Abstract
Central North Carolina (NC) is highly contaminated with per- and polyfluoroalkyl substances (PFAS), in part due to local fluorochemical production. Little is known about the exposure profiles and long-term health impacts for humans and animals that live in nearby communities. In this study, serum PFAS concentrations were determined using liquid chromatography high-resolution mass spectrometry and diagnostic clinical chemistry endpoints were assessed for 31 dogs and 32 horses that reside in Gray’s Creek NC at households with documented PFAS contamination in their drinking water. PFAS were detected in every sample, with 12 of the 20 PFAS detected in ≥50% of samples from each species. The average total PFAS concentrations in horses were lower compared to dogs who had higher concentrations of PFOS (dogs 2.9 ng/mL; horses 1.8 ng/mL), PFHxS (dogs 1.43 ng/mL, horses < LOD), and PFOA (dogs 0.37 ng/mL; horses 0.10 ng/mL). Regression analysis highlighted alkaline phosphatase, glucose, and globulin proteins in dogs and gamma glutamyl transferase in horses as potential biomarkers associated with PFAS exposure. Overall, the results of this study support the utility of companion animal and livestock species as sentinels of PFAS exposure differences inside and outside of the home. As in humans, renal and hepatic health in domestic animals may be sensitive to long-term PFAS exposures.
Keywords: canine, drinking water, equine, fluoroether, One Health, nafion by-product 2, GenX chemicals, PFOA, PFOS
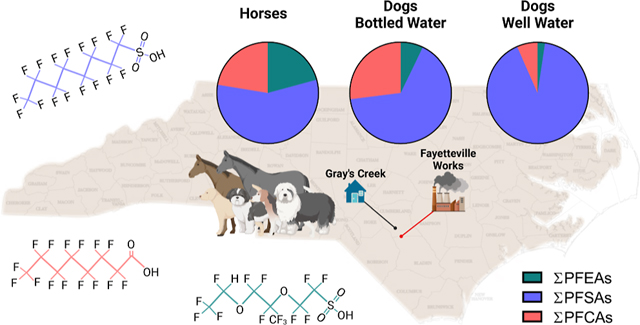
Graphical Abstract
INTRODUCTION
Per- and polyfluoroalkyl substances (PFAS) are a diverse group of synthetic organofluorine chemicals that are characterized by the presence of one or more perfluoroalkyl or polyfluoroalkyl groups. Many are amphiphilic, heat resistant, and chemically stable, properties that make them useful for a wide variety of industrial and consumer applications.1,2 High volume production and widespread use of PFAS-containing products, along with global transport of these chemicals through air and water, has led to ubiquitous contamination of indoor and outdoor environments.3–10 Because many PFAS are non-degradable in the environment, or degrade to terminal perfluorinated end products, they persist in the environment and accumulate in biota.11 Such ubiquity, persistence, high-mobility, and ability to bioaccumulate, combined with the robust evidence of toxicity, necessitates biomonitoring efforts to characterize the exposure and long-term health consequences of PFAS.
For decades, Fayetteville Works, a fluorochemical manufacturing facility located along the border of Cumberland County in central North Carolina (NC; Figure S1), has discharged PFAS into the surrounding environment through vent stack emissions and direct discharge of process wastewater into the Cape Fear River.12–15 As a result, elevated levels of PFAS are present in residential drinking water and blood samples from fish, wildlife, and humans living near the Cape Fear River.15–22 Owing to concerns regarding the persistence and toxicity of long-chain PFAS, US manufacturers participating in the 2010/2015 perfluorooctanoic acid (PFOA) stewardship program, put forth by the Environmental Protection Agency (EPA), report that they no longer produce PFOA and perfluorooctanesulfonic acid (PFOS).23 Consequently, the production and use of shorter-chain alternatives, such as the perfluoroalkyl ether acid (PFEA) hexafluoropropylene oxide dimer acid (HFPO-DA), colloquially known as a GenX chemical, has increased.11,24,25 Recent monitoring efforts have detected PFEAs in thousands of private wells in the eastern half of the state, including many in the Gray’s Creek area of southern Cumberland County NC, with concentrations of HFPO-DA exceeding the EPA’s drinking water health advisory limit of 10 parts per trillion (ppt; Table S1; Figure S2).26–28 In conjunction with toxicity assessments for GenX chemicals finding evidence of adverse health effects in the liver, kidneys, thyroid, and immune system, there is considerable concern surrounding the potential long-term health impacts resulting from chronic consumption of PFAS-contaminated water.29–31 However, the impacts of long-term exposure to the complex mixtures of PFAS found in the Gray’s Creek area remain unknown.
Animal sentinels are animals that have ecological and physiological adaptations that make them sensitive to environmental hazards such as toxins, pollutants, or pathogens and can exhibit signs of adverse effects before humans would notice any effects. As a result, sentinel animals are indicators useful for detecting and providing advanced indication of environmental health hazards.32 In a One Health context, there is increasing appreciation and use of domestic animals as sentinels of human exposure and health risks associated with household sources of chemical contamination. For example, studies of dogs and cats demonstrate that the prevalence and abundance of PFAS, pesticides, and flame retardants often parallel and, in some cases, match those of their owners.33–37 Bolstering their utility as sentinels for human health effects, domestic animals have substantial overlap in shared health risks, with 360 diseases in pet dogs that are analogous to human diseases.38 Combined with their shorter lifespans, and therefore shorter disease latencies, companion animals can provide critical insights into human-relevant, exposure-associated adverse health outcomes. In addition to dogs and cats, horses represent a potentially useful, but underutilized, residential sentinel. Horses live in close proximity to their owner’s homes but often spend their entire life outside, which gives them a unique exposure environment that is relevant to both humans and local wildlife. By studying sentinel animals that occupy both indoor and outdoor residential environments, we can ascertain information on situational environmental exposures and potential health risks.
The “One Health” concept emphasizes the importance of understanding how shared environmental experiences contribute to the etiology of human and animal disease and ecosystem degradation. Although this approach has typically been applied to studies of zoonotic disease, there is growing interest in its application for identifying chemical-induced health risks to inform regulatory and public health response to chemical hazards like PFAS.39 In the present study, we utilized a collaborative “One Health” approach, combining community-based participatory research with the expertise of veterinary, analytical, and toxicological scientists, to assess PFAS exposures and health biomarkers in pet dogs and horses living in the Gray’s Creek area of Cumberland County NC. We tested multiple hypotheses in this study. First, because PFAS are ubiquitous contaminants of both the indoor and outdoor environment, we hypothesized that pets would have detectable levels of PFAS in their serum. Second, recent publications have shown that PFAS, including HFPO-DA, can have longer half-lives in male rodents;31,40,41 therefore, we hypothesized that male study animals would have higher serum concentrations of PFAS. Third, because dogs and horses occupy different living environments, we hypothesized that differences in primary habitat contamination would influence serum PFAS composition and abundance. Specifically, we hypothesized that dogs would have higher overall PFAS exposure reflecting the indoor environment with PFAS exposure dominated by congeners found in drinking water and house dust contamination (i.e., PFOS, PFOA, and perfluorohexanesulfonic acid; PFHxS).33,42,43 Fourth, due to concerns about drinking water contamination in this area, approximately half of the households that participated in this study gave their dogs bottled water, rather than well water, as their primary source of drinking water. We hypothesized that dogs provided with bottled water would have lower serum PFAS concentrations compared to those given well water. Finally, we hypothesized that the differences in serum PFAS would be associated with disparate health biomarker profiles across (i.e., horse vs dog) and within (i.e., dog bottled vs well) species.
MATERIALS AND METHODS
Study Population.
Owners provided informed consent prior to sample collection, completed a questionnaire about their companion and/or livestock animal’s age, sex, weight, and source of drinking water, bottled or well, shared results confirming well water PFAS contamination, and allowed a veterinarian to take a blood sample for serum PFAS and health biomarker analysis (Figure S1). Details regarding HFPO-DA concentrations detected in well water from participating households can be found in Table S1 and Figure S2. Pet demographic information is provided in Table S2. Owners and their animals (n = 31, dog; n = 32, horses) were recruited through social media and recruitment emails from August through September 2020. Dogs and horses were considered eligible if they lived in the Gray’s Creek area and PFAS analysis had been completed on their drinking water source by the North Carolina Department of Environmental Quality (NCDEQ) between 2017 and 2021. The final study cohort comprised 22 households from an area that was within 18 kilometers of the fluorochemical production facility. A flow diagram detailing the stratification of samples across species, source of drinking water, household, and sex is shown in Figure S3. All study protocols and associated materials were approved by the NCSU Institutional Review Board (protocol number 21069) and Institutional Animal Care Use Committee (protocol number 20-336).
Sample Collection.
Blood samples (3–5 mL) were collected in September 2020 by a registered veterinary technician or licensed veterinarian using a 5 mL Luer lock syringe (BD, Franklin Lakes, NJ) with a 20-gauge 1 in. needle from the cephalic or jugular vein through standard venipuncture. Blood was transferred from the syringe into serum blood collection tubes (BD Vacutainer, Cat# 368045, plastic, Franklin Lakes, NJ) and allowed to clot at ambient temperature for at least 30 min. Serum was aliquoted into Teflon-free cryovials following centrifugation (1800g for 10 min at 4 °C) and transported on ice to NCSU. Serum fractions were stored at −80 °C until analysis.
Sample Preparation for PFAS Analysis.
Details on the materials used for sample preparation and PFAS analysis can be found in supplemental methods (Supporting Information Document 1). Serum (50 μL) was transferred into 2 mL polypropylene tubes, 10 μL of 0.1 M formic acid spiked with 24 isotopically labeled internal PFAS standards was added, and each sample was vortex mixed. Cold acetonitrile (300 μL; −20 °C) was added to denature and precipitate proteins. The sample was then vortex mixed and centrifuged at 10,000 rpm for 5 min, and the process was repeated. Following the second centrifugation, 100 μL of supernatant was transferred to a liquid chromatography vial containing 300 μL of 0.4 mM ammonium formate in diH2O.
Mass Spectrometry.
The PFAS analyzed were chosen based on compounds that were previously detected in the NC Cape Fear River basin and the availability of appropriate authentic standards.15,16,44,45 At the time of analysis, analytical standards were available for 33 PFAS of interest (Table S3). Methods for the preparation of standard curves, mass spectrometry conditions, and quality controls were identical to those used previously.16,17 Additional details are also contained in supplemental methods (Supporting Information Document 1).
Analysis of Blood Chemistry Biomarkers.
