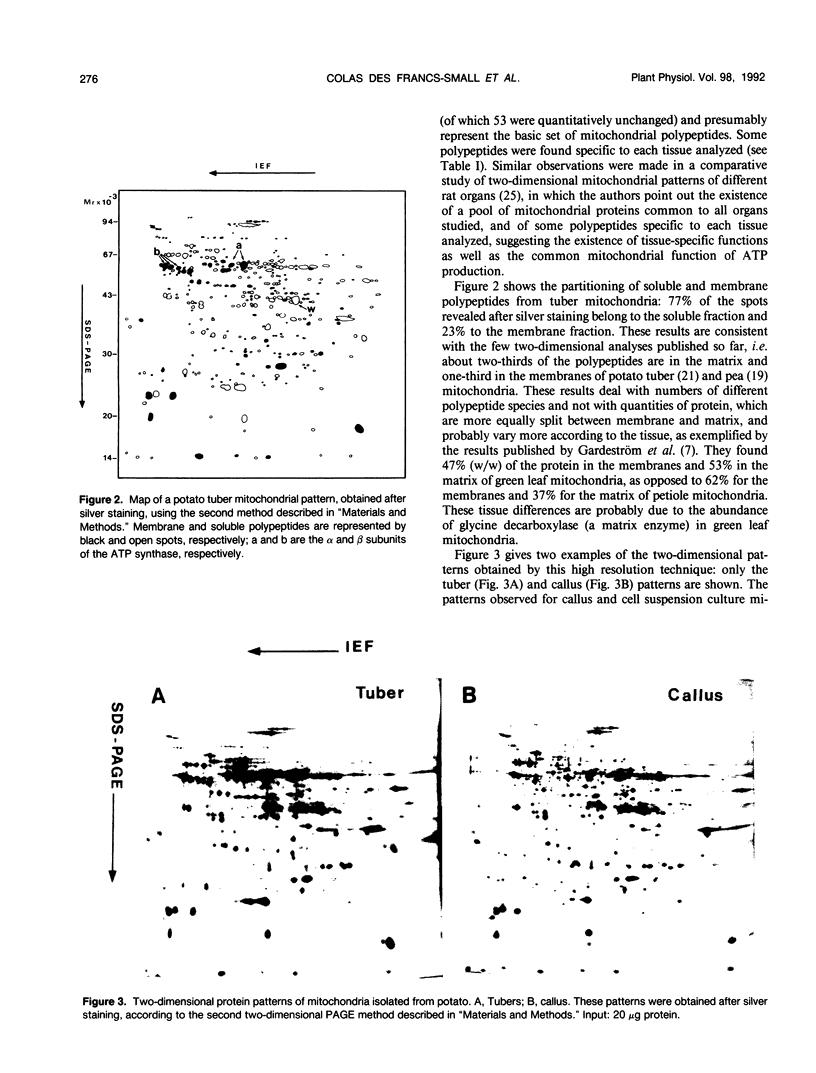
Abstract
The protein contents of mitochondria from different potato (Solanum tuberosum L.) tissues (tubers, dark-grown shoots, and green leaves) grown in a greenhouse or in vitro were compared by two-dimensional polyacrylamide gel electrophoresis. Two different methods were used: using the method that gave the highest resolution, an average number of 360 polypeptides was revealed on the mitochondrial patterns after silver staining. The mitochondrial protein patterns of etiolated tissues (tubers, dark-grown shoots) are roughly similar but distinct from those of green leaves. The four subunits of the glycine decarboxylase complex (involved in photorespiration) and a few other polypeptides are very abundant in green tissues, compared with nonphotosynthetic tissues. Conversely, some other polypeptides that are abundant in tubers and dark-grown shoots are hardly detectable in green leaf mitochondria. A rabbit antiserum was raised against a 40 kilodalton polypeptide that is among the most characteristic of these nonphotosynthetic tissue-specific polypeptides, and the N-terminal sequence of this polypeptide was determined. No effect of in vitro culture was observed on the protein composition of mitochondria isolated from differentiated tissues. However, the protein patterns of callus and cell suspension mitochondria are distinct from those of any differentiated tissues, although their basic pattern is clearly mitochondrial.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry M., Briquet M. Mitochondrial modifications associated with the cytoplasmic male sterility in faba beans. Eur J Biochem. 1982 Sep;127(1):129–135. doi: 10.1111/j.1432-1033.1982.tb06846.x. [DOI] [PubMed] [Google Scholar]
- Boutry M., Chua N. H. A nuclear gene encoding the beta subunit of the mitochondrial ATP synthase in Nicotiana plumbaginifolia. EMBO J. 1985 Sep;4(9):2159–2165. doi: 10.1002/j.1460-2075.1985.tb03910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardeström P., Bergman A., Ericson I. Oxidation of Glycine via the Respiratory Chain in Mitochondria Prepared from Different Parts of Spinach. Plant Physiol. 1980 Feb;65(2):389–391. doi: 10.1104/pp.65.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack E., Leaver C. J. The alpha-subunit of the maize F(1)-ATPase is synthesised in the mitochondrion. EMBO J. 1983;2(10):1783–1789. doi: 10.1002/j.1460-2075.1983.tb01658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neuburger M., Journet E. P., Bligny R., Carde J. P., Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch Biochem Biophys. 1982 Aug;217(1):312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Newton K. J., Walbot V. Maize mitochondria synthesize organ-specific polypeptides. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6879–6883. doi: 10.1073/pnas.82.20.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzgaber-Muller J., Kunapuli S. P., Douglas M. G. Nuclear genes coding the yeast mitochondrial adenosine triphosphatase complex. Isolation of ATP2 coding the F1-ATPase beta subunit. J Biol Chem. 1983 Oct 10;258(19):11465–11470. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayssiere J. L., Cordeau L., Larcher J. C., Gros F., Croizat B. Tissue-specific mitochondrial proteins. Biochimie. 1989 Jul;71(7):787–791. doi: 10.1016/0300-9084(89)90041-2. [DOI] [PubMed] [Google Scholar]