Abstract
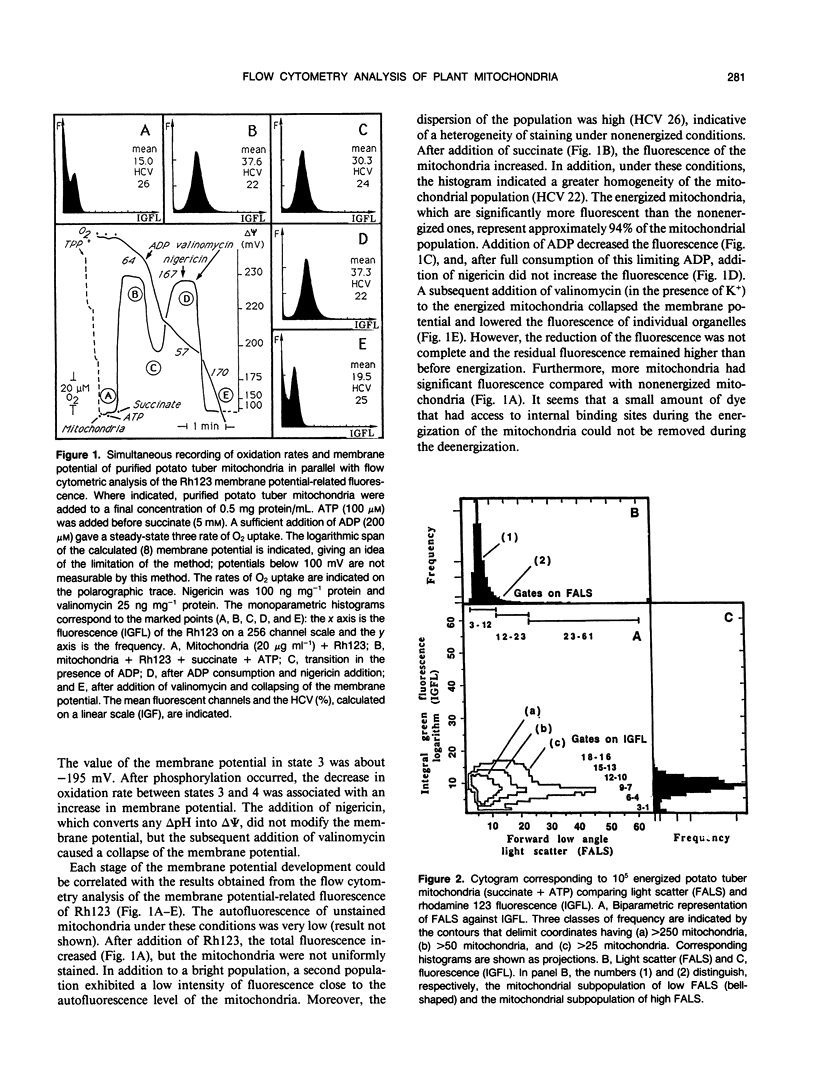
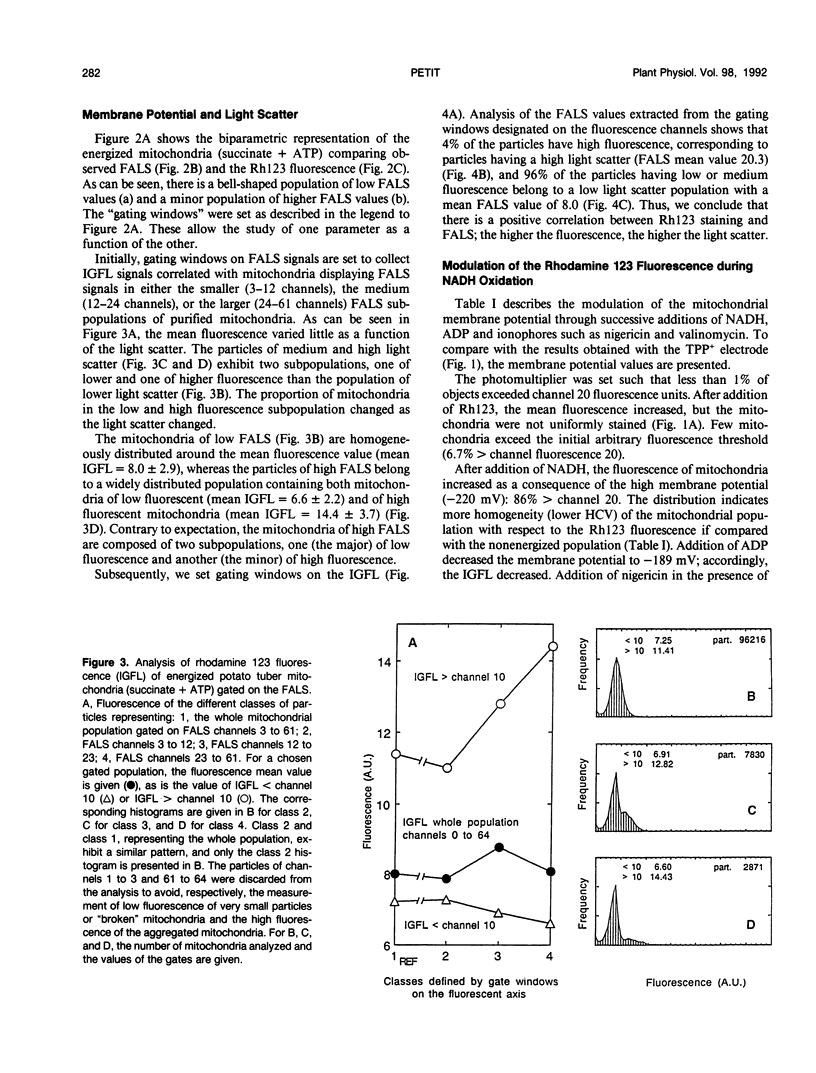
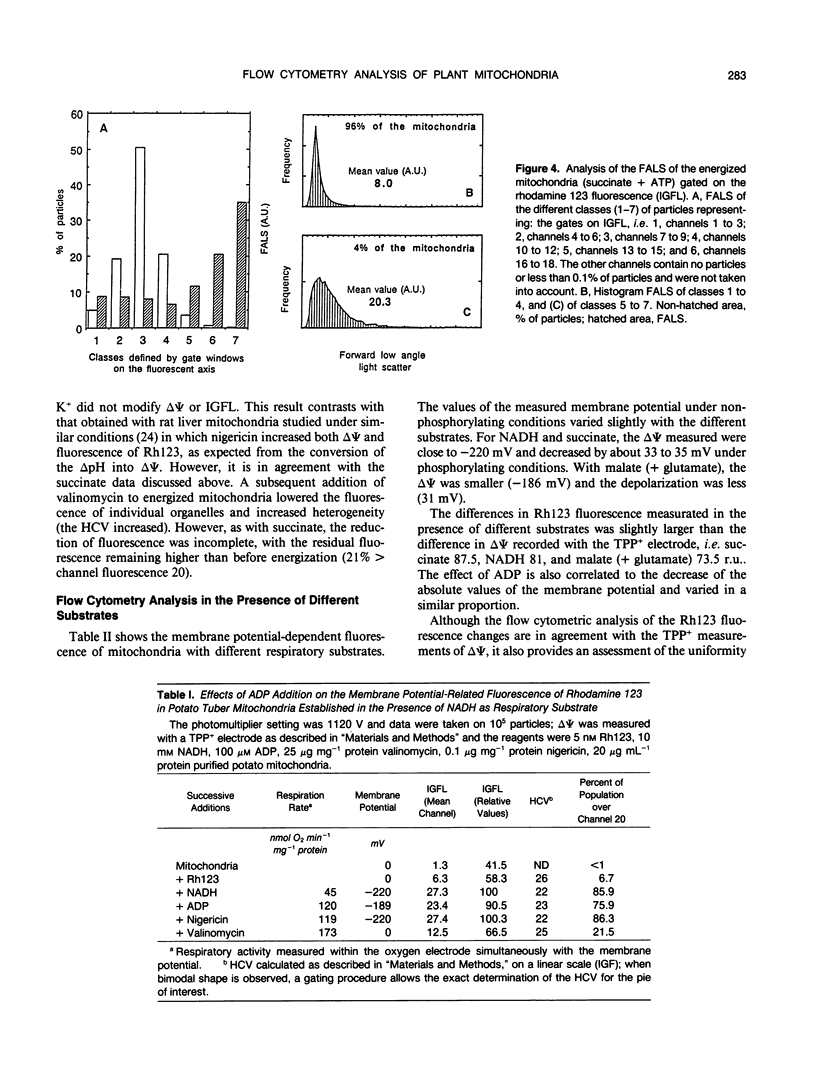
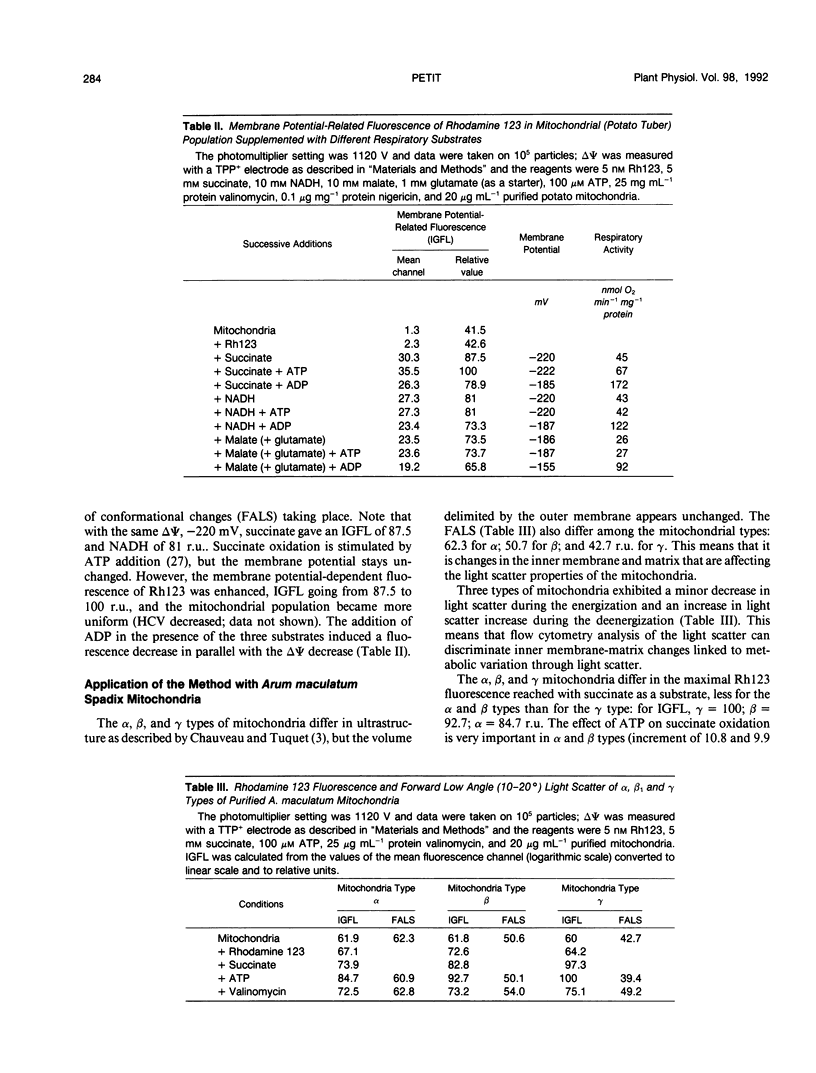
The fluorescent dye rhodamine 123, which selectively accumulates in mitochondria based on the membrane potential, was used with flow cytometry to evaluate variations in activity of mitochondria isolated from plant tissues. In the presence of succinate and ATP, potato (Solanum tuberosum L.) tuber mitochondrial activity was affected by metabolic inhibitors and compounds that modify the membrane potential. The more uniform the mitochondrial population, the higher the observed membrane potential. The reactive population corresponds to the proportion of intact mitochondria (94-97%) defined by classic methods. Changes in the light-scattering properties are more related to internal modifications affecting the inner membrane-matrix system of the mitochondria during metabolic modulation than to specific volume change or outer membrane surface modifications. We tested our approach using an Arum maculatum preparation that contains three different types of mitochondria and demonstrated the validity of the light-scatter measurements to distinguish the α, β, and [ill] mitochondria and to measure their ability to built up a membrane potential in the presence of succinate. These results demonstrate clearly that flow cytometric techniques using rhodamine 123 can be employed to study the activity in isolated plant mitochondria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashford C. L., Smith J. C. The use of optical probes to monitor membrane potential. Methods Enzymol. 1979;55:569–586. doi: 10.1016/0076-6879(79)55067-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M. Optical measurement of membrane potential. Rev Physiol Biochem Pharmacol. 1978;83:35–88. doi: 10.1007/3-540-08907-1_2. [DOI] [PubMed] [Google Scholar]
