Abstract
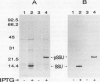
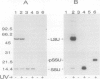
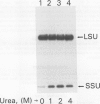
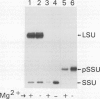
The small subunit (SSU) of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) possesses a binding site that can be photoaffinity labeled with [32P]8-azidoadenosine 5′ triphosphate (N3ATP). In the present study, photoaffinity labeling was used to compare the nucleotide analog binding properties of SSU in the Rubisco holoenzyme complex (holoE SSU) with the properties of isolated SSU and the precursor form (pSSU) that contains a transit peptide. To facilitate these studies, the complete coding regions of tobacco (Nicotiana tabacum L.) SSU and pSSU were cloned into pET expression vectors and the polypeptides were synthesized in Escherichia coli. Protein import studies showed that cloned pSSU polypeptides were imported into intact chloroplasts, where they were processed to the mature form and assembled into the Rubisco holoenzyme. Cloned SSU and pSSU isolated from E. coli were photoaffinity labeled with N3ATP. The apparent Kd value for SSU and pSSU, 18 micromolar N3ATP, was identical to the value determined for holoE SSU. However, differences in photolabeling between cloned SSU or pSSU and holoE SSU were apparent in the level of protection afforded by ATP and UTP, in the response of photolabeling to free Mg2+, and in the higher photolabeling efficiency that characterized the cloned SSU. Treatment of the Rubisco holoenzyme with a concentration of urea sufficient to disassociate the subunits markedly increased photoincorporation into SSU, indicating that intersubunit associations within the holoenzyme complex may be the major factor influencing photolabeling efficiency of SSU. Thus, differences in SSU conformation between the isolated and assembled states affect photolabeling efficiency and other nucleotide analog binding properties of the SSU, but not the apparent affinity for N3ATP.
