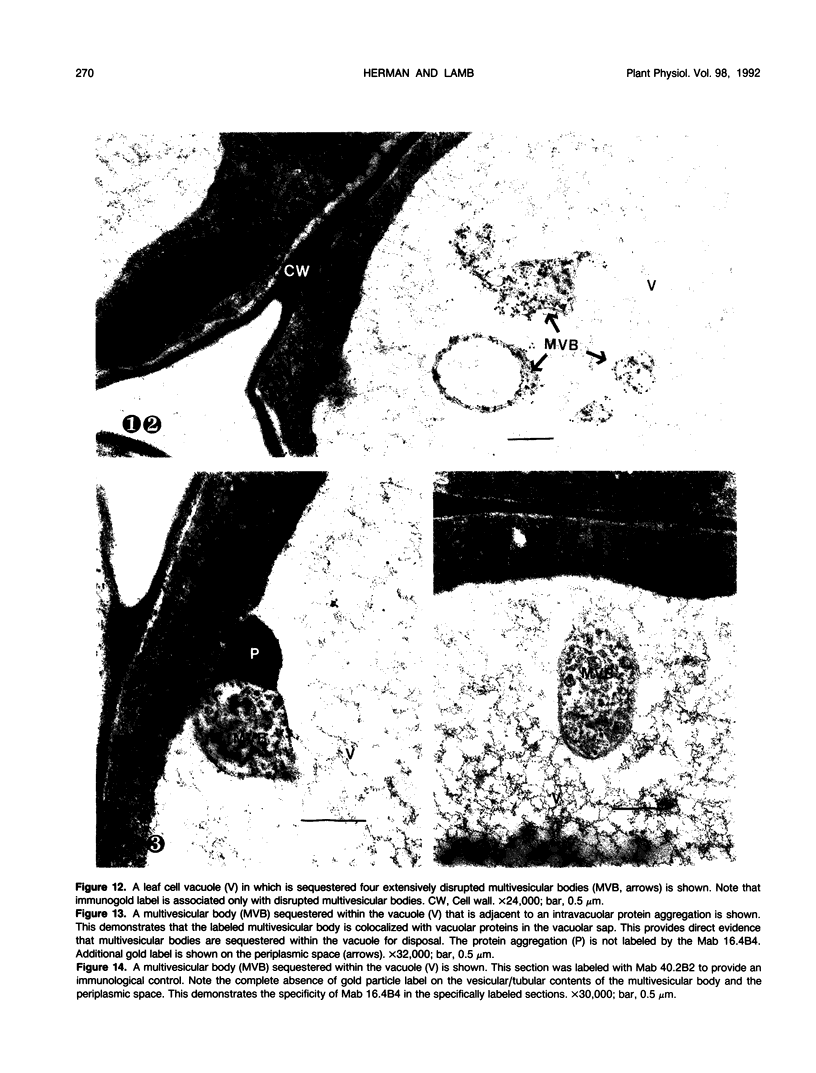
We investigated the subcellular distribution of antigenic sites immunoreactive to the monoclonal antibody 16.4B4 (PM Norman, VPM Wingate, MS Fitter, CJ Lamb [1986] Planta 167: 452-459) in tobacco (Nicotiana tabacum) leaf cells. This antibody is directed against a glycan epitope in a family of plasma membrane arabinogalactan proteins of 135 to 180 kilodaltons, elaborated from a polypeptide of relative molecular mass 50 kilodaltons (PM Norman, P Kjellbom, DJ Bradley, MG Hahn, CJ Lamb [1990] Planta 181: 365-373). We demonstrated by immunogold electron microscopy that the epitope reactive with monoclonal antibody 16.4B4 is localized on the cell surface in the leaf parenchyma cell periplast. The 16.4B4 antigen is also localized in multivesicular invaginations of the plasma membrane also known as plasmalemmasomes, implying a biochemical and, hence, functional interrelationship between these structures. Monoclonal antibody 16.4B4 also labels intracellular multivesicular bodies that appear to represent internalized plasmalemmasomes. Antibody reactivity was also observed in partially degraded multivesicular bodies sequestered within the central vacuole. We propose that the subcellular distribution of the epitope reactive with monoclonal antibody 16.4B4 defines a plasmalemmasome (or multivesicular body-mediated) pathway for the internalization of the periplasmic matrix for vacuolar mediated disposal. The multivesicular bodies appear to be equivalent to the well-characterized endosomes and multivesicular bodies of animal cells involved in the internalization and lysosome-mediated degradation of extracellular materials.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpita N., Sabularse D., Montezinos D., Delmer D. P. Determination of the pore size of cell walls of living plant cells. Science. 1979 Sep 14;205(4411):1144–1147. doi: 10.1126/science.205.4411.1144. [DOI] [PubMed] [Google Scholar]
- Herman E. M., Baumgartner B., Chrispeels M. J. Uptake and apparent digestion of cytoplasmic organelles by protein bodies (protein storage vacuoles) in mung bean cotyledons. Eur J Cell Biol. 1981 Jun;24(2):226–235. [PubMed] [Google Scholar]
- Horn M. A., Heinstein P. F., Low P. S. Receptor-Mediated Endocytosis in Plant Cells. Plant Cell. 1989 Oct;1(10):1003–1009. doi: 10.1105/tpc.1.10.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell R. I., Knox J. P., Scofield G. N., Selvendran R. R., Roberts K. A family of abundant plasma membrane-associated glycoproteins related to the arabinogalactan proteins is unique to flowering plants. J Cell Biol. 1989 May;108(5):1967–1977. doi: 10.1083/jcb.108.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson W. W., Platt K. A., Campbell N. The use of lanthanum to delineate the apoplastic continuum in plants. Cytobios. 1973 Sep-Oct;8(29):57–62. [PubMed] [Google Scholar]