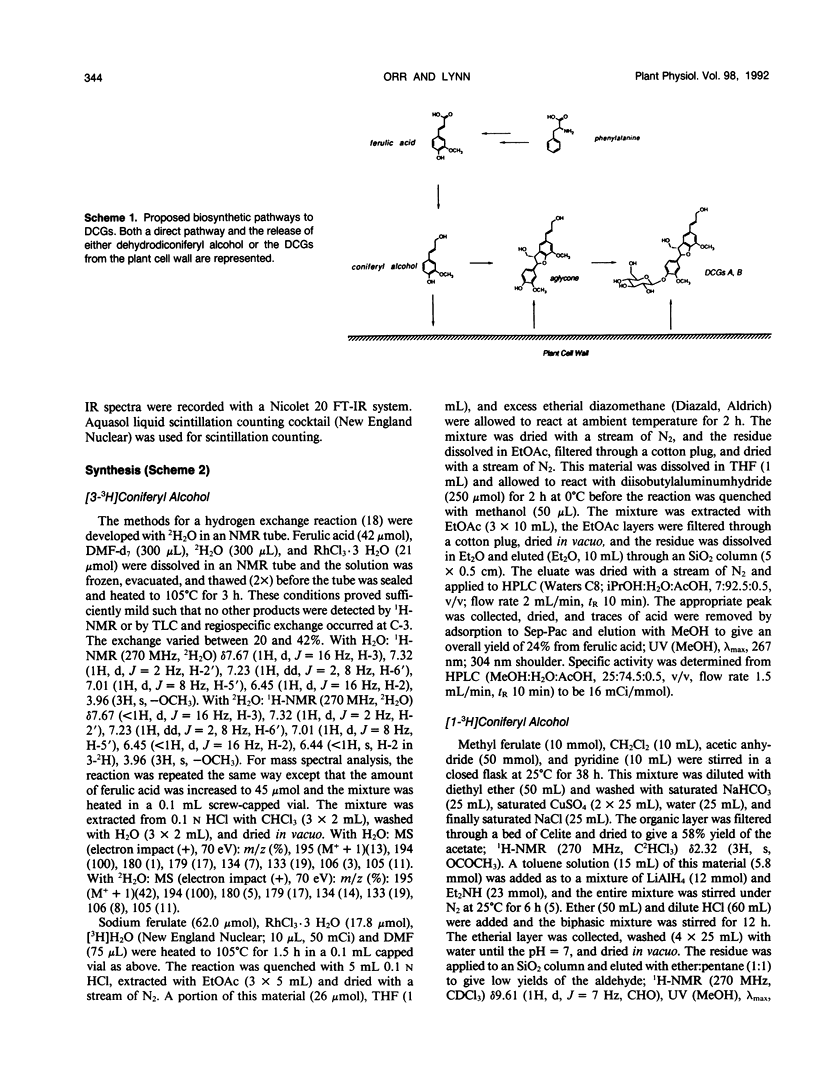
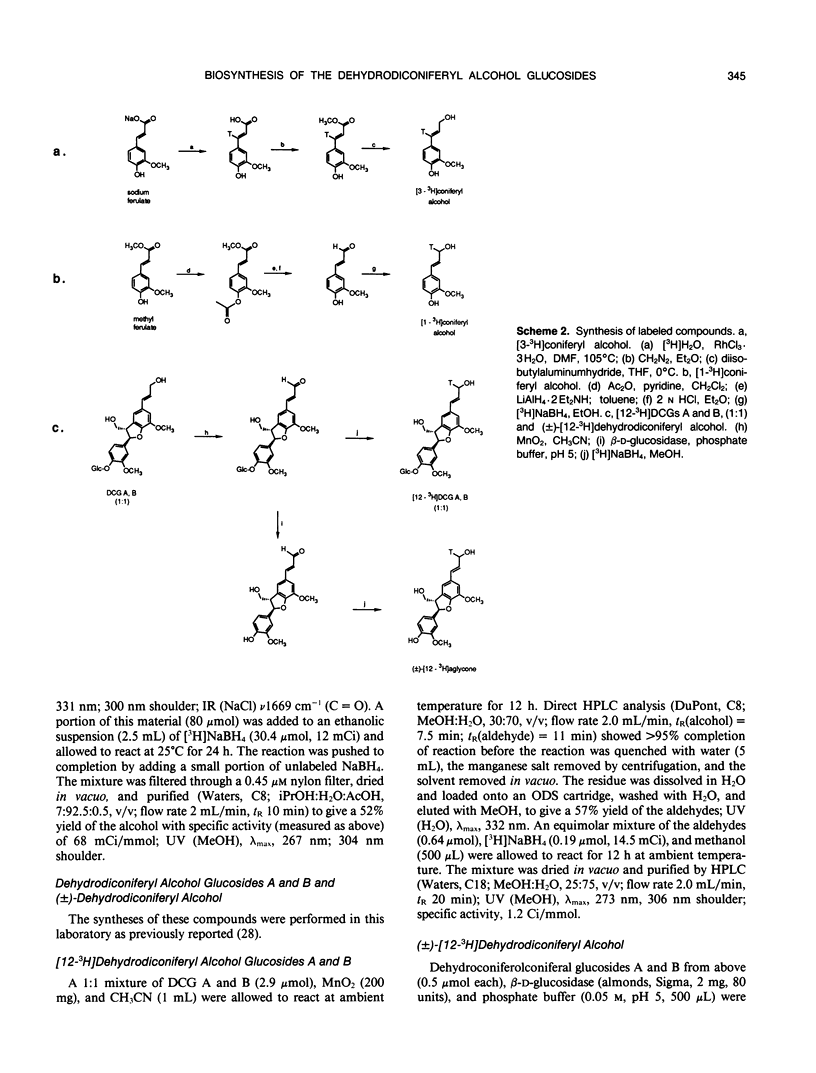
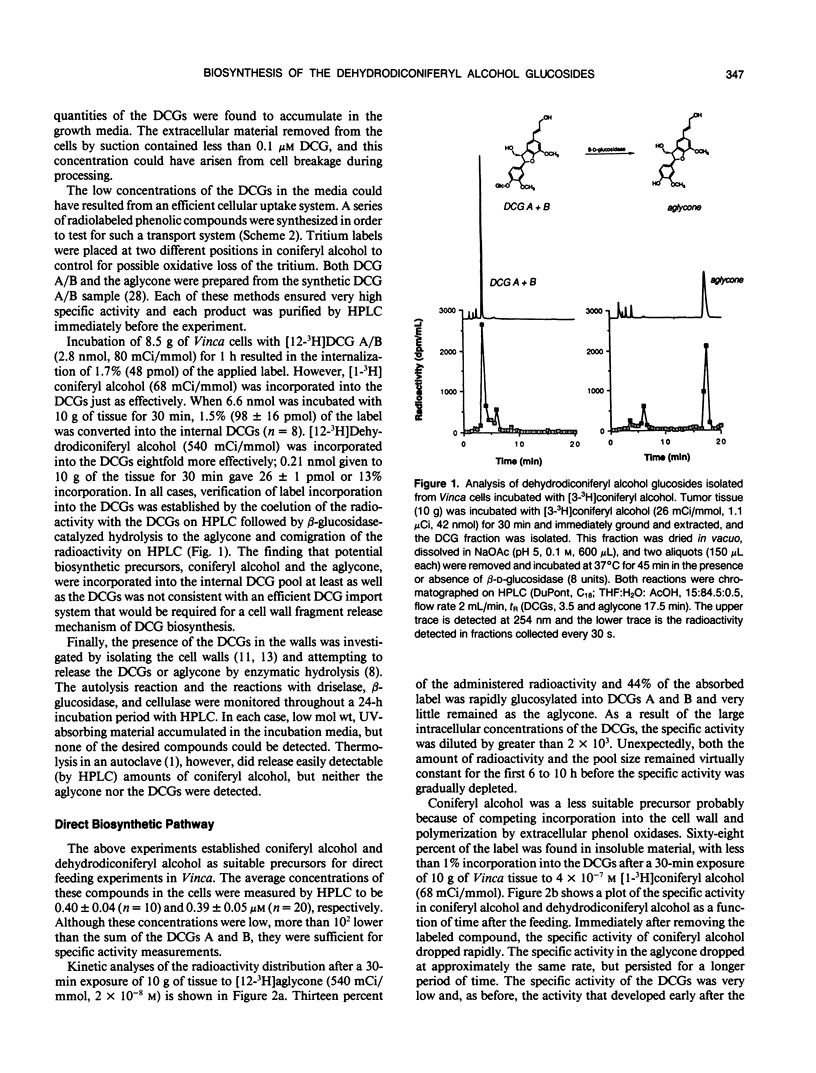
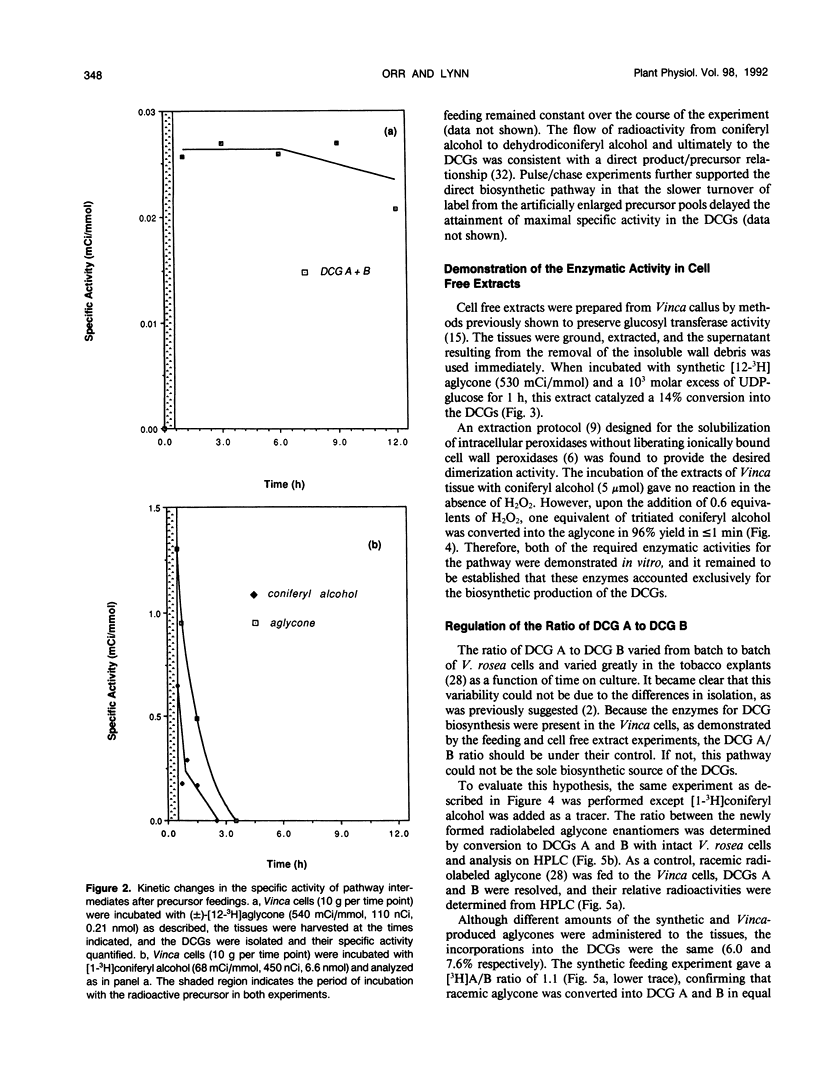
Abstract
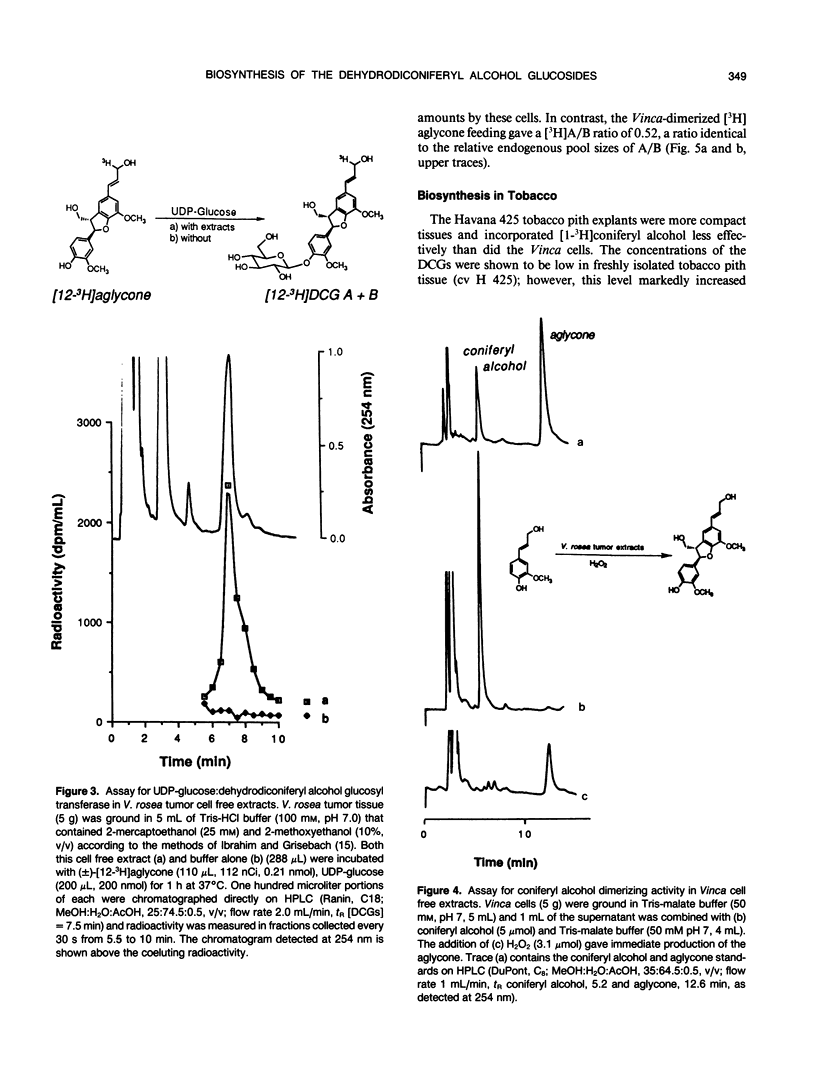
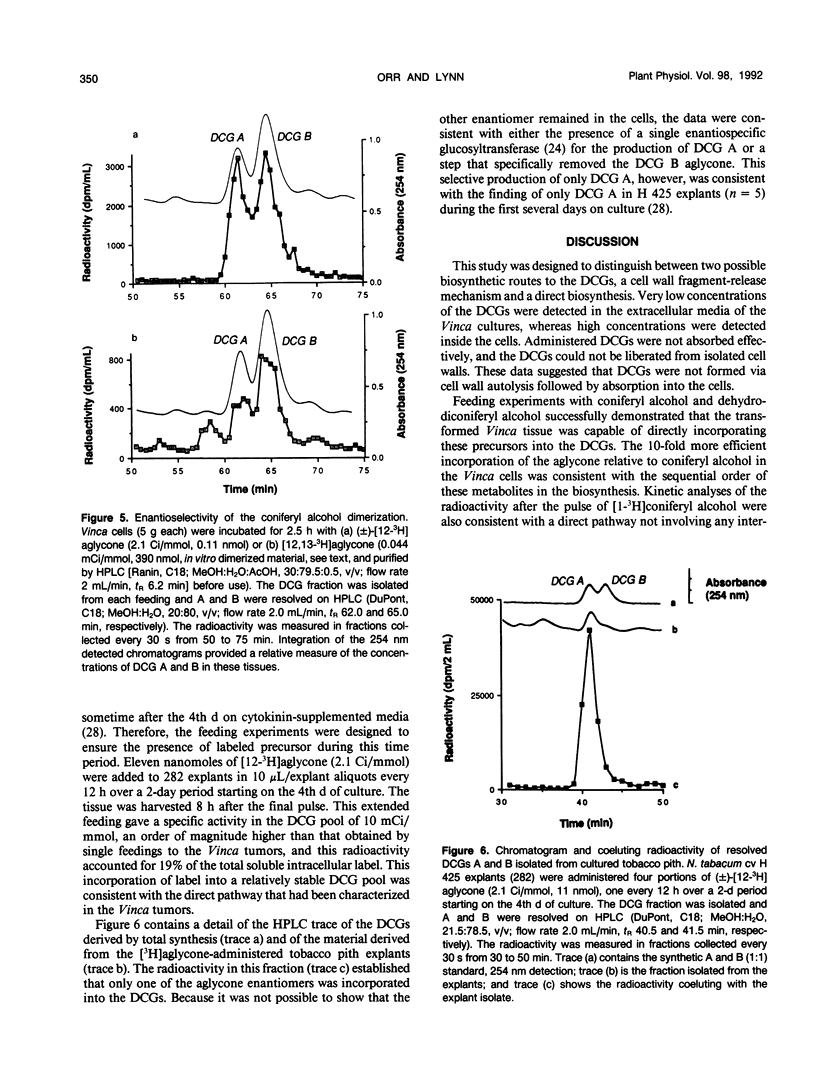
The dehydrodiconiferyl alcohol glucosides A and B are factors isolated from transformed Vinca rosea tumor cells that can replace the cytokinin requirement for growth of tobacco (Nicotiana tabacum) pith and callus cells in culture. These factors, present in tobacco pith cells, have their concentrations elevated approximately 2 orders of magnitude after cytokinin exposure. Biosynthesis experiments showed that these compounds are not cell wall fragments, as previously suggested, but are produced directly from coniferyl alcohol. Their synthesis is probably associated with the existing pathway for cell wall biosynthesis in both Vinca tumors and tobacco pith explants. The pathway requires only two steps, the dimerization of coniferyl alcohol by a soluble intracellular peroxidase and subsequent glycosylation. Biosynthetic