Full text
PDF

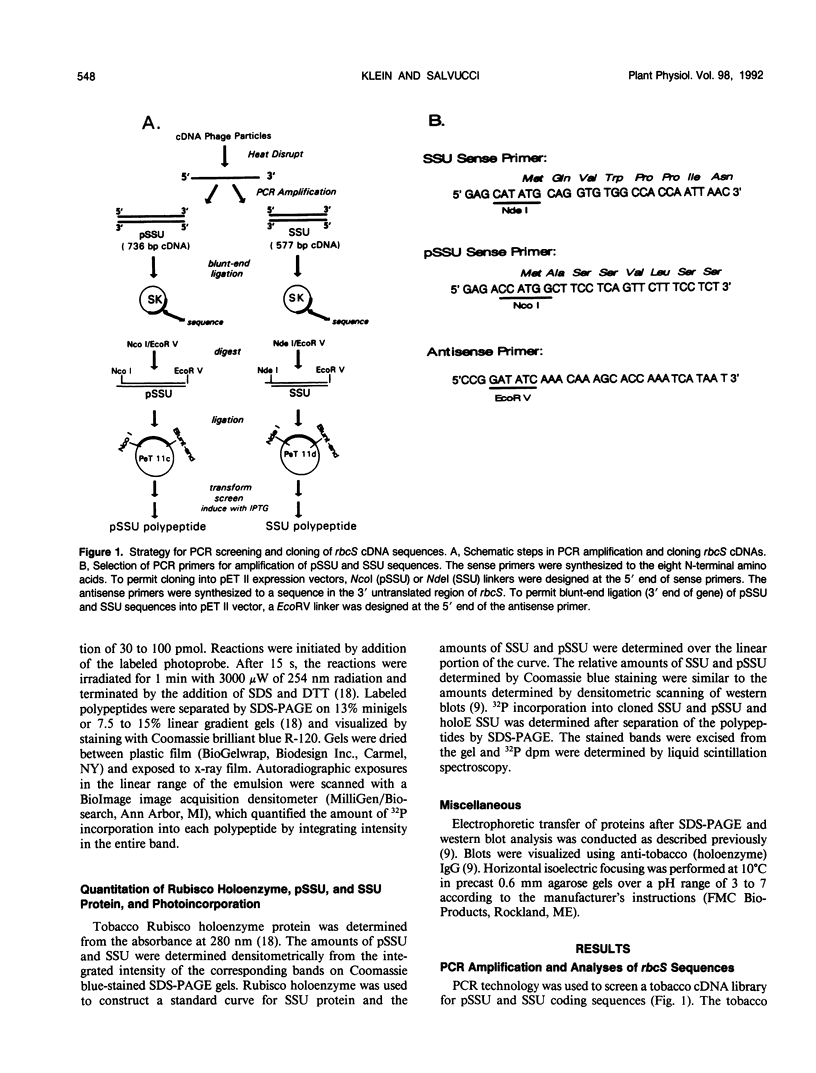

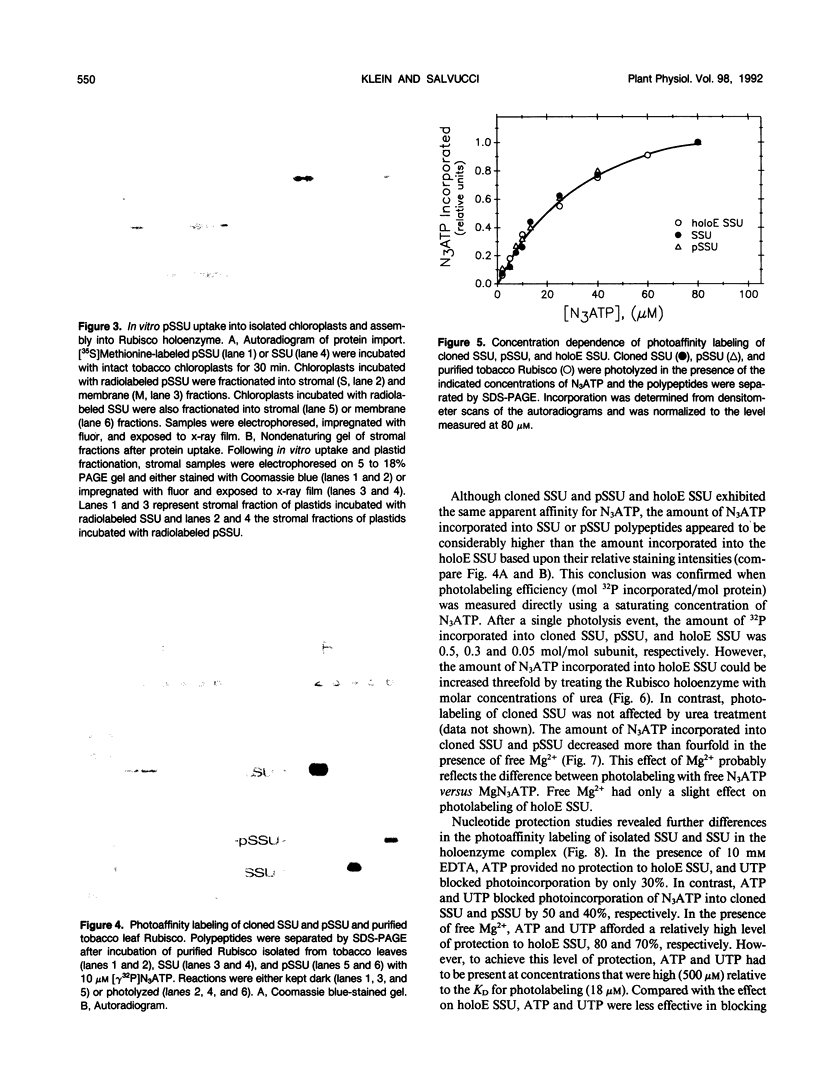

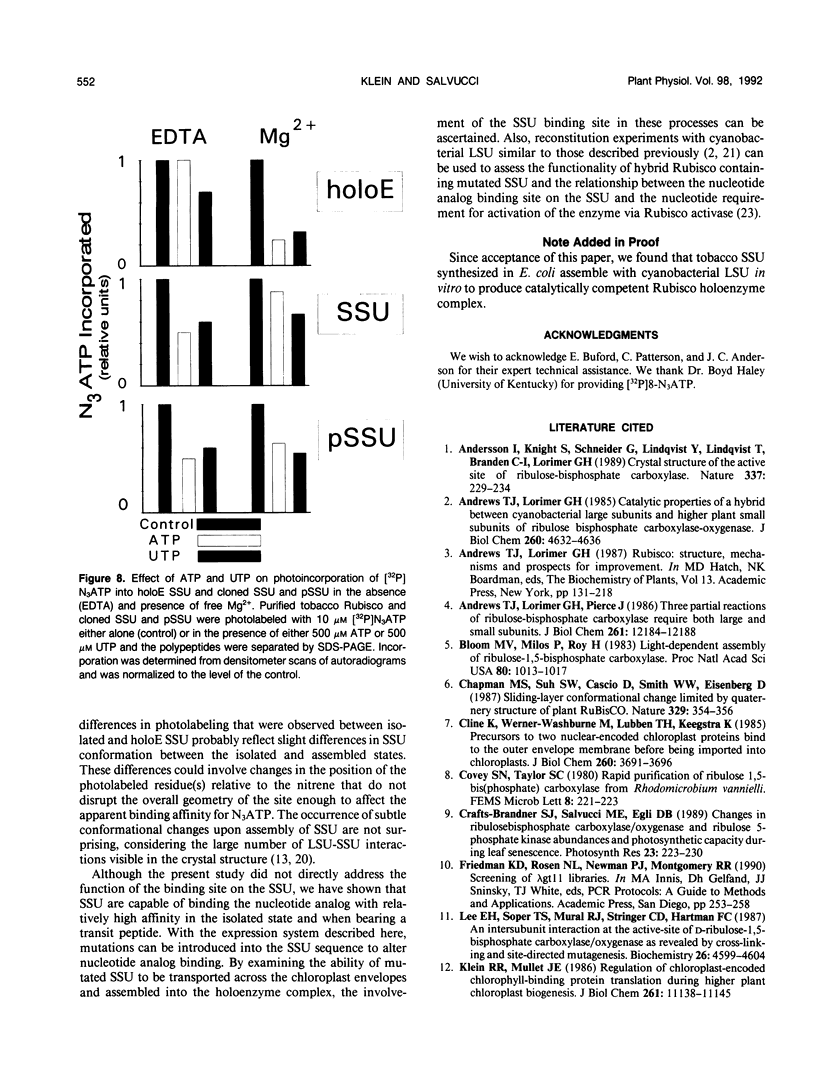

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Lorimer G. H. Catalytic properties of a hybrid between cyanobacterial large subunits and higher plant small subunits of ribulose bisphosphate carboxylase-oxygenase. J Biol Chem. 1985 Apr 25;260(8):4632–4636. [PubMed] [Google Scholar]
- Andrews T. J., Lorimer G. H., Pierce J. Three partial reactions of ribulose-bisphosphate carboxylase require both large and small subunits. J Biol Chem. 1986 Sep 15;261(26):12184–12188. [PubMed] [Google Scholar]
- Bloom M. V., Milos P., Roy H. Light-dependent assembly of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1013–1017. doi: 10.1073/pnas.80.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman M. S., Suh S. W., Cascio D., Smith W. W., Eisenberg D. Sliding-layer conformational change limited by the quaternary structure of plant RuBisCO. Nature. 1987 Sep 24;329(6137):354–356. doi: 10.1038/329354a0. [DOI] [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Lubben T. H., Keegstra K. Precursors to two nuclear-encoded chloroplast proteins bind to the outer envelope membrane before being imported into chloroplasts. J Biol Chem. 1985 Mar 25;260(6):3691–3696. [PubMed] [Google Scholar]
- Klein R. R., Mullet J. E. Regulation of chloroplast-encoded chlorophyll-binding protein translation during higher plant chloroplast biogenesis. J Biol Chem. 1986 Aug 25;261(24):11138–11145. [PubMed] [Google Scholar]