Blood chemistry values for 14 parameters (Table S4) were obtained for all samples with sufficient volume and quality of serum necessary for analysis (dogs: n = 29, Table S5; horses: n = 26, Table S6) using a VetScan VS2 Whole Blood chemistry analyzer (Abaxis, Union City, CA) with commercially available diagnostic veterinary panels optimized for dogs (Abaxis, Comprehensive Diagnostic Profile Cat #500-1043) or horses (Abaxis, Equine Profile Plus Cat #500-1038). Two dogs, 1020 and 1036, and six horses, 1142–1147, were not analyzed due to insufficient serum volume for the dogs and missing metadata needed for statistical analysis for the horses (Supporting Information Document 1; Tables S7 and S8). In dogs, hemolysis hindered our ability to accurately measure total bilirubin (n = 24) and potassium (n = 20) in a subset of samples. Calcium (n = 28) concentration was also suppressed for a single dog in this study. All samples analyzed passed individual assay control QC assessments of the instrument and rotors. All procedures followed the manufacturer’s protocols using 100 μL of serum.
Statistical Analysis.
Each blood sample was assigned a randomized number code by NCSU researchers at the time of collection. Samples were decoded for location, sex, age, weight, and drinking water source only after PFAS measurement and analysis was completed. Data were analyzed using Prism (version 9.3, GraphPad La Jolla, CA), JMP Pro (Version 16, SAS, Raleigh, NC, USA), SPSS (version 28.0, IBM, Armonk, NY, United States), and the R statistical environment (Version 4.1.1; R Core Team 2021). For all statistical analyses, a minimal level of statistical significance for differences in values among or between groups was considered (p ≤ 0.05).
Where necessary, PFAS detected at concentrations less than the LOD were assigned an interpolated concentration value of prior to statistical analyses (Tables S7 and S8). Shapiro–Wilk’s test was used to assess normality of data; however, no PFAS, except for perfluoro(3,5-dioxahexanoic) acid (PFO2HxA) in horses, passed normality. Individual PFAS concentrations were transformed using , where the addition of 1 allows for the inclusion of non-detects (0) and quantifiable values < 1 following data transform.46
Principal components analysis (PCA) was performed to dimensionally reduce PFAS concentrations using a restricted maximum likelihood estimation. Ward’s general agglomerative hierarchical clustering was performed on transformed PFAS concentrations. Differences between PFAS concentration means were analyzed by two-way ANOVA with Sidak’s multiple comparisons post-hoc test. Fisher’s exact test was used to compare individual PFAS detection frequencies across and within species. General linear modeling (GLM) was used to examine the impact of water source (dogs only), household, sex, age, and weight, on serum concentrations of individual PFAS with detection frequencies ≥50% for individual compounds. Water source and sex were considered fixed factors, household was considered a fixed factor within water source, and age and weight were treated as covariates (Tables S9 and S10). Adjusted correlation coefficients were considered negligible (±0.0–0.1), weak (±0.1–0.39), moderate (±0.4–0.69), strong (±0.7–0.89), and very strong (±0.9–1) based on previously established criteria.47
To evaluate the magnitude of effect for differences between means and GLM analyses, effect sizes were calculated. ANOVA effect size was determined by calculating Eta squared (η2), effects of which are defined as small at 0.01, medium at 0.06, and large at 0.14.48 Effect sizes for post-hoc Sidak’s corrections for multiple comparisons were calculated by Cohen’s d, effects of which are defined as small at 0.2, medium at 0.5, and large at 0.81.48
To identify the associations between serum PFAS concentrations and health parameters, multiple linear regression analysis was performed using PFAS concentrations as independent variables and biochemical parameters as dependent variables, with household and water source as covariates (Tables S11 and S12). Shapiro–Wilk’s test was used to assess normality of data. Health parameters that did not pass normality were natural log transformed for regression analysis. In dogs, because serum PFAS concentrations were significantly impacted by the source of drinking water, separate regression analyses were run for dogs given bottled water and dogs given well water.
RESULTS
Unique PFAS Exposure Profiles of Dogs and Horses.
From the targeted list of the 33 PFAS analyzed, we identified 20 different PFAS including PFEAs, perfluorosulfonic acids (PFSAs), and perfluorocarboxylic acids (PFCAs, Table S3). Of those 20, 16 had a ≥50% detection in one species. The PFSAs, specifically PFOS and PFHxS, had the highest concentrations in all serum samples, with the highest levels observed for PFHxS in dogs and PFOS in horses. Two congeners were detected only in dogs, PFHxS and perfluoroethocysulfonic acid (NVHOS), while three congeners, perfluoropentanoic acid (PFPeA), HFPO-DA, and perfluoro(3,5,7,9,11-pentaoxadodecanoic) acid (PFO5DoDA), were detected only in horses (Table 1). Species-specific differences in detection frequency were observed for perfluorohexanoic acid (PFHxA; p < 0.0001), perfluoropentanesulfonic acid (PFPeS; p = 0.05), PFHxS (p < 0.0001), PFOS (p = 0.003), and perfluorododecanoic acid (PFDoDA; p = 0.0006), with PFHxA, PFPeS, PFHxS, and PFOS more frequently detected in dogs and PFDoDA more frequently detected in horses.
Table 1.
Detection Limits, Detection Frequency, and Concentrations of PFAS in Dog and Horse Seruma
| PFAS |
LOD (ng/mL) |
detection frequency (% >LOD) |
concentration (ng/mL, GM and range) |
detection frequency (% >LOD) |
concentration (ng/mL, GM and range) |
||||
|---|---|---|---|---|---|---|---|---|---|
| dog (n = 31) | horse (n = 32) | dog (n = 31) | horse (n = 32) | dog bottle (n = 17) | dog well (n = 14) | dog bottle (n = 17) | dogwell (n = 14) | ||
| PFCA | |||||||||
| PFPeA | 0.03 | 0 | 3 | <LOD | <LOD (<LOD-0.05) | 0 | 0 | <LOD | <LOD |
| PFHxA | 0.03 | 94c | 31 | 0.064 (0.02–0.34) | 0.02 (<LOD-0.05) | 100 | 86 | 0.07 (0.03–0.34) | 0.05 (0.02–0.21) |
| PFHpA | 0.03 | 97 | 97 | 0.097 (<LOD-0.13) | 0.09 (0.02–0.11) | 94 | 100 | 0.10 (<LOD-0.13) | 0.10 (0.09–0.11) |
| PFOA | 0.01 | 100 | 97 | 0.37 (0.09–2.63) | 0.10(0.01–0.13) | 100 | 100 | 0.43 (0.09–2.63) | 0.28 (0.11–0.55) |
| PFNA | 0.01 | 100 | 91 | 0.28 (0.10–0.64) | 0.09 (<LOD-0.16) | 100 | 100 | 0.29 (0.10–0.40) | 0.27 (0.12–0.56) |
| PFDA | 0.01 | 100 | 97 | 0.21 (0.10–0.57) | 0.25 (0.01–0.88) | 100 | 100 | 0.20 (0.10–0.40) | 0.22 (0.13–0.57) |
| PFUnDA | 0.04 | 100 | 97 | 0.15 (0.08–0.42) | 0.17 (0.03–0.36) | 100 | 100 | 0.13 (0.08–0.21) | 0.17 (0.09–0.42) |
| PFDoDA | 0.05 | 42 | 84c | 0.07 (0.04–0.16) | 0.12 (0.04–0.23) | 35 | 50 | 0.06 (0.04–0.15) | 0.08 (0.04–0.16) |
| PFSA | |||||||||
| PFBS | 0.01 | 100 | 97 | 0.30 (0.12–2.08) | 0.13 (0.01–0.24) | 100 | 100 | 0.28 (0.12–2.08) | 0.31 (0.15–1.88) |
| PFPeS | 0.02 | 100a | 84 | 0.14 (0.11–0.25) | 0.10 (<LOD-0.18) | 100 | 100 | 0.13 (0.11–0.17) | 0.16 (0.12–0.25) |
| PFHxS | 0.06 | 77c | 0 | 1.43 (0.04–80.55) | <LOD | 65 | 93 | 0.54 (0.04–4.01) | 3.26 (0.04–80.55) |
| PFHpS | 0.03 | 100 | 97 | 0.23 (0.06–0.83) | 0.09 (0.02–0.27) | 100 | 100 | 0.13 (0.06–0.33) | 0.36 (0.08–0.83) |
| PFOS | 0.77 | 87b | 50 | 2.88 (0.54–17.60) | 1.82 (0.54–11.77) | 82 | 93 | 1.74 (0.54–8.78) | 4.93 (0.54–17.60) |
| PFEA | |||||||||
| PMPA | 0.01 | 55 | 38 | 0.06 (<LOD-0.16) | 0.04 (<LOD-0.11) | 47 | 64 | 0.06 (<LOD-0.16) | 0.07 (<LOD-0.13 |
| PEPA | 0.02 | 100 | 89 | 0.10 (0.08–0.14) | 0.07 (<LOD-0.11) | 100 | 100 | 0.09 (0.08–0.14) | 0.10 (0.08–0.14) |
| HFPO-DA | 0.01 | 0 | 6 | <LOD | 0.11 (<LOD-3.25) | 0 | 0 | <LOD | <LOD |
| PFO2HxA | 0.05 | 94 | 97 | 0.10 (<LOD-0.15) | 0.10 (0.04–0.16) | 88 | 100 | 0.10 (<LOD-0.15) | 0.11 (0.06–0.15) |
| PFO5DoDA | 0.1 | 0 | 3 | <LOD | 0.02 (<LOD-0.49) | 0 | 0 | <LOD | <LOD |
| NVHOS | 0.1 | 3 | 0 | 0.007 (<LOD-0.25) | <LOD | 0 | 7 | <LOD | 0.02 (<LOD-0.25) |
| NBP2 | 0.01 | 65 | 63 | 0.12 (ND-0.26) | 0.57 (ND-2.38) | 59 | 71 | 0.11 (<LOD-0.24) | 0.13 (<LOD-0.26) |
| Totals | |||||||||
| ΣPFAS | 6.93 (1.74–101.90) | 3.75 (1.52–14.02) | 4.41 (1.74–13.50) | 11.61 (2.58–101.90) | |||||
| ΣPFEAs | 0.39 (0.09–0.61) | 0.78 (0.14–2.63) | 0.36 (0.09–0.61) | 0.43 (0.29–0.61) | |||||
| ΣPFSAs | 5.03 (0.91–99.33) | 2.13 (0.75–12.19) | 2.79 (0.91–10.49) | 9.59 (1.25–99.3) | |||||
| ΣPFCAs | 1.23 (0.54–3.62) | 0.84 (0.11–1.60) | 1.28 (0.54–3.62) | 1.16 (0.80–2.08) | |||||
Letters indicate statistical differences in detection frequencies across species based on Fisher’s exact test. a = p ≤ 0.05. b = p ≤ 0.01. c = p ≤ 0.001.
Abbreviations: GM, geometric mean; LOD, limit of detection; PFCA, perfluorocarboxylic acid; PFSA, perfluorosulfonic acid; PFEA, perfluoroalkyl ether acid.
