Abstract
Bovine trichomoniasis is a sexually transmitted disease caused by Tritrichomonas foetus and characterized by early embryo loss. The mechanism of this loss is not known, although the parasite is known to cause inflammation and to have the ability to kill host cells by a contact-dependent cytotoxic mechanism. Antibody specific for a 190,000-Da surface complex (Tf190) was previously shown to inhibit this adhesion. In this study we used immunoaffinity chromatography to purify Tf190 from T. foetus in order to analyze its composition and examine its expression. Analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of purified Tf190 followed by silver staining revealed three components of Tf190. Western blotting and antibody-binding experiments showed that the 140- and 60-kDa bands were immunogenic. By using a battery of monoclonal antibodies (MAbs) periodate-sensitive epitopes were identified on Tf190, suggesting that these epitopes contained carbohydrate structures. Analyses of affinity-purified Tf190 by high-performance liquid chromatography and gas-liquid chromatography demonstrated the presence of the monosaccharides and lipids known to be prominent constituents of the lipophosphoglycan (LPG) of T. foetus. Flow cytometry experiments on several isolates of T. foetus with Tf190-specific antibodies revealed that Tf190 was present on subpopulations of all isolates but that not all epitopes were present on every isolate. This pattern of reactivities on the different parasite isolates was confirmed by Western blots of whole-parasite extracts probed with MAbs and antiserum. These results suggest that although variation in the expression of epitopes of Tf190 occurs in different strains of T. foetus, the Tf190 adhesion complex is widespread in different populations of the parasite. The data further suggest that immunogenic structures, important in the adhesion of T. foetus to mammalian cells, are located in the LPG-like component of Tf190.
Tritrichomonas foetus is an extracellular, flagellate, protozoan parasite of domestic cattle and the cause of bovine trichomoniasis, which can cause early-gestation abortions (4). Trichomoniasis is a sexually transmitted disease, and T. foetus lives on the mucosal surfaces of the reproductive tract, although it can invade deeper tissues, particularly in pregnant animals (20). T. foetus can attach to host cells (10) and kill them via a contact-dependent cytotoxic mechanism (8, 9), suggesting that such host cell destruction may be important in the pathogenesis of bovine trichomoniasis. The mechanism of this pathogenesis is not fully understood, and the molecular interactions between T. foetus and host cells and tissues are currently under investigation.
Previously we used monoclonal antibodies (MAbs) that react with a surface structure of T. foetus Tf190 to inhibit the killing of targets by T. foetus and parasite adhesion to target cells (9). These results suggested that Tf190 had an important role in the adhesion/killing process and that it is immunogenic. Therefore, Tf190 may be considered an adhesin and an important target for immune responses that could protect against the pathogenic effects of trichomoniasis through elicitation of antibodies that block parasite adhesion to host cells.
In this paper we report that high-performance liquid chromatography (HPLC) and gas-liquid chromatography (GLC) analyses of purified Tf190 indicate that this adhesin contains carbohydrate and lipid constituents strikingly similar to those in the lipophosphoglycan (LPG)-like structure of T. foetus (21). We demonstrate by flow cytometry that while Tf190 is expressed on the surface of the parasite, epitopes on Tf190 vary among geographically distinct isolates. Finally, we show that immunization of cattle with affinity-purified Tf190 elicits antibodies primarily directed toward carbohydrate structures on Tf190.
MATERIALS AND METHODS
Parasites.
T. foetus was cultured at 37°C in Diamond’s medium containing 5% fetal bovine serum and 20 μg of gentamicin per ml as previously described (6). The following parasite strains were used in this work: MU-Y-32 (Y-32), MU-17905 (17905), and MU-BQM-3948 (BQM), all of which were isolates from Missouri (kindly provided by Reuel Hook); MT 85-330.1 (330.1), a clone of a 1985 isolate from Montana (6); and BP-4, a 1954 Maryland isolate (ATCC 30003; American Type Culture Collection, Rockville, Md.).
Purification of Tf190.
T. foetus cells were washed twice with ice-cold phosphate-buffered saline (PBS), pH 7.2, by centrifugation (400 × g, 10 min) and then extracted by resuspension for 30 min at 108 parasites/ml in 10 volumes of ice-cold extraction buffer (50 mM Tris [pH 8], 100 mM NaCl, 100 μM leupeptin, 10 μM E-64, 5 mM EDTA, 0.5% Nonidet P-40 [NP-40]). Extracts were centrifuged (13,000 × g, 5 min) to remove cellular debris, and the supernatants were filtered (0.45-μm-pore-size filter). To prepare purified Tf190, filtered supernatants were subjected to affinity chromatography with a MAb-protein G-Sepharose column. MAb 32.3B3.5 (ascitic fluid or culture supernatant) was bound to protein G-Sepharose (Pharmacia Biotech) and cross-linked with dimethylpimelimidate by standard methods (13). Parasite extracts were then mixed with affinity matrix by gentle rocking overnight; the matrix was poured into a syringe column and washed first with 10 bed volumes of 50 mM Tris, pH 8.0, then with 10 volumes of 10 mM phosphate, pH 6.8, and finally with 5 volumes of 100 mM glycine, pH 2.7, all containing 0.5% NP-40. Chromatographic fractions were tested for antigen presence by a dot immunobinding assay (DIA) or sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by Western blotting and probing with MAbs or bovine serum (5).