- Colnna R., Massari S., Azzone G. F. The problem of cation-binding sites in the energized membrane of intact mitochondria. Eur J Biochem. 1973 May 2;34(3):577–585. doi: 10.1111/j.1432-1033.1973.tb02798.x. [DOI] [PubMed] [Google Scholar]
- Emaus R. K., Grunwald R., Lemasters J. J. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta. 1986 Jul 23;850(3):436–448. doi: 10.1016/0005-2728(86)90112-x. [DOI] [PubMed] [Google Scholar]
- Garlid K. D., Beavis A. D. Swelling and contraction of the mitochondrial matrix. II. Quantitative application of the light scattering technique to solute transport across the inner membrane. J Biol Chem. 1985 Nov 5;260(25):13434–13441. [PubMed] [Google Scholar]
- Kamo N., Muratsugu M., Hongoh R., Kobatake Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol. 1979 Aug;49(2):105–121. doi: 10.1007/BF01868720. [DOI] [PubMed] [Google Scholar]
- Knight V. A., Wiggins P. M., Harvey J. D., O'Brien J. A. The relationship between the size of mitochondria and the intensity of light that they scatter in different energetic states. Biochim Biophys Acta. 1981 Aug 12;637(1):146–151. doi: 10.1016/0005-2728(81)90220-6. [DOI] [PubMed] [Google Scholar]
- Kovác L., Böhmerová E., Butko P. Ionophores and intact cells. I. Valinomycin and nigericin act preferentially on mitochondria and not on the plasma membrane of Saccharomyces cerevisiae. Biochim Biophys Acta. 1982 Dec 30;721(4):341–348. doi: 10.1016/0167-4889(82)90088-x. [DOI] [PubMed] [Google Scholar]