Unsupervised hierarchical clustering analysis and PCA of Log10(X + 1) transformed concentration data demonstrated unique exposure profiles between dogs and horses, with principal components (PC) 1 and PC2 accounting for 38% of the variance (Figure 1A,B). Analysis of the difference between mean Log10(X + 1) PFAS concentrations revealed a significant effect of species [F (1, 1220) = 47.92; p < 0.0001; η2 = 0.02] and congener [F (29, 1220) = 60.77; p < 0.0001; η2 = 0.42] and a significant interaction between species and congener [F (19, 1220) = 16.54; p < 0.0001; η2 = 0.12]. Šidák’s multiple comparisons test indicated that mean ΣPFAS, PFOS, PFHxS, and PFOA were significantly higher in dogs (p < 0.0001; d = 0.97; p < 0.0001; d = 0.90; p < 0.0001; d = 1.26; p = 0.006; d = 1.19), and Nafion Byproduct 2 (NBP2) was higher in horses (p = 0.001; d = 0.79; Figure 1C).
Figure 1.
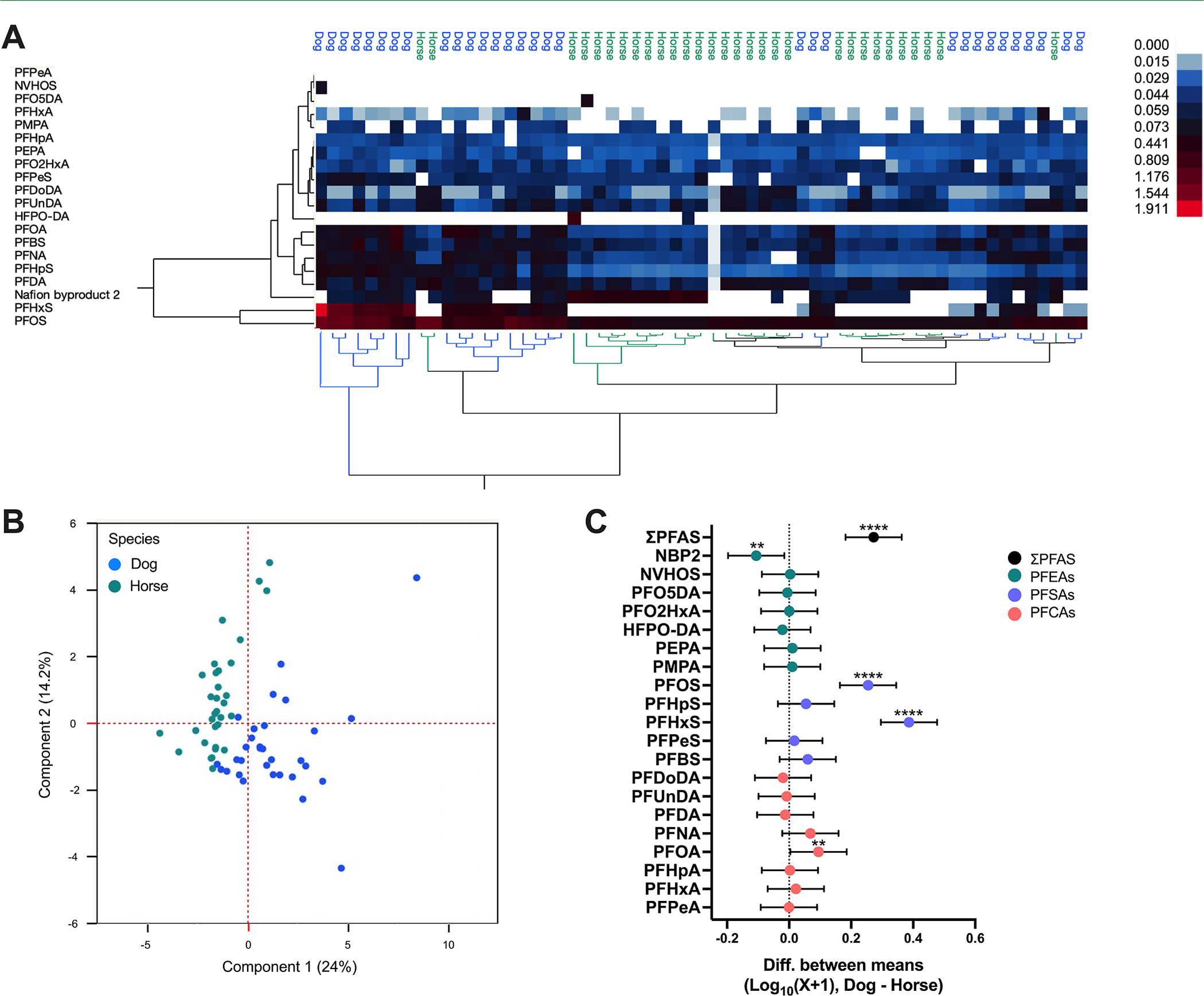
Comparison of PFAS in dogs and horses. Results of (A) unsupervised hierarchical clustering and (B) principal component analysis of Log10(X + 1) transformed serum PFAS concentrations from dogs and horses. (C) Differences between mean Log10(X + 1) transformed serum PFAS concentrations with 95% confidence intervals, dog–horse (fluoroethers, green; sulfonics, purple; carboxylics, orange; **p ≤ 0.01; ****p ≤ 0.0001); dog n = 31 and horse n = 32.
Unique PFAS Exposure Profiles in Dogs given Drinking Well or Bottled Water.
No significant differences in detection frequencies for any individual PFAS were observed between dogs given bottled water or well water. However, the detection frequency of all PFAS congeners except PFHxA was higher for dogs consuming primarily well water. Notably, ΣPFSA concentrations were higher in dogs given well water, and ΣPFCA concentrations, particularly PFOA, were moderately higher in dogs drinking bottled water (Table 1). Unsupervised hierarchical clustering analysis and PCA of Log10(X + 1) transformed data demonstrated unique exposure profiles between dogs given bottled water and dogs given well water, with 46% of the variance explained by PC1 and PC2 (Figure 2A,B). A significant effect of water source [F (1, 580) = 24.59; p < 0.0001; η2 = 0.01], congener [F (19, 580) = 56.91; p < 0.0001; η2 = 0.6], and interaction [F (19, 580) = 9.42; p < 0.0001; η2 = 0.10] on the difference between mean Log10(X + 1) PFAS concentrations was observed. Sidák’s multiple comparisons test indicated that mean ΣPFAS, PFOS, and PFHxS are significantly higher in dogs that consumed well water compared to those in dogs consuming bottled water (p < 0.0001; d = 1.26; p < 0.0001; d = 1.32; p < 0.0001; d = 1.18; Figure 2C).
Figure 2.
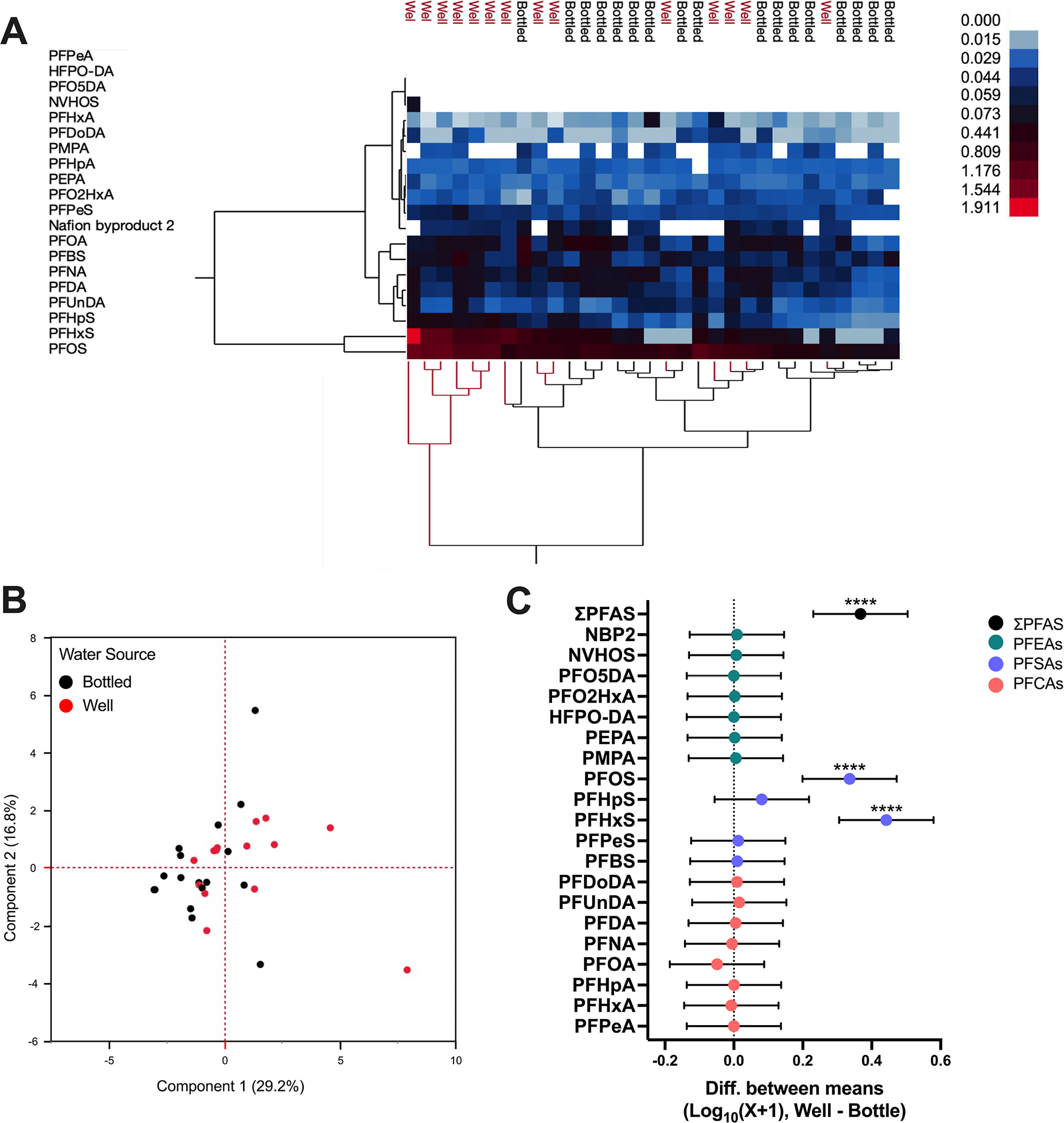
Comparison of PFAS in dogs’ drinking well or bottled water. Results of (A) unsupervised hierarchical clustering and (B) principal component analysis of Log10(X + 1) transformed serum PFAS concentrations from dogs given bottled water and well water. (C) Difference between mean Log10(X + 1) transformed serum PFAS concentrations with 95% confidence intervals, well–bottled (fluoroethers, green; sulfonics, purple; carboxylics, orange; ****p ≤ 0.0001). Dogs given well water (n = 14) compared to bottled water (n = 17).