In some experiments whole-cell extracts of T. foetus were subjected to affinity chromatography on columns of concanavalin A-Sepharose (ConAS; Sigma Chemical Co., St. Louis, Mo.). Whole-cell extract containing approximately 5 mg of protein/ml was adjusted to pH 6, and 1 volume of this sample was added to 4 volumes of ConAS in acetate buffer (0.1 M sodium acetate [pH 6], 0.5 M NaCl, 1 mM MgCl2, 0.5% NP-40). This preparation was mixed (end-over-end) overnight, and the matrix was placed in a column and washed with 20 volumes of acetate buffer. The matrix was then mixed overnight with 4 ml of acetate buffer containing 0.25 M α-d-mannose, transferred to a column, and further eluted with acetate-mannose buffer.
Carbohydrate and lipid analysis.
HPLC was used to analyze the carbohydrate composition of Tf190. Affinity-purified Tf190 was treated with 2.5 N trifluoroacetic acid at 100°C for 3.5 h, dried under a stream of nitrogen with the addition of isopropanol, and desalted on a C18 Sep-Pak column (Waters Chromatography). The hydrolysate was subjected to anion exchange chromatography on a CarboPac PA1 column by using 15 mM NaOH as the eluent on a Dionex HPLC equipped with a pulse amperometric detector (21).
Fatty acid analysis of affinity-purified Tf190 was done as follows. Dried Tf190 (≈20 μg) was hydrolyzed with 1 M anhydrous methanolic HCl, and the reaction mixture was heated at 75°C for 16 h under nitrogen, cooled, and extracted with methylene chloride as described previously (22). The lower organic phase was dried under nitrogen, and an aliquot was subjected to GLC analysis. The same method was used to identify fatty acid in LPG from T. foetus. Additional analysis was done by phosphoinositol-specific phospholipase C (PI-PLC) digestion of Tf190. Approximately 25 μg of Tf190 was treated with 0.8 U of PI-PLC from Bacillus thuringiensis (Oxford Glyco Systems) for 18 h at 37°C. The reaction was stopped by the addition of benzene-ethanol (1:1), and the sample was dried under nitrogen. The mixture was dissolved in aqueous solvent containing 0.8 M sodium acetate and subjected to C18 Sep-Pak (Waters Chromatography) chromatography as suggested by the manufacturer. Briefly, the C18 Sep-Pak column containing the reaction mixture was washed with 30 ml of water, and the lipid moiety was eluted with 1 ml of methanol followed by 5 ml of methanol-chloroform (1:1). The organic solvent mixture containing the lipid product was dried and further subjected to methanolic HCl hydrolysis as previously described (22). The hydrolysate was dried and purified on a Biosil-A column, and the fatty acid methyl esters eluted with chloroform were identified by GLC. The same method was used to identify the lipid moiety in LPG from T. foetus.
Antibodies and flow cytometry.
Anti-T. foetus MAbs 32.3B3.3, 32.3B3.5, 32.8D3.2, 34.1C6.4, 34.5D4.5, and 34.5D4.10 used in this study were prepared against T. foetus 330.1 as described previously (6, 7). The rabbit anti-Tf190 was prepared by immunizing rabbits with affinity-purified Tf190 prepared as described above. Flow cytometry analysis of the rabbit anti-Tf190 was done with four strains of T. foetus: 330.1, Y-32, BQM, and BP-4. Live parasites were treated with the desired dilution of either anti-T. foetus MAbs or rabbit anti-Tf190 serum in PBS on ice as described previously (7, 9). The binding of MAbs or rabbit anti-Tf190 was detected with fluorescein-conjugated anti-mouse immunoglobulin G (IgG) or fluorescein-conjugated goat anti-rabbit IgG (Cappel Laboratories, Malvern, Pa.), respectively, by using a FACScan instrument (Becton Dickinson, Mountain View, Calif.).
Western blots and antibody reactions.
Whole parasites, whole-parasite extracts, and affinity-purified Tf190 were subjected to SDS-PAGE (18). Some gels were stained with silver stain (Silver Express; NOVEX) according to the manufacturer’s protocol. Gels were electroblotted (24) and probed with antibodies as previously described (6) or treated with proteinase K or periodate followed by reaction with antibodies. For periodate treatment blots were soaked in 50 mM acetate buffer, pH 4.5, for 5 min and then treated with acetate buffer containing 20 mM sodium periodate in the dark for 1 h. Blots were then rinsed twice in acetate buffer for 10 min and once more for 5 min and were treated with 0.15 M NaCl containing 1% (wt/vol) glycine for 30 min. Control blots were treated identically except that no periodate was used. For treatment with proteinase K, blots were placed in a plastic bag containing 66 μg of proteinase K (Sigma Chemical) per ml in digestion buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA) and placed in a water bath at 56°C for 1 h. Control blots were treated identically except that no proteinase K was used. After treatment, blots were rinsed in distilled water and probed with antibodies.