- Knight S., Andersson I., Brändén C. I. Crystallographic analysis of ribulose 1,5-bisphosphate carboxylase from spinach at 2.4 A resolution. Subunit interactions and active site. J Mol Biol. 1990 Sep 5;215(1):113–160. doi: 10.1016/S0022-2836(05)80100-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of a genomic DNA clone for the small subunit of ribulose bis-phosphate carboxylase-oxygenase from tobacco. Nucleic Acids Res. 1985 Apr 11;13(7):2373–2386. doi: 10.1093/nar/13.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter R. L., Haley B. E. Photoaffinity labeling of nucleotide binding sites with 8-azidopurine analogs: techniques and applications. Methods Enzymol. 1983;91:613–633. doi: 10.1016/s0076-6879(83)91054-6. [DOI] [PubMed] [Google Scholar]
- Rychlik W., Rhoads R. E. A computer program for choosing optimal oligonucleotides for filter hybridization, sequencing and in vitro amplification of DNA. Nucleic Acids Res. 1989 Nov 11;17(21):8543–8551. doi: 10.1093/nar/17.21.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G., Knight S., Andersson I., Brändén C. I., Lindqvist Y., Lundqvist T. Comparison of the crystal structures of L2 and L8S8 Rubisco suggests a functional role for the small subunit. EMBO J. 1990 Jul;9(7):2045–2050. doi: 10.1002/j.1460-2075.1990.tb07371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrcka A. V., Ramage R. T., Bohnert H. J., Jensen R. G. Purification and characterization of large and small subunits of ribulose 1,5-bisphosphate carboxylase expressed separately in Escherichia coli. Arch Biochem Biophys. 1991 Apr;286(1):6–13. doi: 10.1016/0003-9861(91)90002-z. [DOI] [PubMed] [Google Scholar]
- Streusand V. J., Portis A. R. Rubisco Activase Mediates ATP-Dependent Activation of Ribulose Bisphosphate Carboxylase. Plant Physiol. 1987 Sep;85(1):152–154. doi: 10.1104/pp.85.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]