Correlations between Clinical Biomarkers and PFAS Concentrations.
For dogs, we identified a significant overall effect of water source, with household as a nested factor, on PFAS concentrations (F = 8818.22; p < 0.001; η2 = 1.0). No significant overall effect of sex, age, or weight were observed (Table S9). A significant effect of sex was identified for NBP2, with males having higher levels than females [difference between means (ΔM) = 0.04; 95% CI: 0.02, 0.06; p = 0.003].
Multiple linear regression analysis for dogs was adjusted for household and water source to evaluate the associations between health biomarkers and PFAS concentrations (Tables 2; S11). Significant positive correlations between PFHpS and alkaline phosphatase (ALP), perfluorobutanesulfonic acid (PFBS) and alanine aminotransferase (ALT), PFO2HxA and blood urea nitrogen (BUN), ΣPFAS and BUN, perfluoroethoxypropyl carboxylic acid (PEPA) and glucose, PFO2HxA and total protein, and PFO2HxA and globulin were observed. Significant negative correlations were observed for PFHxS and ALP, NBP2 and ALP, PMPA and BUN, and PFO2HxA and albumin/globulin ratio.
Table 2.
Correlations between PFAS Concentration [Log10(X + 1)] in Dogs and each Biomarkera
| biomarker |
N
|
PFAS β (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PFBS | PFHxS | PFHpS | PMPA | PEPA | PFO2HxA | nbp2 | ΣPFAS | ||
| albumin (g/dL) | 29 | 1.03 | −0.22 | −1.03 | −2.30 | −3.26 | −1.43 | 2.74 | −2.30 |
| (−3.13, 5.37) | (−1.10, 0.67) | (−8.52, 6.45) | (−10.64, 6.05) | (−33.03, 26.51) | (−15.78, 12.92) | (−4.79, 10.27) | (−10.64, 6.05) | ||
| alkaline phosphatase (U/L) | 29 | 3.07 | −3.79 b | 21.12 a | −17.58 | 15.39 | −10.49 | −21.46 a | −17.58 |
| (−9.34, 15.47) | (−6.31, −1.27) | (−0.26, 42.50) | (−41.43, 6.27) | (−69.65, 100.43) | (−51.48, 30.51) | (−42.96, 0.04) | (−41.43, 6.27) | ||
| alanine aminotransferase (U/L) | 29 | 10.93 a | −1.33 | 14.31 | 0.84 | 19.78 | 6.59 | −6.21 | 0.84 |
| (0.41, 21.45) | (−3.47, 0.81) | (−3.81, 32.44) | (−10.39, 21.06) | (−52.31, 91.88) | (−28.16, 41.35) | (−24.44, 12.02) | (−19.39, 21.06) | ||
| amylase (U/L) | 29 | 1678.36 | −377.37 | 245.28 | −2453.74 | −5551.19 | −4001.65 | −1669.17 | −2453.74 |
| (−2219.66, 5576.38) | (−1169.45, 414.71) | (−6470.10, 6960.65) | (−9947.19, 5039.71) | (−32,266.75, 21,164.37) | (−16,881.31, 8878.01) | (−8423.44, 5085.10) | (−9947.19, 5039.71) | ||
| total bilirubin (mg/dL) | 24 | 3.95 | −2.27 | 3.09 | −18.19 | 25.63 | −14.86 | −1.16 | −18.19 |
| (−7.43, 15.33) | (−5.82, 1.28) | (−21.47, 27.64) | (−43.90, 7.53) | (−40.76, 92.02) | (−61.31, 31.59) | (−19.83, 17.50) | (−43.90, 7.53) | ||
| blood urea nitrogen (mg/dL) | 29 | 1.39 | 0.42 | −2.89 | −9.65 b | −12.88 | 14.19 b | 0.46 | −9.65 |
| (−2.22, 5.00) | (−0.32, 1.15) | (−9.11, 3.33) | (−16.59, −2.71) | (−37.63, 11.87) | (2.26, 26.13) | (−5.80, 6.71) | (−16.59, −2.71) | ||
| calcium (mg/dL) | 28 | 5.11 | 0.22 | 3.57 | −1.90 | 22.43 | 9.46 | 10.44 | −1.90 |
| (−10.89, 21.10) | (−2.95, 3.38) | (−25.09, 32.22) | (−33.07, 29.27) | (−84.59, 129.45) | (−41.98, 60.91) | (−16.55, 37.44) | (−33.07, 29.27) | ||
| phosphorus (mg/dL) | 29 | −0.81 | −0.23 | 1.80 | −0.66 | −7.23 | −4.93 | −3.63 | −0.66 |
| (−3.94, 2.31) | (−0.86, 0.41) | (−3.59, 7.18) | (−6.67, 5.35) | (−28.65, 14.20) | (−15.26, 5.40) | (−9.05, 1.79) | (−6.67, 5.35) | ||
| creatinine (mg/dL) | 29 | −1.38 | 0.72 | −4.23 | −1.75 | −7.52 | 12.10 | 4.79 | −1.75 |
| (−5.59, 2.84) | (−0.13, 1.58) | (−11.49, 3.03) | (−9.85, 6.35) | (−36.40, 21.35) | (−0.183, 26.02) | (−2.52, 12.08) | (−9.85, 6.35) | ||
| glucose (mg/dL) | 29 | 1.66 | 0.35 | −0.49 | 3.18 | 49.99a | −1.29 | 9.49 | 3.18 |
| (−5.19, 8.52) | (−1.05, 1.74) | (−12.30, 11.31) | (−9.99, 16.35) | (3.03, 96.96) | (−23.94, 21.37) | (−2.38, 21.35) | (−9.99, 16.35) | ||
| sodium (mmol/L) | 29 | −0.04 | 0.00 | 0.39 | 0.29 | −1.89 | 0.27 | −0.14 | 0.29 |
| (−0.34, 0.27) | (−0.06, 0.06) | (−0.14, 0.93) | (−0.31, 0.88) | (−4.01, 0.22) | (−0.75, 1.29) | (−0.67, 0.39) | (−0.31, 0.88) | ||
| potassium (mmol/L) | 20 | 10.29 | −0.27 | 7.51 | −5.69 | −64.57 | 30.57 | −17.47 | −5.69 |
| (−21.32, 41.90) | (−4.37, 3.82) | (−20.53, 35.56) | (−37.59, 26.21) | (−147.09, 17.95) | (−26.34, 87.48) | (−37.70, 2.77) | (−37.59, 26.21) | ||
| total Protein (g/dL) | 29 | 8.65 | 0.69 | −1.45 | 1.02 | −44.83 | 42.09 b | −3.16 | 1.02 |
| (−0.41, 17.71) | (−1.15, 2.54) | (−17.06, 14.16) | (−16.40, 18.44) | (−106.93, 17.27) | (12.16, 72.03) | (−18.86, 12.54) | (−16.40, 18.44) | ||
| globulin (g/dL) | 29 | 7.31 | 0.81 | −0.42 | 3.21 | −43.75 | 43.03 b | −6.26 | 3.21 |
| (−1.07, 15.68) | (−0.89, 2.52) | (−14.85, 14.01) | (−12.89, 19.32) | (−101.16, 13.66) | (15.36, 70.71) | (−20.77, 8.26) | (−12.89, 19.32) | ||
| Albumin/Globulin | |||||||||
| ratio | 29 | −3.37 | −0.23 | −1.46 | −2.38 | 16.89 | −22.28 b | 3.90 | −2.38 |
| (−8.28, 1.53) | (−1.23, 0.76) | (−9.91, 6.99) | (−11.81, 7.05) | (−16.72, 50.51) | (−38.49, −6.08) | (−4.59, 12.40) | (−11.81, 7.05) | ||
Data in bold indicate a significant association. Letters indicate p-values with a = p ≤ 0.05 and b = p ≤ 0.01. β represents the beta coefficients and CI represents the 95% confidence intervals.
Because 51.4% of the horses in this study resided at the same location, a significant overall effect of household (F = 58.77; p < 0.001; η2 = 1.0) was evident in our GLM results. However, no overall effects of sex, age, or weight were observed (Table S10). Weak to moderate effects of body weight were observed for PFDoDA (F = 5.52; r = 0.42; p = 0.03; η2 = 0.25), PFO2HxA (F = 6.92; r = 0.18; p = 0.02; η2 = 0.29), and PFPeS (F = 5.99; r = 0.20; p = 0.03; η2 = 0.26). Sensitivity analysis conducted by removing samples with non-detectable concentrations of PFPeS (n = 5), did not identify a significant relationship between PFPeS and animal body weight.
For biomarker analysis, our multiple linear regression analysis for horses was adjusted for household and identified significant positive correlations between PFBS and creatine kinase, and PFUnDA and calcium. Negative correlations for PFHpA and total carbon dioxide, PFOA and creatine kinase, PFDoDA and gamma glutamyl transferase (GGT), and PFHpA and albumin/globulin ratio were observed (Tables 3 and S12).
Table 3.