Blots were probed with antibodies as described previously (6) by reaction with the desired dilutions of primary MAbs in a 5% (wt/vol) powdered milk-PBS buffer solution (BLOTTO) (16) overnight followed by development with affinity-purified, horseradish peroxidase (HRP)-labeled goat anti-mouse α, γ, and μ (no. 55570; Cappel Laboratories) and TMB membrane substrate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.). Blots probed with bovine antibodies were treated identically except that bound bovine IgG1 was detected by using monoclonal anti-bovine IgG1 (BIG715A; VMRD, Pullman, Wash.), followed by HRP-antimouse conjugate and TMB substrate.
RESULTS
Composition of Tf190.
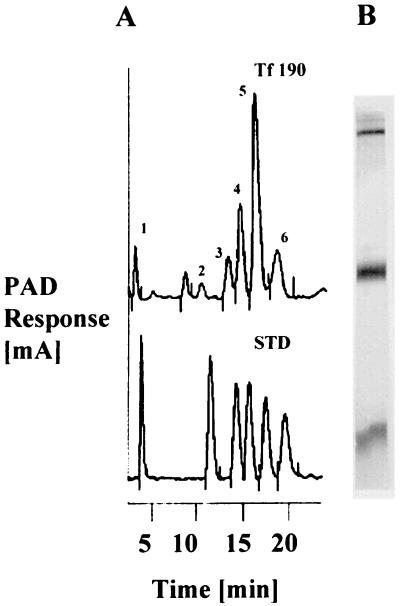
To examine the composition of Tf190, the complex was purified by immunoaffinity chromatography and subjected to HPLC analysis. HPLC analysis of Tf190 indicated the presence of fucose, galactosamine, glucosamine, galactose, glucose, and mannose (Fig. 1A). The pattern of silver-stained gels illustrated two major subunits of Tf190, which were present at approximately 140 and 60 kDa, as well as a component of about 20 kDa (Fig. 1B). Immunoaffinity-purified Tf190 was also subjected to affinity chromatography on ConAS, and the material that eluted with α-d-mannose reacted with MAb 32.3B3.5 in a DIA, indicating that Tf190 bound to ConA via a mannose residue.
FIG. 1.
Purified Tf190 contains carbohydrate structures. (A) HPLC chromatogram of affinity-purified Tf190. Peaks: 1, fucose; 2, galactosamine; 3, glucosamine; 4, galactose; 5, glucose; 6, mannose. (B) Silver staining pattern of affinity-purified Tf190 after SDS-PAGE. Tf190 was electrophoresed on a 10% T gel. PAD, pulse amperometric detector. STD, sugar standards.
Carbohydrate composition analysis by HPLC of affinity-purified Tf190 revealed peaks that correlated with fucose (Fig. 1A, peak 1), galactosamine (peak 2), glucosamine (peak 3), galactose (peak 4), glucose (peak 5), and mannose (peak 6). These are the principal monosaccharides previously shown to be present in the LPG of T. foetus (21).
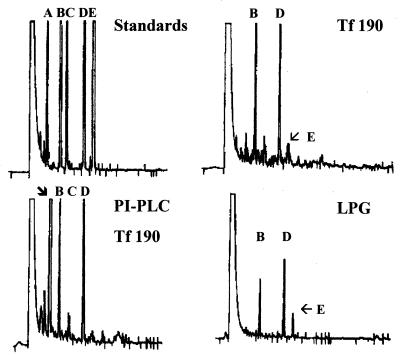
GLC analysis of fatty acid methyl esters obtained after acid methanolysis of native Tf190 antigen and the lipid product(s) derived from PI-PLC-treated Tf190 antigen showed palmitic (45%) and stearic (44%) acids to be the major fatty acids (Fig. 2). The retention times of fatty acid methyl esters from Tf190 antigen are identical to those of palmitic and stearic acid methyl ester standards. The detailed nature of the lipid moiety will be the subject of future studies. T. foetus LPG also contains palmitic and stearic acids as the major fatty acids (Fig. 2) (20).
FIG. 2.
Purified Tf190 and LPG have similar fatty acid residues. Affinity-purified Tf190 was subjected to acid hydrolysis (Tf190) or PI-PLC digestion prior to acid hydrolysis (PI-PLC Tf190). Biochemically purified LPG from T. foetus was saponified prior to analysis (LPG), and samples were compared to fatty acid standards (Standards). Samples were methylated and subjected to GLC. Letters indicate fatty acid chain sizes: A, 14:0; B, 16:0; C, 16:1; D, 18:0; E, 18:1. A peak due to deoxycholate in the panel PI-PLC Tf190 sample is indicated by an arrow.