- Liu Z., Bushnell W. R., Brambl R. Pontentiometric cyanine dyes are sensitive probes for mitochondria in intact plant cells : kinetin enhances mitochondrial fluorescence. Plant Physiol. 1987 Aug;84(4):1385–1390. doi: 10.1104/pp.84.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Mediavilla C., Orfao A., Gonzalez M., Medina J. M. Identification by flow cytometry of two distinct rhodamine-123-stained mitochondrial populations in rat liver. FEBS Lett. 1989 Aug 28;254(1-2):115–120. doi: 10.1016/0014-5793(89)81020-8. [DOI] [PubMed] [Google Scholar]
- Mai M. S., Allison W. S. Inhibition of an oligomycin-sensitive ATPase by cationic dyes, some of which are atypical uncouplers of intact mitochondria. Arch Biochem Biophys. 1983 Mar;221(2):467–476. doi: 10.1016/0003-9861(83)90165-0. [DOI] [PubMed] [Google Scholar]
- Modica-Napolitano J. S., Weiss M. J., Chen L. B., Aprille J. R. Rhodamine 123 inhibits bioenergetic function in isolated rat liver mitochondria. Biochem Biophys Res Commun. 1984 Feb 14;118(3):717–723. doi: 10.1016/0006-291x(84)91453-0. [DOI] [PubMed] [Google Scholar]
- Neuburger M., Journet E. P., Bligny R., Carde J. P., Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch Biochem Biophys. 1982 Aug;217(1):312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- O'Connor J. E., Vargas J. L., Kimler B. F., Hernandez-Yago J., Grisolia S. Use of rhodamine 123 to investigate alterations in mitochondrial activity in isolated mouse liver mitochondria. Biochem Biophys Res Commun. 1988 Feb 29;151(1):568–573. doi: 10.1016/0006-291x(88)90632-8. [DOI] [PubMed] [Google Scholar]