Correlations between PFAS Concentration [Log10(X + 1)] in Horses and each Biomarkera
| biomarker |
N |
PFAS β (95% CI) |
||||
|---|---|---|---|---|---|---|
| PFHpA | PFOA | PFUnDA | PFDoDA | PFBS | ||
| sodium (mmol/L) | 26 | −3.30 | −1.98 | 2.39 | 0.49 | 1.46 |
| (−11.88, 5.28) | (−8.45, 4.49) | (−0.88, 5.66) | (−1.78, 2.77) | (−1.02, 3.95) | ||
| potassium (mmol/L) | 26 | −4.96 | −66.67 | 53.16 | −4.79 | 19.33 |
| (−198.13, 188.22) | (−212.46, 79.12) | (−20.43, 79.12) | (−56.00, 46.43) | (−36.59, 75.24) | ||
| total carbon dioxide (mmol/L) | 26 | −378.26 a | −16.80 | 110.83 | 44.28 | 5.52 |
| (−758.30, 1.77) | (−303.61, 270.02) | (−33.95, 255.61) | (−56.47, 145.00) | (−104.48, 115.53) | ||
| creatine kinase (U/L) | 26 | 13.48 | −53.48 b | 18.59 | −4.38 | 19.78 a |
| (−38.59, 65.55) | (−92.78, −14.18) | (−1.25, 38.43) | (−18.18, 9.43) | (4.71, 34.85) | ||
| glucose (mg/dL) | 26 | −2869.64 | 2743.38 | −335.55 | −192.46 | −95.69 |
| (6596.67, 857.38) | (−69.46, 5556.21) | (−1755.44, 1084.34) | (−1180.54, 795.62) | (−1174.50, 983.13) | ||
| calcium (mg/dL) | 26 | −2.27 | −3.49 | 5.39 a | −0.32 | 1.93 |
| (−16.52, 11.98) | (−14,24, 7.27) | (−0.04, 10.82) | (−4.10, 3.45) | (−2.20, 6.06) | ||
| bBlood urea nitrogen (mg/dL) | 26 | −187.74 | −68.39 | 37.08 | −12.75 | 61.91 |
| (−617.09, 241.62) | (−392.43, 255.65) | (−126.49, 200.66) | (−126.58, 101.08) | (−62.37, 186.19) | ||
| creatinine (mg/dL) | 26 | −18.39 | 11.91 | 5.23 | 0.75 | 6.11 |
| (−59.30, 22.52) | (−18.97, 42.78) | (−10.36, 20.82) | (−10.10, 11.59) | (−5.74, 17.95) | ||
| aspartate Aminotransferase (U/L) | 26 | −3581.06 | −3507.56 | 3039.93 | −1599.30 | 1411.47 |
| (−13,041.88, 5879.77) | (−3507.56, 3632.64) | (−564.37, 6644.24) | (−4107.49, 908.89) | (−1327.03, 4149.97) | ||
| total bilirubin (mg/dL) | 26 | 3.29 | −2.12 | 1.51 | 1.09 | −0.45 |
| (−53.03, 59.60) | (−44.62, 40.39) | (−19.95, 23.00) | (−13.84, 16.02) | (−16.76, 15.85) | ||
| GGT (U/L) | 26 | 30.38 | −15.39 | 23.92 | −17.46 a | 2.04 |
| (−35.65, 96.41) | (−65.22, 34.45) | (−1.24, 49.08) | (−34.96, 0.05) | (−17.07, 21.15) | ||
| albumin (g/dL) | 26 | −2.82 | −5.74 | 1.66 | 2.11 | 3.26 |
| (−14.68, 9.04) | (−14.69, 3.21) | (−2.86, 6.18) | (−1.03, 5.26) | (−0.18, 6.69) | ||
| total protein (g/dL) | 26 | 52.81 | −49.95 | 3.62 | 2.33 | 10.69 |
| (−69.44, 175.06) | (−142.22, 42.31) | (−42.96, 50.19) | (−30.08, 34.74) | (−24.69, 46.08) | ||
| globulin (g/dL) | 26 | 61.39 | −29.40 | −0.11 | −4.76 | −0.87 |
| (−34.92, 157.71) | (−102.08, 43.29) | (−36.80, 36.59) | (−30.29, 20.78) | (−28.75, 27.01) | ||
| Albumin/Globulin | ||||||
| ratio | 26 | −37.41 a | 11.41 | 2.92 | 4.76 | 2.04 |
| (−72.81, −2.00) | (−15.31, 38.13) | (−10.57, 16.41) | (−4.63, 14.15) | (−8.21, 12.29) | ||
Data in bold indicate a significant association. letters indicate p-values with a = p < 0.05 and b = p < 0.01. β represents the beta coefficients and CI represents the 95% confidence intervals.
DISCUSSION
Analysis of serum PFAS concentrations in domestic dogs and horses from Gray’s Creek NC revealed that these companion animals and livestock are ubiquitously exposed to PFAS. Of the 20 PFAS detected, 12 were detected in more than half the samples from the two species. The PFSAs were measured at the highest concentrations, with PFOS accounting for 43% of PFSAs and 41% of ΣPFAS in dogs. Those findings are consistent with previous studies of domestic cats in NC where PFOS (G.M = 8.89 ng/mL) and PFHxS (G.M = 6.91 ng/mL) were about twofold higher than the concentrations we observed in pet dogs drinking well water (PFOS G.M = 4.93 ng/mL; PFHxS G.M = 3.26 ng/mL).33 Further, the median concentrations of PFOS and PFHxS in dogs consuming well water (PFOS 4.65 ng/mL; PFHxS 3.25 ng/mL) were similar to the concentrations found in children residing in Wilmington NC (PFOS 5.1 ng/mL; PFHxS 1.9 ng/mL).44 This finding suggests that dogs may serve as an important sentinel of household PFAS exposure that is particularly relevant to humans during sensitive developmental windows.49,50 PFAS concentrations in horse serum was also dominated by PFSAs, with PFOS accounting for 85% of PFSAs and 48% of ΣPFAS, but the total concentrations were threefold lower than the concentrations found in dogs. Compared to dogs, horse serum was enriched for PFEAs; this difference was the result of increased concentrations of NBP2, which is a production intermediate of Nafion fluoropolymer manufacture. Collectively, these findings highlight the utility of companion animal and livestock species as sentinels of PFAS exposure differences inside and outside of the home.
The relative overall composition of PFAS detected in pet serum appears reflective of their primary living environment and source of drinking water. Exposure to an indoor environment was associated with higher ΣPFAS concentrations in dogs, which is consistent with previous reports showing lower concentrations of PFAS in feral cats compared to indoor domestic cats.33 Notably, dog serum was dominated by PFOS, PFHxS, and PFOA, an exposure profile reflective of the dominant perfluorinated compounds previously reported in human serum samples.44,51 These similarities likely relate to shared exposure routes within indoor environments including water, house dust, and PFAS treated textiles.42,43,52,53 While we did not specifically look at PFAS concentrations in dog food, others have reported concentrations in the parts per billion (i.e., ng/g) range for PFOS, PFOA, and PFHxS. Dog food often contains food industry by-products and fish meal, which in part may explain the higher concentrations of these PFAS in dog serum compared to horses.54–56 Follow-up studies looking at the total daily intake of PFAS from food and water sources in dogs may clarify the contribution of the ambient environment to circulating PFAS concentrations as opposed to dietary sources.54
Source of drinking water was also a major contributing factor to serum PFAS concentrations in dogs, with about half of the dogs in this study given well water and the other half provided bottled water for drinking. Dogs whose primary drinking water source was from the well had higher concentrations of PFSAs, mainly PFOS and PFHxS, as compared to dogs given bottled water. Those two PFSAs are consistently detected as the primary contaminants found in biota living near the Cape Fear River watershed, a drinking water source that services up to 1.5 million North Carolinians. Further, PFCAs, including PFOA, PFHxA, and PFHpA, have also been frequently detected in surface water samples from the Cape Fear River often at concentrations higher than PFSAs.20 Nevertheless, recent findings from our lab and others have reported PFOS as the PFAS found at the highest concentrations in wildlife and humans that co-utilize the Cape Fear River, potentially demonstrating high degrees of bioaccumulation for this perfluoroalkyl acid and/or decomposition of polyfluoroalkyl compounds into PFOS as a terminal breakdown product.16,17,21,22,44 Additional studies are needed to determine if these findings are driven by (1) unidentified sources of PFOS exposure, including dietary sources that may retain PFOS (e.g., fish and vegetation), (2) differences in biological half-lives (PFOS > PFOA) in these species, and/or (3) terminal transformation of longer-chain precursors into PFOS that result in the high concentrations detected in serum.57–59
In contrast to dogs, horses living in the same area had higher concentrations of NBP2 in their serum and unique, albeit limited, detection of HFPO-DA and PFO5DoDA. Some of these PFEAs, like HFPO-DA, have been shown to have substantially shorter half-lives than their longer-chain predecessors (e.g., PFOA), on the order of hours rather than years. Therefore, it is possible that we only see detectable levels of HFPO-DA and PFO5DoDA in horses due to contamination of the outdoor environment, more time spent outdoors, and consumption of grasses, hay, or other forage, which may contain PFAS that are taken up from contaminated water and soil or deposited from contaminated air and rainwater.60–64 However, it is also important to consider that differences in the latency between water consumption and the time of blood collection, in conjunction with reduced half-lives of shorter-chain PFAS, may have contributed to the limited detection of HFPO-DA in horses and NVHOS in dogs. This seems particularly relevant in the case of HFPO-DA, where we only see detectable levels in horses that reside at the same household, which also had the highest concentrations of HFPO-DA in well water.
Since NBP2 was first identified in the Cape Fear River in 2017 it has been consistently detected in humans and animals that reside in and around this river basin.14,16,17,44 To our knowledge, this is the first study to report PFAS exposure in horses. The relatively high frequency of detection of NBP2 (64.2%) and the higher geometric mean concentration of NBP2 in horses (0.57 ng/mL) compared to those in dogs (0.12 ng/mL) emphasize the utility of these animals as sentinels of contaminant exposure in the natural environment from a point source. It is notable that PFHxS, one of the most commonly detected PFAS, was not detected in any of the horses in this study. While more work is needed to understand why PFHxS was not detected in horses, this finding highlights the importance of looking at sentinel species that occupy different environments and may suggests that behavioral or physiological differences of species may contribute to differences in the ability to detect even common PFAS congeners.
In addition to living environment and source of drinking water, we identified sex and body weight as factors influencing serum concentrations for some PFAS. In dogs, we observed a significant effect of sex on NBP2 concentration, with males having higher serum NBP2 concentrations than females, a trend that has been previously shown for other PFEAs in rodent models.31,40,41 In horses, negative correlations between body weight and serum concentrations of PFDoDA and PFO2HxA were observed. Previously, we reported similar findings in Striped Bass, with negative correlations for PFOS, PFDA, and NBP2 with increasing body weight and size.16 While a more robustly powered population study is required to evaluate the biological significance of these relationships in each species, our findings do suggest and support previous studies showing that factors including sex and body weight contribute to differences in absorption, distribution, and/or excretion of PFAS.25,65
Our multiple linear regression analysis found few changes across blood chemistry parameters that were correlated with individual PFAS and no significant correlations, except for BUN in dogs, with total PFAS. Within dogs, two key diagnostic biomarkers used to assess liver function, ALP and ALT, were correlated with serum PFAS concentrations. Specifically, positive correlations between ALP and PFHpS and ALT and PFBS were observed. Although ALT was within the normal range for the majority of animals, ALP was found to be above the normal range in 38% of dogs. Previous studies, including studies in domestic dogs, have shown that long-term exposure to PFAS can increase ALP, highlighting the liver as a critical target of PFAS toxicity.54,66–70 In addition to elevations in ALP, elevated glucose levels may also be a sign of liver toxicity as chronic liver damage can lead to glucose intolerance and even diabetes.71,72 Glucose levels were above the expected normal range in 21% of animals; however, animals in this study were not fasted prior to blood collection, which may confound the positive relationship observed between PEPA and glucose. Levels of BUN, total protein, and globulin, diagnostic indicators of liver and kidney diseases, were all positively correlated with PFO2HxA, but concentrations of these biomarkers primarily fell within the normal range, except for globulin, which was lower in 31% of dogs. This finding is consistent with numerous human and animals studies that found suppression of adaptive immune activities associated with PFAS; however, future studies looking at markers of both innate and adaptive immune system in these animals are warranted to better characterize the physiological basis for the observed changes in globulins.73–76 Few studies have evaluated the impact of environmentally relevant mixtures and replacement PFEAs on the kidneys or liver.16,70,77–79 Our findings provide further suggestive evidence of renal and hepatic sensitivity to PFAS exposure, and have identified ALP, glucose, and globulins as potential biomarkers of environmentally relevant PFAS exposures in dogs.