Immunogenicity of Tf190.
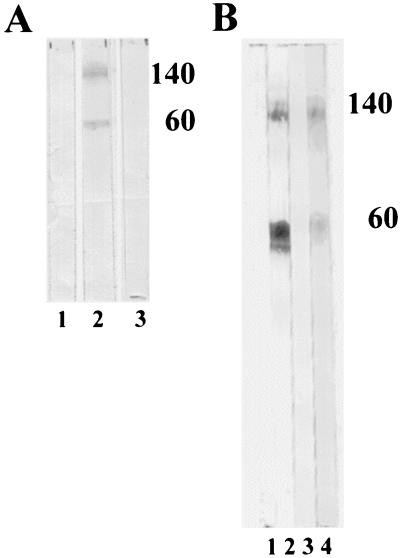
Results of DIA experiments in which sera from animals immunized with affinity-purified Tf190 were tested on dot blots indicated that Tf190 was immunogenic. These sera reacted specifically with Tf190 when used to probe Western blots of whole-parasite antigens separated by SDS-PAGE (Fig. 3).
FIG. 3.
Antibodies from animals immunized with purified Tf190 recognize heterodimeric, periodate-sensitive structures on this antigen. (A) Western blot analysis of T. foetus whole-cell extract with HRP–anti-rabbit IgG plus preimmune rabbit sera (lane 1), rabbit anti-Tf190 sera at a dilution of 1:1,000 (lane 2), or no primary antibody (lane 3). (B) Western blot analysis of T. foetus whole-cell extract reacted with ascitic fluid of MAb 32.3B3.5 (lanes 1 and 2) or bovine anti-Tf190 serum (lanes 3 and 4) treated with periodate (lanes 1 and 3) or acetate buffer (lanes 2 and 4). Molecular sizes in thousands are indicated by the numbers to the right of each panel.
The epitopes recognized by such polyvalent sera and by MAbs specific for Tf190 also appeared to contain carbohydrate determinants. As shown in Fig. 3 periodate treatment destroyed the epitopes recognized by 32.3B3.5 (Fig. 3B; compare lane 1 to lane 2) and the determinants reacting with polyvalent bovine serum from animals immunized with affinity-purified Tf190 (Fig. 3B; compare lane 3 to lane 4). The reactions of two other MAbs specific for Tf190 were also ablated by treatment of the blot with periodate (Table 1). While most of the binding of bovine anti-Tf190 IgG1 antibodies from serum was destroyed by periodate treatment (Fig. 3B, lane 3), treatment with proteinase K caused little effect on the binding (data not shown). The reactivities of antibodies with Western blots of Tf190 are summarized in Table 1.
TABLE 1.
Reaction characteristics of antibodies used to analyze Tf190a
| Antibody | DIA result | Periodate sensitivityb | Pattern on Western blot (kDa) |
|---|---|---|---|
| 32.3B3.5 | + | + | 140, 60 |
| 32.3B3.3 | + | + | 140, 60 |
| 32.1B4.2 | + | ND | 140, 60 |
| 32.8D3.2 | + | + | 140, 60 |
| 34.5D4.10 | + | ND | 140 |
| Rabbit anti-Tf190 | + | ND | 140, 60 |
| Bovine anti-Tf190 | + | + | 140, 60 |
Antibodies were reacted with blots by the procedure described for Western blotting in Materials and Methods.
+, sensitive; ND, not determined.
Epitope variation.
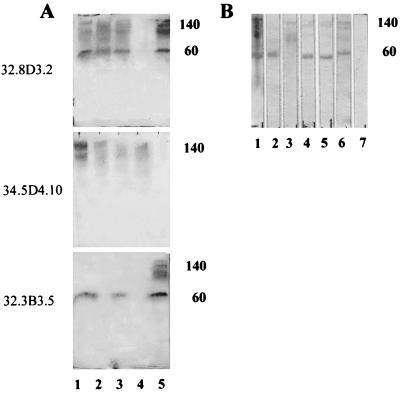
Whole-cell extracts of five strains of T. foetus, Y-32, BP-4, 17905, BQM, and 330.1, were subjected to SDS-PAGE followed by Western blotting, and blots were probed with the anti-T. foetus MAbs 32.8D3.2, 34.5D4.10, and 32.3B3.5. Large (140-kDa) and small (60-kDa) subunits of Tf190 were detected in all strains of T. foetus except BQM by 32.8D3.2 (Fig. 4A). MAb 34.5D4.10 reacted primarily with the heavy 140-kDa subunit of Tf190 in four strains but not in the 330.1 strain (compare lanes 1 to 4 with lane 5 in Fig. 4A). MAb 32.3B3.5 detected the small subunit of Tf190 in strains Y-32, 17509, and 330.1 (Fig. 4A, lanes 1, 3, and 5) and the 140-kDa subunit in strain 330.1 (lane 5). Thus, differential expression of certain epitopes seems to occur in different strains of T. foetus. Distinct immunogenic epitopes also occur on Tf190 in one strain of T. foetus. This epitope variation was demonstrated within the 330.1 strain by the different reaction patterns of MAbs on a blot of 330.1. Some MAbs reacted more strongly with the 60-kDa band (Fig. 4B, lanes 2, 4, and 5), and one MAb reacted with components larger than 60 kDa, including the 140-kDa subunit (Fig. 4B, lane 3). Other MAbs reacted strongly with both bands (Fig. 4B, lanes 1 and 6).