experiments suggested that dehydrodiconiferyl alcohol glucoside breakdown was very slow and control of its concentration was exerted through restricted availability of coniferyl alcohol.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson-Prouty A. J., Albersheim P. Host-Pathogen Interactions: VIII. Isolation of a Pathogen-synthesized Fraction Rich in Glucan That Elicits a Defense Response in the Pathogen's Host. Plant Physiol. 1975 Aug;56(2):286–291. doi: 10.1104/pp.56.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church D. L., Galston A. W. 4-Coumarate:coenzyme A ligase and isoperoxidase expression in Zinnia mesophyll cells induced to differentiate into tracheary elements. Plant Physiol. 1988;88:679–684. doi: 10.1104/pp.88.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R., Satterwhite D. M., Cane D. E., Chang C. C. Biosynthesis of monoterpenes. Enantioselectivity in the enzymatic cyclization of (+)- and (-)-linalyl pyrophosphate to (+)- and (-)-pinene and (+)- and (-)-camphene. J Biol Chem. 1988 Jul 25;263(21):10063–10071. [PubMed] [Google Scholar]
- Hanley M. R. Mitogenic neurotransmitters. Nature. 1989 Jul 13;340(6229):97–97. doi: 10.1038/340097a0. [DOI] [PubMed] [Google Scholar]
- Hess K. M., Dudley M. W., Lynn D. G., Joerger R. D., Binns A. N. Mechanism of phenolic activation of Agrobacterium virulence genes: development of a specific inhibitor of bacterial sensor/response systems. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7854–7858. doi: 10.1073/pnas.88.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim R. K., Grisebach H. Purification and properties of UDP-glucose: coniferyl alcohol glucosyltransferase from suspension culturesof Paul's scarlet rose. Arch Biochem Biophys. 1976 Oct;176(2):700–708. doi: 10.1016/0003-9861(76)90214-9. [DOI] [PubMed] [Google Scholar]
- Lagrimini L. M., Bradford S., Rothstein S. Peroxidase-Induced Wilting in Transgenic Tobacco Plants. Plant Cell. 1990 Jan;2(1):7–18. doi: 10.1105/tpc.2.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. W., Dron M., Schmid J., Dixon R. A., Lamb C. J. Developmental and environmental regulation of a phenylalanine ammonia-lyase-beta-glucuronidase gene fusion in transgenic tobacco plants. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9284–9288. doi: 10.1073/pnas.86.23.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Lynn D. G., Chen R. H., Manning K. S., Wood H. N. The structural characterization of endogenous factors from Vinca rosea crown gall tumors that promote cell division of tobacco cells. Proc Natl Acad Sci U S A. 1987 Feb;84(3):615–619. doi: 10.1073/pnas.84.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn D. G., Chen R. H., Manning K. S., Wood H. N. The structural characterization of endogenous factors from Vinca rosea crown gall tumors that promote cell division of tobacco cells. Proc Natl Acad Sci U S A. 1987 Feb;84(3):615–619. doi: 10.1073/pnas.84.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G. K. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990 Feb 1;4(2):176–187. [PubMed] [Google Scholar]
- Roth S. Are glycosyltransferases the evolutionary antecedents of the immunoglobulins? Q Rev Biol. 1985 Jun;60(2):145–153. doi: 10.1086/414313. [DOI] [PubMed] [Google Scholar]
- Sih C. J., Ravikumar P. R., Huang F. C., Buckner C., Whitlock H., Jr Letter: Isolation and synthesis of pinoresinol diglucoside, a major antihypertensive principle of Tu-Chung(Eucommia ulmoides, Oliver). J Am Chem Soc. 1976 Aug 18;98(17):5412–5413. doi: 10.1021/ja00433a070. [DOI] [PubMed] [Google Scholar]
- Teutonico R. A., Dudley M. W., Orr J. D., Lynn D. G., Binns A. N. Activity and accumulation of cell division-promoting phenolics in tobacco tissue cultures. Plant Physiol. 1991 Sep;97(1):288–297. doi: 10.1104/pp.97.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. J., Hrazdina G. Endoplasmic reticulum as a site of phenylpropanoid and flavonoid metabolism in hippeastrum. Plant Physiol. 1984 Apr;74(4):901–906. doi: 10.1104/pp.74.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambryski P., Tempe J., Schell J. Transfer and function of T-DNA genes from agrobacterium Ti and Ri plasmids in plants. Cell. 1989 Jan 27;56(2):193–201. doi: 10.1016/0092-8674(89)90892-1. [DOI] [PubMed] [Google Scholar]