In horses, biomarkers of liver and kidney function were also associated with serum concentrations of PFAS. Significant correlations between serum PFAS concentrations and total carbon dioxide, creatine kinase, calcium, GGT, and albumin/globulin ratio were observed. Strikingly, albumin concentration was elevated in 62% of horses. Albumin serves as the primary transport protein for a number of PFAS, including PFOS, PFOA, perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), and PFHxS, which may have important ramifications for the half-lives of PFAS and the function and half-life of serum albumin itself.80–87 Additional samples are needed to establish if there are any interactions between albumin concentrations and PFAS exposure in horses living in the Gray’s Creek area. While GGT was elevated for 23% of animals, the other biomarkers, total carbon dioxide, creatine kinase, and calcium, generally fell within a clinically normal range for the majority of horses in this study. Increases in GGT are a general clinical indicator of liver disease; however, the importance of the negative correlation between GGT and PFDoDA observed in these animals remains unclear but may suggest a subclinical vitamin B6 and magnesium deficiency in the study animals, which we consider unrelated to PFAS exposures.
Companion animals and livestock, like dogs and horses, can provide insight into environmentally relevant human and wildlife exposures due to their shared indoor and outdoor environments. While this sentinel niche has previously been established for dogs, horses have been underutilized in this domain. It is important to reiterate that this study was conducted to explore the potential associations between serum PFAS concentrations and health biomarkers that can be used as clinical indicators of underlying conditions. Therefore, reported associations are unable to define causal relationships. Nonetheless, our findings build upon previous studies of PFAS exposure in sentinel animals and help to establish horses as a useful biomonitoring tool of residential PFAS contamination from a nearby point source. A more robustly powered study, with a greater diversity of participating households, is needed to ascertain the strength of associations between the observed changes in diagnostic biomarkers and PFAS exposure in dogs and horses living in Gray’s Creek NC. The ubiquity of domestic animals, availability of diagnostic tools for health assessments, and overlap with human diseases emphasizes the importance of utilizing a “One Health” approach to study shared environmental exposures and adverse health outcomes for humans and animals in the industrialized world.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the residents of Gray’s Creek and their canine and equine companions that participated in this study. Research reported in this publication was supported by the North Carolina Policy Collaboratory. We also thank John Szoka and Kirk deViere for their support of the Gray’s Creek community and this research. We are grateful for the input of Dr. Thomas Jackson and for his insightful feedback on data analysis. S.M.B. received support from the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number P42 ES031009. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare the following competing financial interest(s): The following authors disclose their associations that could be perceived as potential competing interests with the subject matter discussed in this manuscript: Dr. Kentley Dean is an employee of Southern Oaks Animal Hospital a full-service veterinary medical facility, located in Hope Mills, NC. Mike Watters and Debra Stevens-Stewart are residents of the study area and served as community representatives for this community engage participatory research study. The remaining authors declare no competing interests.
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.est.3c01146
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.3c01146.
Details on supplemental methods for PFAS analysis, mass spectrometry, quality controls, and SRM 1957 analysis (PDF)
Results of residential water testing for HFPO-DA (Table S1), pet demographics (Table S2), targeted PFAS analytes (Table S3), hematological parameters measured by VetScan blood chemistry rotors (Table S4), dog VetScan data (Table S5), horse VetScan data (Table S6), raw PFAS concentrations in dog serum (Table S7), raw PFAS concentrations in horse serum (Table S8), results of general linear model analysis for effect of water source (with household as a nested factor), sex, age, and body weight on serum PFAS concentrations in dogs (Table S9), results of general linear model analysis for effect of household, sex, age, and body weight on serum PFAS concentrations in horses (Table S10), and results of multiple linear regression analysis of log10(X+1) PFAS concentrations and health biomarkers in dogs (Table S11) and horses (Table S12) (XLSX)
Contributor Information
Kylie D. Rock, Center for Environmental and Health Effects of PFAS, Department of Biological Sciences, North Carolina State University, Raleigh, North Carolina 27695, United States
Madison E. Polera, Department of Applied Ecology, North Carolina State University, Raleigh, North Carolina 27695, United States
Theresa C. Guillette, Oak Ridge Institute for Science and Education Research Participation Program, Oak Ridge, Tennessee 37831, United States
Hannah M. Starnes, Center for Environmental and Health Effects of PFAS, Department of Biological Sciences, North Carolina State University, Raleigh, North Carolina 27695, United States
Kentley Dean, Southern Oaks Animal Hospital, Hope Mills, North Carolina 28348, United States.
Mike Watters, Gray’s Creek Residents United against PFAS in Our Wells & Rivers, Gray’s Creek, North Carolina 28348, United States.
Debra Stevens-Stewart, Gray’s Creek Residents United against PFAS in Our Wells & Rivers, Gray’s Creek, North Carolina 28348, United States.
Scott M. Belcher, Center for Environmental and Health Effects of PFAS, Department of Biological Sciences, North Carolina State University, Raleigh, North Carolina 27695, United States
REFERENCES
- (1).Glüge J; Scheringer M; Cousins IT; DeWitt JC; Goldenman G; Herzke D; Lohmann R; Ng CA; Trier X; Wang Z An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci.: Processes Impacts 2020, 22, 2345–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Gaines LGT Historical and current usage of per- and polyfluoroalkyl substances (PFAS): A literature review. Am. J. Ind. Med. 2023, 66, 353–378. [DOI] [PubMed] [Google Scholar]
- (3).Bečanová J; Komprdová K; Vrana B; Klánová J Annual dynamics of perfluorinated compounds in sediment: A case study in the Morava River in Zĺin district, Czech Republic. Chemosphere 2016, 151, 225–233. [DOI] [PubMed] [Google Scholar]
- (4).Boiteux V; Dauchy X; Bach C; Colin A; Hemard J; Sagres V; Rosin C; Munoz JF Concentrations and patterns of perfluoroalkyl and polyfluoroalkyl substances in a river and three drinking water treatment plants near and far from a major production source. Sci. Total Environ. 2017, 583, 393–400. [DOI] [PubMed] [Google Scholar]
- (5).Chen H; Sun R; Zhang C; Han J; Wang X; Han G; He X Occurrence, spatial and temporal distributions of perfluoroalkyl substances in wastewater, seawater and sediment from Bohai Sea, China. Environ. Pollut. 2016, 219, 389–398. [DOI] [PubMed] [Google Scholar]
- (6).Liu B; Zhang H; Xie L; Li J; Wang X; Zhao L; Wang Y; Yang B Spatial distribution and partition of perfluoroalkyl acids (PFAAs) in rivers of the Pearl River Delta, southern China. Sci. Total Environ. 2015, 524–525, 1–7. [DOI] [PubMed] [Google Scholar]
- (7).Saleeby B; Shimizu MS; Sanchez Garcia RI; Avery GB; Kieber RJ; Mead RN; Skrabal SA Isomers of emerging per- and polyfluoroalkyl substances in water and sediment from the Cape Fear River, North Carolina, USA. Chemosphere 2021, 262, 128359. [DOI] [PubMed] [Google Scholar]
- (8).Taniyasu S; Yamashita N; Moon HB; Kwok KY; Lam PKS; Horii Y; Petrick G; Kannan K Does wet precipitation represent local and regional atmospheric transportation by perfluorinated alkyl substances? Environ. Int. 2013, 55, 25–32. [DOI] [PubMed] [Google Scholar]
- (9).US EPA UENC for E. Toward a new comprehensive global database of per- and polyfluoroalkyl substances (PFASs): Summary report on updating the OECD 2007 list of per- and polyfluoroalkyl substances (PFASs) [Internet]. 2009. Available from: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/5099062 (accessed Sep 12, 2022). [Google Scholar]
- (10).Wang Y; Chang W; Wang L; Zhang Y; Zhang Y; Wang M; Wang Y; Li P A review of sources, multimedia distribution and health risks of novel fluorinated alternatives. Ecotoxicol. Environ. Saf. 2019, 182, 109402. [DOI] [PubMed] [Google Scholar]
- (11).Cousins IT; DeWitt JC; Glüge J; Goldenman G; Herzke D; Lohmann R; Ng CA; Scheringer M; Wang Z The high persistence of PFAS is sufficient for their management as a chemical class. Environ. Sci.: Processes Impacts 2020, 22, 2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Pétré MA; Genereux DP; Koropeckyj-Cox L; Knappe DRU; Duboscq S; Gilmore TE; Hopkins ZR Per- and Polyfluoroalkyl Substance (PFAS) Transport from Groundwater to Streams near a PFAS Manufacturing Facility in North Carolina, USA. Environ. Sci. Technol. 2021, 55, 5848–5856. [DOI] [PubMed] [Google Scholar]
- (13).D’Ambro EL; Pye HOT; Bash JO; Bowyer J; Allen C; Efstathiou C; Gilliam RC; Reynolds L; Talgo K; Murphy BN Characterizing the Air Emissions, Transport, and Deposition of Perand Polyfluoroalkyl Substances from a Fluoropolymer Manufacturing Facility. Environ. Sci. Technol. 2021, 55, 862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Hopkins ZR; Sun M; DeWitt JC; Knappe DRU Recently Detected Drinking Water Contaminants: GenX and Other Per- and Polyfluoroalkyl Ether Acids. J. AWWA 2018, 110, 13–28. [Google Scholar]