FIG. 4.
Epitopes of Tf190 vary between strains and within the same strain of T. foetus. (A) Western blots of whole-cell extracts of five strains of T. foetus (Y-32, lane 1; BP-4, lane 2; 17509, lane 3; BQM, lane 4; 330.1, lane 5) were reacted with MAb 32.8D3.2, 34.5D4.10, or 32.3B3.5 as indicated. (B) Western blot of whole 330.1 reacted with six different MAbs. Lane 1, 32.8D3.2; lane 2, 32.1B4.3; lane 3, 34.5D4.10; lane 4, 32.3B3.3; lane 5, 32.3B3.5; lane 6, 32.1B4.2; lane 7, no primary antibody. Molecular sizes in thousands are indicated on the right of each panel.
Expression of Tf190.
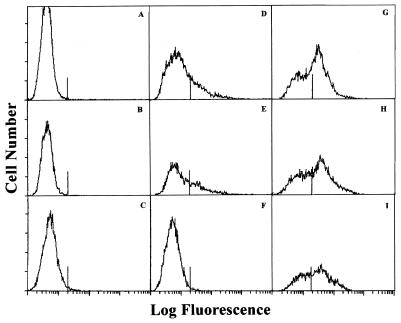
To examine the levels of surface expression of Tf190 in populations of T. foetus, anti-Tf190 antibodies were reacted with live parasites and the parasites were washed, fixed, and reacted with the appropriate fluorescein-conjugated secondary antibodies. These parasites were then analyzed by flow cytometry to determine the relative levels of antibody binding to the surface of T. foetus. Results of flow cytometry analysis confirmed the epitope variation detected by Western blotting using MAbs. While the 32.3B3.5 MAb reacted with a subpopulation of homologous strain 330.1 and heterologous strain Y-32 (Fig. 5D and E), it did not react with another Missouri strain, BQM (Fig. 5F).
FIG. 5.
Flow cytometry analysis of live T. foetus cells indicates differences in expression levels of Tf190 and Tf190 epitopes among isolates of T. foetus. T. foetus isolates 330.1 (A, D, and G), Y-32 (B, E, and H), and BQM (C, F, and I) were probed with fluorescein-conjugated anti-mouse IgG (A to C), 32.3B3.5 plus fluorescein-conjugated anti-mouse IgG (D to F), or rabbit anti-Tf190 plus fluorescein-conjugated anti-rabbit IgG (G to I). The vertical line on each x axis indicates the 1.9 × 101 intensity that 95% or more of control cells (parasites plus conjugate) fell below. Data in each panel are based on an analysis of 10,000 events.
Polyclonal antibodies prepared against affinity-purified Tf190 from clone 330.1 reacted with homologous and heterologous parasite strains (Fig. 5G, H, and I). Additional MAbs were examined by flow cytometry and one, 32.8D3.2, reacted with all three strains of T. foetus (summarized in Table 2). The bimodal pattern of antibody binding suggested that each parasite population contained two subpopulations of parasites: one subpopulation that expressed high levels of Tf190 and another subpopulation that expressed low levels of Tf190. The MAb binding results indicate that certain epitopes defined by these MAbs, including carbohydrate epitopes (periodate sensitive; Fig. 3B, lanes 1 and 3; Table 1), varied among parasite strains. Interestingly, the rabbit polyvalent anti-Tf190 antibody, elicited against Tf190 from strain 330.1, reacted with all parasite strains tested, indicating that stable Tf190 epitopes were expressed on the surfaces of all strains of T. foetus examined (Table 2).
TABLE 2.
Expression of Tf190 on the surfaces of live T. foetus cells as determined by flow cytometry
| Antibody | Intensityb | Mode fluorescence (%)a for strain:
|
||||
|---|---|---|---|---|---|---|
| 330.1 | Y-32 | BQM | 17905 | BP-4 | ||
| 32.3B3.5c,d | − | 9 (64) | 5 (71) | 20 (1) | NDf | ND |
| + | 22 (28) | 35.7 (29) | 5 (98) | 29 (99) | 101 (100) | |
| 32.3B3.3d | − | 6.5 (76) | 5.7 (69) | 20 (2) | ||
| + | 20 (24) | 29.8 (31) | 5.3 (98) | ND | ND | |
| 32.1B4.2d | − | 23 (2) | 26 (2) | 21 (1) | ||
| + | 4.7 (98) | 4.1 (98) | 5.3 (98) | ND | ND | |
| 32.8D3.2d | − | 5.7 (64) | 10 (61) | 7 (84) | ||
| + | 33 (35) | 23 (39) | 21.5 (16) | ND | ND | |
| Rabbit anti-Tf190e | − | 19 (42) | 19 (39) | 13 (40) | ||
| + | 32 (58) | 42.8 (60) | 37 (47) | ND | ND | |
Results are values of mode fluorescence (with percentages of gated events in parentheses) based on 10,000 events. The mean value of mode fluorescence ± the standard deviation for controls without primary antibody was 4.03 ± 1.2 (range, 2.8 to 5.5).