- Petit P. X., O'Connor J. E., Grunwald D., Brown S. C. Analysis of the membrane potential of rat- and mouse-liver mitochondria by flow cytometry and possible applications. Eur J Biochem. 1990 Dec 12;194(2):389–397. doi: 10.1111/j.1432-1033.1990.tb15632.x. [DOI] [PubMed] [Google Scholar]
- Peña A., Uribe S., Pardo J. P., Borbolla M. The use of a cyanine dye in measuring membrane potential in yeast. Arch Biochem Biophys. 1984 May 15;231(1):217–225. doi: 10.1016/0003-9861(84)90381-3. [DOI] [PubMed] [Google Scholar]
- Raison J. K., Laties G. G., Crompton M. The role of state 4 electron transport in the activation of state 3 respiration in potato mitochondria. J Bioenerg. 1973;4(4):409–422. doi: 10.1007/BF01648968. [DOI] [PubMed] [Google Scholar]
- Ronot X., Benel L., Adolphe M., Mounolou J. C. Mitochondrial analysis in living cells: the use of rhodamine 123 and flow cytometry. Biol Cell. 1986;57(1):1–7. doi: 10.1111/j.1768-322x.1986.tb00458.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. Membrane potential and surface potential in mitochondria: uptake and binding of lipophilic cations. J Membr Biol. 1984;81(2):127–138. doi: 10.1007/BF01868977. [DOI] [PubMed] [Google Scholar]
- Sommarin M., Petit P. X., Møller I. M. Endogenous protein phosphorylation in purified plant mitochondria. Biochim Biophys Acta. 1990 Apr 9;1052(1):195–203. doi: 10.1016/0167-4889(90)90076-p. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S. The use of cyanine dyes for the determination of membrane potentials in cells, organelles, and vesicles. Methods Enzymol. 1979;55:689–695. doi: 10.1016/0076-6879(79)55077-0. [DOI] [PubMed] [Google Scholar]
- Waggoner A. Optical probes of membrane potential. J Membr Biol. 1976 Jun 30;27(4):317–334. doi: 10.1007/BF01869143. [DOI] [PubMed] [Google Scholar]