- (15).Sun M; Arevalo E; Strynar M; Lindstrom A; Richardson M; Kearns B; Pickett A; Smith C; Knappe DRU Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environ. Sci. Technol. Lett 2016, 3, 415–419. [Google Scholar]
- (16).Guillette TC; McCord J; Guillette M; Polera ME; Rachels KT; Morgeson C; Kotlarz N; Knappe DRU; Reading BJ; Strynar M; Belcher SM Elevated levels of per- and polyfluoroalkyl substances in Cape Fear River Striped Bass (Morone saxatilis) are associated with biomarkers of altered immune and liver function. Environ. Int. 2020, 136, 105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Guillette TC; Jackson TW; Guillette M; McCord J; Belcher SM Blood concentrations of per- and polyfluoroalkyl substances are associated with autoimmune-like effects in American alligators from Wilmington, North Carolina. Front. Toxicol. 2022, 4, 1010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).McCord J; Strynar M Identification of Per- and Polyfluoroalkyl Substances in the Cape Fear River by High Resolution Mass Spectrometry and Nontargeted Screening. Environ. Sci. Technol. 2019, 53, 4717–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Nakayama S; Strynar MJ; Helfant L; Egeghy P; Ye X; Lindstrom AB Perfluorinated Compounds in the Cape Fear Drainage Basin in North Carolina. Environ. Sci. Technol. 2007, 41, 5271–5276. [DOI] [PubMed] [Google Scholar]
- (20).Pétré MA; Salk KR; Stapleton HM; Ferguson PL; Tait G; Obenour DR; Knappe DRU; Genereux DP Per- and polyfluoroalkyl substances (PFAS) in river discharge: Modeling loads upstream and downstream of a PFAS manufacturing plant in the Cape Fear watershed, North Carolina. Sci. Total Environ. 2022, 831, 154763. [DOI] [PubMed] [Google Scholar]
- (21).Robuck AR; Cantwell MG; McCord JP; Addison LM; Pfohl M; Strynar MJ; McKinney R; Katz DR; Wiley DN; Lohmann R Legacy and Novel Per- and Polyfluoroalkyl Substances in Juvenile Seabirds from the U.S. Atlantic Coast. Environ. Sci. Technol. 2020, 54, 12938–12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Robuck AR; McCord JP; Strynar MJ; Cantwell MG; Wiley DN; Lohmann R Tissue-Specific Distribution of Legacy and Novel Per- and Polyfluoroalkyl Substances in Juvenile Seabirds. Environ. Sci. Technol. Lett. 2021, 8, 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).US EPA. PFOA Stewardship Program Baseline Year Summary Report [Internet]. 2015. Available from: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/pfoa-stewardship-program-baseline-year-summary-report.(accessed Dec 21, 2022) [Google Scholar]
- (24).Cousins IT; DeWitt JC; Glüge J; Goldenman G; Herzke D; Lohmann R; Miller M; Ng CA; Scheringer M; Vierke L; Wang Z Strategies for grouping per- and polyfluoroalkyl substances (PFAS) to protect human and environmental health. Environ. Sci.: Processes Impacts 2020, 22, 1444–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Wang Z; Cousins IT; Scheringer M; Hungerbühler K Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ. Int. 2013, 60, 242–248. [DOI] [PubMed] [Google Scholar]
- (26).US EPA. Questions and Answers: Drinking Water Health Advisories for PFOA, PFOS, GenX Chemicals and PFBS [Internet]. 2020. Available from: https://www.epa.gov/sdwa/questions-and-answers-drinking-water-health-advisories-pfoa-pfos-genx-chemicals-and-pfbs (accessed Nov 23, 2022) [Google Scholar]
- (27).US EPA. Drinking Water Health Advisory: Hexafluoropropylene Oxide (HFPO) Dimer Acid (CASRN 13252-13-6) and HFPO Dimer Acid Ammonium Salt (CASRN 62037-80-3), Also Known as “GenX Chemicals.” [Internet]; EPA, Office of Water, Health and Ecological Criteria Division: Washington, DC, 2022. Available from:. https://www.epa.gov/sdwa/drinking-water-health-advisories-has. [Google Scholar]
- (28).NC DEQ. Chemours Consent Order NC DEQ [Internet]. 2019. Available from: https://deq.nc.gov/news/key-issues/genx-investigation/chemours-consent-order (accessed Feb 7, 2023). [Google Scholar]
- (29).Blake BE; Cope HA; Hall SM; Keys RD; Mahler BW; McCord J; Scott B; Stapleton HM; Strynar MJ; Elmore SA; Fenton SE Evaluation of Maternal, Embryo, and Placental Effects in CD-1 Mice following Gestational Exposure to Perfluorooctanoic Acid (PFOA) or Hexafluoropropylene Oxide Dimer Acid (HFPO-DA or GenX). Environ. Health Perspect. 2020, 128, 027006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Blake BE; Miller CN; Nguyen H; Chappell VA; Phan TP; Phadke DP; Balik-Meisner MR; Mav D; Shah RR; Fenton SE Transcriptional pathways linked to fetal and maternal hepatic dysfunction caused by gestational exposure to perfluorooctanoic acid (PFOA) or hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) in CD-1 mice. Ecotoxicol. Environ. Saf. 2022, 248, 114314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).US EPA. Human Health Toxicity Assessments for GenX Chemicals [Internet]. 2021. Available from: https://www.epa.gov/chemical-research/human-health-toxicity-assessments-genx-chemicals. (accessed Nov 23, 2022). [Google Scholar]
- (32).Milnes MR; Guillette LJ Alligator Tales: New Lessons about Environmental Contaminants from a Sentinel Species. BioScience 2008, 58, 1027–1036. [Google Scholar]
- (33).Bost PC; Strynar MJ; Reiner JL; Zweigenbaum JA; Secoura PL; Lindstrom AB; Dye JAUSUS domestic cats as sentinels for perfluoroalkyl substances: Possible linkages with housing, obesity, and disease. Environ. Res. 2016, 151, 145–153. [DOI] [PubMed] [Google Scholar]
- (34).Wise CF; Hammel SC; Herkert NJ; Ospina M; Calafat AM; Breen M; Stapleton HM Comparative Assessment of Pesticide Exposures in Domestic Dogs and Their Owners Using Silicone Passive Samplers and Biomonitoring. Environ. Sci. Technol. 2022, 56, 1149–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Wise CF; Hammel SC; Herkert N; Ma J; Motsinger-Reif A; Stapleton HM; Breen M Comparative Exposure Assessment Using Silicone Passive Samplers Indicates That Domestic Dogs Are Sentinels To Support Human Health Research. Environ. Sci. Technol. 2020, 54, 7409–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Wang M; Guo W; Gardner S; Petreas M; Park JS Per- and polyfluoroalkyl substances in Northern California cats: Temporal comparison and a possible link to cat hyperthyroidism: Per- and polyfluoroalkyl substances in cats serum. Environ. Toxicol. Chem. 2018, 37, 2523–2529. [DOI] [PubMed] [Google Scholar]
- (37).Weiss JM; Jones B; Koekkoek J; Bignert A; Lamoree MH Per- and polyfluoroalkyl substances (PFASs) in Swedish household dust and exposure of pet cats. Environ. Sci. Pollut. Res. 2021, 28, 39001–39013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Shearin AL; Ostrander EA Leading the way: canine models of genomics and disease. Dis. Models Mech. 2010, 3, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Buttke DE Toxicology, Environmental Health, and the “One Health” Concept. J. Med. Toxicol. 2011, 7, 329–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Fenton SE; Ducatman A; Boobis A; DeWitt JC; Lau C; Ng C; Smith JS; Roberts SM Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Huang MC; Dzierlenga AL; Robinson VG; Waidyanatha S; DeVito MJ; Eifrid MA; Granville CA; Gibbs ST; Blystone CR Toxicokinetics of perfluorobutane sulfonate (PFBS), perfluorohexane-1-sulphonic acid (PFHxS), and perfluorooctane sulfonic acid (PFOS) in male and female Hsd:Sprague Dawley SD rats after intravenous and gavage administration. Toxicol. Rep. 2019, 6, 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Egeghy PP; Lorber M An assessment of the exposure of Americans to perfluorooctane sulfonate: A comparison of estimated intake with values inferred from NHANES data. J. Exposure Sci. Environ. Epidemiol. 2011, 21, 150–168. [DOI] [PubMed] [Google Scholar]
- (43).Strynar MJ; Lindstrom AB Perfluorinated Compounds in House Dust from Ohio and North Carolina, USA. Environ. Sci. Technol. 2008, 42, 3751–3756. [DOI] [PubMed] [Google Scholar]
- (44).Kotlarz N; McCord J; Collier D; Lea CS; Strynar M; Lindstrom AB; Wilkie AA; Islam JY; Matney K; Tarte P; Polera ME; Burdette K; DeWitt J; May K; Smart RC; Knappe DRU; Hoppin JA Measurement of Novel, Drinking Water-Associated PFAS in Blood from Adults and Children in Wilmington, North Carolina. Environ. Health Perspect. 2020, 128, 077005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Strynar M; Dagnino S; McMahen R; Liang S; Lindstrom A; Andersen E; McMillan L; Thurman M; Ferrer I; Ball C Identification of Novel Perfluoroalkyl Ether Carboxylic Acids (PFECAs) and Sulfonic Acids (PFESAs) in Natural Waters Using Accurate Mass Time-of-Flight Mass Spectrometry (TOFMS). Environ. Sci. Technol. 2015, 49, 11622–11630. [DOI] [PubMed] [Google Scholar]
- (46).O’Hara R, Kotze J. Do not log-transform count data. Nat Preced [Internet] 2010. Available from: https://www.nature.com/articles/npre.2010.4136.1 (accessed Feb 7, 2023). [Google Scholar]
- (47).Schober P; Boer C; Schwarte LA Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [DOI] [PubMed] [Google Scholar]
- (48).Lakens D Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Carroquino MJ; Posada M; Landrigan PJ Environmental Toxicology: Children at Risk. In Environmental Toxicology; Laws EA, Ed.; Springer New York: New York, NY, 2013; pp 239–291. [Google Scholar]
- (50).Heyer DB; Meredith RM Environmental toxicology: Sensitive periods of development and neurodevelopmental disorders. NeuroToxicology 2017, 58, 23–41. [DOI] [PubMed] [Google Scholar]
- (51).Guo P; Furnary T; Vasiliou V; Yan Q; Nyhan K; Jones DP; Johnson CH; Liew Z Non-targeted metabolomics and associations with per- and polyfluoroalkyl substances (PFAS) exposure in humans: A scoping review. Environ. Int. 2022, 162, 107159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Björklund JA; Thuresson K; de Wit CA Perfluoroalkyl Compounds (PFCs) in Indoor Dust: Concentrations, Human Exposure Estimates, and Sources. Environ. Sci. Technol. 2009, 43, 2276–2281. [DOI] [PubMed] [Google Scholar]