+, fluorescence intensity above 1.9 × 101; −, fluorescence intensity below 1.9 × 101.
MAb originally defining Tf190 and used to affinity purify the molecule.
MAbs reacting with affinity-purified Tf190 by DIA.
Rabbit antiserum prepared against affinity-purified Tf190.
ND, not determined.
DISCUSSION
The Tf190 complex has been purified by immunoaffinity chromatography, and biochemical characterization indicates that it contains several immunogenic carbohydrate epitopes apparently located on an LPG-like structure in the complex. The presence of carbohydrate components in Tf190 was suggested by the fact that Tf190 could be bound to ConAS and by the destruction of antibody epitopes (Fig. 3B) by periodate treatment. Detection of mannose, fucose, glucosamine, glucose, galactosamine, and galactose by HPLC of affinity-purified Tf190 (Fig. 1A) confirmed the presence of substantial amounts of carbohydrates, which are the principal monosaccharides known to be in LPG of T. foetus (21). The majority of the lipid content of Tf190 (89%) was in two lipids, palmitic acid and stearic acid (Fig. 2), which are prominent lipid constituents of the LPG of T. foetus (22). The lipid structure of Tf190 was also shown to be susceptible to cleavage by PI-PLC as is that of LPG (22). Although it should be stressed that the proportion of monosaccharides in Tf190 is slightly different from that in biochemically purified T. foetus LPG, these results suggest striking similarities between LPG and the carbohydrate component of Tf190.
The multimeric structure of Tf190, first demonstrated by immunoprecipitation and Western blotting (9), was confirmed by the protein staining patterns of SDS-PAGE gels of affinity-purified Tf190 under reducing conditions. In addition to the 140- and 60-kDa bands, a low-molecular-weight band is evident in the gel of Tf190 stained with silver stain (Fig. 1). The presence of the low-molecular-weight band, which was not recognized by polyvalent antibodies from rabbits or cattle immunized with Tf190 prepared by the same affinity chromatography method (Fig. 3), may indicate proteolytic degradation during cell disruption since this parasite is known to have multiple proteases, particularly cysteine proteinases (19). Another source of the smaller band could be portions of the side chains of the LPG of Tf190 that may have been partially degraded by endogenous hydrolases during purification. The untreated glycoconjugate of Leishmania donovani promastigotes contains LPG that is greater than 150 kDa but that shifts to 11 kDa after mild acid treatment to remove the LPG component (15). Additional structural heterogeneity of the LPGs of Leishmania spp. is due to differences in the LPG side chain carbohydrate composition (25), which could also be present in the LPG of T. foetus. Alternatively, this band may be present in undegraded Tf190 but may simply not be immunogenic. Further analysis of this component will be required to definitively determine the antigenicity and structure of this third component.
LPG may function in the adhesion of T. foetus to mammalian cells. This is suggested by the fact that MAb 32.3B3.5 (which was used to purify Tf190) has been shown to block parasite adhesion (9) and reacts with purified LPG (22a). The LPG of promastigotes of L. donovani is thought to function in the adherence of parasites to macrophages (25). LPGs have also been reported in other kinetoplastids (15, 22) and in Entamoeba histolytica (23), suggesting a conservation of this structural feature in several parasitic protozoan genera and suggesting that these parasites may use LPG glycoconjugates for adhesion to host surfaces.
The fact that periodate treatment destroyed determinants on Tf190 that bound MAb 32.3B3.5 and most of the determinants recognized by bovine polyvalent anti-Tf190 antibodies (Fig. 3B) suggests that epitopes on the LPG-like structure are rather immunogenic. The reaction pattern of rabbit anti-Tf190 antibodies on Western blots of whole-cell extracts and bovine serum (Fig. 3) indicates that epitopes on the 140- and 60-kDa subunits of Tf190 elicit strong antibody responses. Since MAbs reactive with the 60-kDa subunit block adhesion of T. foetus to mammalian cells and reduce the parasite cytotoxicity toward these targets (9), antibodies elicited by immunization with purified Tf190 may be similarly protective (11).