- (53).Hall SM; Patton S; Petreas M; Zhang S; Phillips AL; Hoffman K; Stapleton HM Per- and Polyfluoroalkyl Substances in Dust Collected from Residential Homes and Fire Stations in North America. Environ. Sci. Technol. 2020, 54, 14558–14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).You D; Chang X; Guo L; Xie W; Huang S; Li X; Chai H; Wang Y Per- and polyfluoroalkyl substances (PFASs) in the blood of police and Beagle dogs from Harbin, China: Concentrations and associations with hematological parameters. Chemosphere 2022, 299, 134367. [DOI] [PubMed] [Google Scholar]
- (55).Sévère S; Marchand P; Guiffard I; Morio F; Venisseau A; Veyrand B; Le Bizec B; Antignac JP; Abadie J Pollutants in pet dogs: a model for environmental links to breast cancer. SpringerPlus 2015, 4, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Brake HD; Wilkins MJ; Kaneene JB Per- and polyfluoroalkyl substances: using comparative medicine to understand exposure and adverse health outcomes in people and their pets. Am. J. Vet. Res. 2023, 1–11. [DOI] [PubMed] [Google Scholar]
- (57).Evich MG; Davis MJB; McCord JP; Acrey B; Awkerman JA; Knappe DRU; Lindstrom AB; Speth TF; Tebes-Stevens C; Strynar MJ; Wang Z; Weber EJ; Henderson WM; Washington JW Per- and polyfluoroalkyl substances in the environment. Science 2022, 375, No. eabg9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Xu Y; Fletcher T; Pineda D; Lindh CH; Nilsson C; Glynn A; Vogs C; Norstrom K; Lilja K; Jakobsson K; Li Y Serum Half-Lives for Short- and Long-Chain Perfluoroalkyl Acids after Ceasing Exposure from Drinking Water Contaminated by Firefighting Foam. Environ. Health Perspect. 2020, 128, 077004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Shi Y; Wang J; Pan Y; Cai Y Tissue distribution of perfluorinated compounds in farmed freshwater fish and human exposure by consumption. Environ. Toxicol. Chem. 2012, 31, 717–723. [DOI] [PubMed] [Google Scholar]
- (60).Gannon SA; Fasano WJ; Mawn MP; Nabb DL; Buck RC; Buxton LW; Jepson GW; Frame SR Absorption, distribution, metabolism, excretion, and kinetics of 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid ammonium salt following a single dose in rat, mouse, and cynomolgus monkey. Toxicology 2016, 340, 1–9. [DOI] [PubMed] [Google Scholar]
- (61).Zafeiraki E; Vassiliadou I; Costopoulou D; Leondiadis L; Schafft HA; Hoogenboom RLAP; van Leeuwen SPJ Perfluoroalkylated substances in edible livers of farm animals, including depuration behaviour in young sheep fed with contaminated grass. Chemosphere 2016, 156, 280–285. [DOI] [PubMed] [Google Scholar]
- (62).Mikkonen AT; Martin J; Upton RN; Barker AO; Brumley CM; Taylor MP; Mackenzie L; Roberts MS Spatio-Temporal Trends in Livestock Exposure to Per- and Polyfluoroalkyl Substances (Pfas) Inform Risk Assessment and Management Measures. Environ Res. 2023, 225, 115518. [DOI] [PubMed] [Google Scholar]
- (63).Zhang W; Liang Y Changing bioavailability of per- and polyfluoroalkyl substances (PFAS) to plant in biosolids amended soil through stabilization or mobilization. Environ. Pollut. 2022, 308, 119724. [DOI] [PubMed] [Google Scholar]
- (64).Tian Y; Yao Y; Chang S; Zhao Z; Zhao Y; Yuan X; Wu F; Sun H Occurrence and Phase Distribution of Neutral and Ionizable Per- and Polyfluoroalkyl Substances (PFASs) in the Atmosphere and Plant Leaves around Landfills: A Case Study in Tianjin, China. Environ. Sci. Technol. 2018, 52, 1301–1310. [DOI] [PubMed] [Google Scholar]
- (65).Tao L; Kannan K; Kajiwara N; Costa MM; Fillmann G; Takahashi S; Tanabe S Perfluorooctanesulfonate and Related Fluorochemicals in Albatrosses, Elephant Seals, Penguins, and Polar Skuas from the Southern Ocean. Environ. Sci. Technol. 2006, 40, 7642–7648. [DOI] [PubMed] [Google Scholar]
- (66).Wang Y; Yao J; Dai J; Ma L; Liu D; Xu H; Cui Q; Ma J; Zhang H Per- and polyfluoroalkyl substances (PFASs) in blood of captive Siberian tigers in China: Occurrence and associations with biochemical parameters. Environ. Pollut. 2020, 265, 114805. [DOI] [PubMed] [Google Scholar]
- (67).Crebelli R; Caiola S; Conti L; Cordelli E; De Luca G; Dellatte E; Eleuteri P; Iacovella N; Leopardi P; Marcon F; Sanchez M; Sestili P; Siniscalchi E; Villani P Can sustained exposure to PFAS trigger a genotoxic response? A comprehensive genotoxicity assessment in mice after subacute oral administration of PFOA and PFBA. Regul. Toxicol. Pharmacol. 2019, 106, 169–177. [DOI] [PubMed] [Google Scholar]
- (68).Zhou X; Wang J; Sheng N; Cui R; Deng Y; Dai J Subchronic reproductive effects of 6:2 chlorinated polyfluorinated ether sulfonate (6:2 Cl-PFAES), an alternative to PFOS, on adult male mice. J. Hazard. Mater. 2018, 358, 256–264. [DOI] [PubMed] [Google Scholar]
- (69).Zhang H; Zhou X; Sheng N; Cui R; Cui Q; Guo H; Guo Y; Sun Y; Dai J Subchronic Hepatotoxicity Effects of 6:2 Chlorinated Polyfluorinated Ether Sulfonate (6:2 Cl-PFESA), a Novel Perfluorooctanesulfonate (PFOS) Alternative, on Adult Male Mice. Environ. Sci. Technol. 2018, 52, 12809–12818. [DOI] [PubMed] [Google Scholar]
- (70).Salihovic S; Stubleski J; Kärrman A; Larsson A; Fall T; Lind L; Lind PM Changes in markers of liver function in relation to changes in perfluoroalkyl substances - A longitudinal study. Environ. Int. 2018, 117, 196–203. [DOI] [PubMed] [Google Scholar]
- (71).Petrides AS; Vogt C; Schulze-Berge D; Matthews D; Strohmeyer G Pathogenesis of glucose intolerance and diabetes mellitus in cirrhosis. Hepatology 1994, 19, 616–627. [DOI] [PubMed] [Google Scholar]
- (72).Petrides AS; Stanley T; Matthews DE; Vogt C; Bush AJ; Lambeth H Insulin resistance in cirrhosis: Prolonged reduction of hyperinsulinemia normalizes insulin sensitivity. Hepatology 1998, 28, 141–149. [DOI] [PubMed] [Google Scholar]
- (73).Grandjean P Delayed discovery, dissemination, and decisions on intervention in environmental health: a case study on immunotoxicity of perfluorinated alkylate substances. Environ. Health 2018, 17, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Peden-Adams MM; Keller JM; EuDaly JG; Berger J; Gilkeson GS; Keil DE Suppression of Humoral Immunity in Mice following Exposure to Perfluorooctane Sulfonate. Toxicol. Sci. 2008, 104, 144–154. [DOI] [PubMed] [Google Scholar]
- (75).Woodlief T; Vance S; Hu Q; DeWitt J Immunotoxicity of Per- and Polyfluoroalkyl Substances: Insights into Short-Chain PFAS Exposure. Toxics 2021, 9, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).DeWitt JC; Blossom SJ; Schaider LA Exposure to perfluoroalkyl and polyfluoroalkyl substances leads to immunotoxicity: epidemiological and toxicological evidence. J. Exposure Sci. Environ. Epidemiol. 2019, 29, 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Chen J; Li H; Yao J; Guo H; Zhang H; Guo Y; Sheng N; Wang J; Dai J Chronic exposure to PFO4DA and PFO5DoDA, two perfluoroalkyl ether carboxylic acids (PFECAs), suppresses hepatic stress signals and disturbs glucose and lipid metabolism in male mice. J. Hazard. Mater. 2021, 411, 124963. [DOI] [PubMed] [Google Scholar]
- (78).Conley JM; Lambright CS; Evans N; Medlock-Kakaley E; Hill D; McCord J; Strynar MJ; Wehmas LC; Hester S; MacMillan DK; Gray LE Developmental toxicity of Nafion byproduct 2 (NBP2) in the Sprague-Dawley rat with comparisons to hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) and perfluorooctane sulfonate (PFOS). Environ. Int. 2022, 160, 107056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Lang JR; Strynar MJ; Lindstrom AB; Farthing A; Huang H; Schmid J; Hill D; Chernoff N Toxicity of Balb-c mice exposed to recently identified 1,1,2,2-tetrafluoro-2-[1,1,1,2,3,3-hexafluoro-3-(1,1,2,2-tetrafluoroethoxy)propan-2-yl]oxyethane-1-sulfonic acid (PFESA-BP2). Toxicology 2020, 441, 152529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Forsthuber M; Kaiser AM; Granitzer S; Hassl I; Hengstschläger M; Stangl H; Gundacker C Albumin is the major carrier protein for PFOS, PFOA, PFHxS, PFNA and PFDA in human plasma. Environ. Int. 2020, 137, 105324. [DOI] [PubMed] [Google Scholar]
- (81).Beesoon S; Martin JW Isomer-Specific Binding Affinity of Perfluorooctanesulfonate (PFOS) and Perfluorooctanoate (PFOA) to Serum Proteins. Environ. Sci. Technol. 2015, 49, 5722–5731. [DOI] [PubMed] [Google Scholar]
- (82).Jackson TW; Scheibly CM; Polera ME; Belcher SM Rapid Characterization of Human Serum Albumin Binding for Per- and Polyfluoroalkyl Substances Using Differential Scanning Fluorimetry. Environ. Sci. Technol. 2021, 55, 12291–12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Lu Y; Meng L; Ma D; Cao H; Liang Y; Liu H; Wang Y; Jiang G The occurrence of PFAS in human placenta and their binding abilities to human serum albumin and organic anion transporter 4. Environ. Pollut. 2021, 273, 116460. [DOI] [PubMed] [Google Scholar]
- (84).Alesio JL; Slitt A; Bothun GD Critical new insights into the binding of poly- and perfluoroalkyl substances (PFAS) to albumin protein. Chemosphere 2022, 287, 131979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Sagiv SK; Rifas-Shiman SL; Webster TF; Mora AM; Harris MH; Calafat AM; Ye X; Gillman MW; Oken E Sociodemographic and Perinatal Predictors of Early Pregnancy Per- and Polyfluoroalkyl Substance (PFAS) Concentrations. Environ. Sci. Technol. 2015, 49, 11849–11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Pan Y; Zhu Y; Zheng T; Cui Q; Buka SL; Zhang B; Guo Y; Xia W; Yeung LWY; Li Y; Zhou A; Qiu L; Liu H; Jiang M; Wu C; Xu S; Dai J Novel Chlorinated Polyfluorinated Ether Sulfonates and Legacy Per-/Polyfluoroalkyl Substances: Placental Transfer and Relationship with Serum Albumin and Glomerular Filtration Rate. Environ. Sci. Technol. 2017, 51, 634–644. [DOI] [PubMed] [Google Scholar]
- (87).Bangma J; Guillette TC; Bommarito PA; Ng C; Reiner JL; Lindstrom AB; Strynar MJ Understanding the dynamics of physiological changes, protein expression, and PFAS in wildlife. Environ. Int. 2022, 159, 107037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