In vivo expression of Tf190 was characterized by the presence of high- and low-level expression subpopulations of parasites, and epitope variation was evident among different isolates of T. foetus. The bimodal peaks obtained by flow cytometry analysis with MAbs or polyvalent anti-Tf190 (Fig. 5) suggested the presence of two T. foetus subpopulations: high-level Tf190 expression and low-level Tf190 expression subpopulations. Epitope variation in vivo was detected in experiments in which the binding of MAbs to live T. foetus cells was examined by flow cytometry (Fig. 5). It should be noted that polyvalent anti-Tf190 serum seemed to stain a larger number of T. foetus cells than did MAbs (Fig. 5, panels D and E compared to panels G and H; Table 2), suggesting that multiple epitopes not recognized by individual MAbs were detected by the polyvalent serum. One isolate from Missouri, BQM, seemed particularly devoid of several epitopes defined by anti-330.1 Tf190 MAbs, although MAb 32.8D3.2, and polyvalent anti-330.1 Tf190 antibodies (Table 2) reacted with this isolate in vivo.
Epitope variation was also suggested by the results of experiments obtained when anti-Tf190 MAbs were reacted with Western blots of T. foetus. The results indicate that strain-to-strain epitope variation (Fig. 4A) as well as variation within the same strain (Fig. 4B) occur. Since many MAbs specific for Tf190 appear to bind to the LPG-like moiety of the molecule, variability in T. foetus LPG epitopes among isolates of the parasites may explain most of the observed epitope variation. This is similar to the case in Leishmania major in which epitopes in the repeating phosphosaccharide core of the LPG of L. major elicited one MAb that recognized an oligosaccharide repeat unit, PO4-6 [Ara (β 1-2) Gal (β 1-3)] Gal (β 1-4) Man α 1-, known to be expressed in larger amounts in the metacyclic stage than in the procyclic stage (17). Although it seems unlikely that the variation detected in Tf190 is artifactual since expression of different epitopes in vivo among parasite strains was detected on live T. foetus cells (Table 2), nothing is known of the regulatory control of expression of Tf190 or LPG in T. foetus. In addition, much of the structure of the LPG (and Tf190) of T. foetus has not been determined to the precision of the Leishmania LPG (25) so that the variable epitopes and adhesion determinants have yet to be precisely mapped.
The results of previous experiments in which Tf190 was metabolically labeled and immunoprecipitated with 32.3B3.5 indicated that the adhesin complex contained polypeptide components (9). The presence of protein in affinity-purified Tf190, indicated by protein assay and gel analysis (Fig. 1), confirms these findings and raises the possibility that the adhesion function(s) of Tf190 may also involve peptide determinants. Polypeptide adhesins have been reported in Trichomonas vaginalis, and antibodies to these surface polypeptides blocked adhesion (1, 12). Adhesin expression was upregulated after contact of T. vaginalis with targets (2, 3). The presence of immunogenic polypeptide adhesins in T. vaginalis and of the 11-kDa peptide that copurifies with LPG of L. donovani (14) implies that the peptide structures in Tf190 may be immunogenic. Both the L. donovani 11-kDa peptide (14) and Tf190 (26) elicit antigen-specific T-cell responses, suggesting immunological memory against these antigens is likely to be present and could lead to protective responses upon challenge.
Although cattle produce immune responses to T. foetus (4) and although some protection against infection is afforded by immunization, the mechanism of protection is not understood. The effects of antibody responses on possible virulence mechanisms, such as parasite adhesion to host tissues, are not known. Therefore, it will be important to determine the precise roles of the LPG and peptide components of the Tf190 complex in the adhesion of T. foetus to host tissues and to determine whether the immune responses to this surface structure are indeed protective.
ACKNOWLEDGMENTS
We thank Sandy Kurk for her assistance with the flow cytometry analysis and Reuel Hook for the Missouri isolates of T. foetus used in this work.
This work was supported by USDA grants 92-03829 and 95-02170, USDA Animal Health Funds, and the Montana Agricultural Experiment Station.
Footnotes
Publication J5171 of the Montana Agricultural Experiment Station.
REFERENCES
- 1.Alderete J F, Garza G E. Identification and properties of Trichomonas vaginalis proteins involved in cytadherence. Infect Immun. 1988;56:28–33. doi: 10.1128/iai.56.1.28-33.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderete J F, Lehker M W, Arroyo R. The mechanisms and molecules involved in cytoadherence and pathogenesis of Trichomonas vaginalis. Parasitol Today. 1995;11:70–74. [Google Scholar]
- 3.Arroyo R, Gonzalez-Robles A, Martinez-Palomo A, Alderete J F. Signaling of Trichomonas vaginalis for amoeboid transformation and adhesion synthesis follows cytoadherence. Mol Microbiol. 1993;7:299–309. doi: 10.1111/j.1365-2958.1993.tb01121.x. [DOI] [PubMed] [Google Scholar]
- 4.BonDurant R H, Honigberg B M. Trichomonads of veterinary importance. In: Kreier J P, editor. Parasitic protozoa. Vol. 9. New York, N.Y: Academic Press; 1994. pp. 111–188. [Google Scholar]
- 5.Burgess D E, Jerrells T. Molecular identity and location of invariant antigens on Trypanosoma brucei rhodesiense defined with monoclonal antibodies reactive with sera from trypanosomiasis patients. Infect Immun. 1985;50:893–899. doi: 10.1128/iai.50.3.893-899.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess D E. Tritrichomonas foetus: preparation of monoclonal antibodies with effector function. Exp Parasitol. 1986;62:266–274. doi: 10.1016/0014-4894(86)90031-7. [DOI] [PubMed] [Google Scholar]
- 7.Burgess D E. Clonal and geographic distribution of a surface antigen of Tritrichomonas foetus. J Protozool. 1988;35:119–122. doi: 10.1111/j.1550-7408.1988.tb04090.x. [DOI] [PubMed] [Google Scholar]
- 8.Burgess D E, Knoblock K F, Daugherty T, Robertson N P. Cytotoxic and hemolytic effects of Tritrichomonas foetus on mammalian cells. Infect Immun. 1990;58:3627–3632. doi: 10.1128/iai.58.11.3627-3632.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess D E, McDonald C M. Analysis of adhesion and cytotoxicity of Tritrichomonas foetus towards mammalian cells with monoclonal antibodies. Infect Immun. 1992;60:4253–4259. doi: 10.1128/iai.60.10.4253-4259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbeil L B, Hodson J L, Jones D W, Corbeil R R, Widders P R, Stephens L R. Adherence of Tritrichomonas foetus to bovine vaginal epithelial cells. Infect Immun. 1989;57:2158–2165. doi: 10.1128/iai.57.7.2158-2165.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbeil L B. Vaccination strategies against Tritrichomonas foetus. Parasitol Today. 1994;10:103–106. doi: 10.1016/0169-4758(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 12.Enbring J, Alderete J F. Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol Microbiol. 1992;6:853–862. doi: 10.1111/j.1365-2958.1992.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 13.Harlow E, Lane D, editors. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. pp. 521–523. [Google Scholar]
- 14.Jardim A, Tolson D L, Turco S J, Pearson T W, Olafson R W. The Leishmania donovani lipophosphoglycan T lymphocyte-reactive component is a tightly associated protein complex. J Immunol. 1991;147:3538–3544. [PubMed] [Google Scholar]
- 15.Jardim A, Funk V, Caprioli R M, Olafson R W. Isolation and structural characterization of the Leishmania donovani kinetoplastid membrane protein-11, a major immunoreactive membrane glycoprotein. Biochem J. 1995;305:307–313. doi: 10.1042/bj3050307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson D, Elder J. Antibody directed to determinants of a Moloney virus-derived MCF GP 70 recognizes a thymic differentiation antigen. J Exp Med. 1983;159:1751–1756. doi: 10.1084/jem.158.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelleher M, Curtis J M, Sacks D L, Handman E, Bacic A. Epitope mapping of monoclonal antibodies directed against lipophosphoglycan of Leishmania major promastigotes. Mol Biochem Parasitol. 1994;66:187–200. doi: 10.1016/0166-6851(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Lockwood B C, North M J, Scott K I, Bremer A F, Coombs G H. The use of a highly sensitive electrophoretic method to compare proteinases of trichomonads. Mol Biochem Parasitol. 1987;24:89–95. doi: 10.1016/0166-6851(87)90119-8. [DOI] [PubMed] [Google Scholar]
- 20.Rhyan J C, Wilson K L, Burgess D E, Stackhouse L L, Quinn W J. Immunohistochemical detection of Tritrichomonas foetus in formalin-fixed, paraffin-embedded sections of bovine placenta and fetal lung. J Vet Diagn Invest. 1995;7:98–101. doi: 10.1177/104063879500700116. [DOI] [PubMed] [Google Scholar]
- 21.Singh B N. Lipophosphoglycan-like glycoconjugate of Tritrichomonas foetus and Trichomonas vaginalis. Mol Biochem Parasitol. 1993;57:281–294. doi: 10.1016/0166-6851(93)90204-b. [DOI] [PubMed] [Google Scholar]
- 22.Singh B N, Beach D H, Lindmark D G, Costella C E. Identification of the lipid moiety and further characterization of the novel lipophosphoglycan-like glycoconjugates of Trichomonas vaginalis and Tritrichomonas foetus. Arch Biochem Biophys. 1994;309:273–280. doi: 10.1006/abbi.1994.1113. [DOI] [PubMed] [Google Scholar]
- 22a.Singh, B. N., and D. E. Burgess. Unpublished data.
- 23.Stanley S L, Huizenga H, Li E. Isolation and partial characterization of a surface glycoconjugate of Entamoeba histolytica. Mol Biochem Parasitol. 1992;50:127–138. doi: 10.1016/0166-6851(92)90250-n. [DOI] [PubMed] [Google Scholar]
- 24.Towbin H, Straehlin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turco S J, Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- 26.Voyich J, Burgess D E. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Cytokine expression of cattle immunized with purified Tf190 adhesin of Tritrichomonas foetus, abstr. E-7; p. 35. [Google Scholar]