Abstract
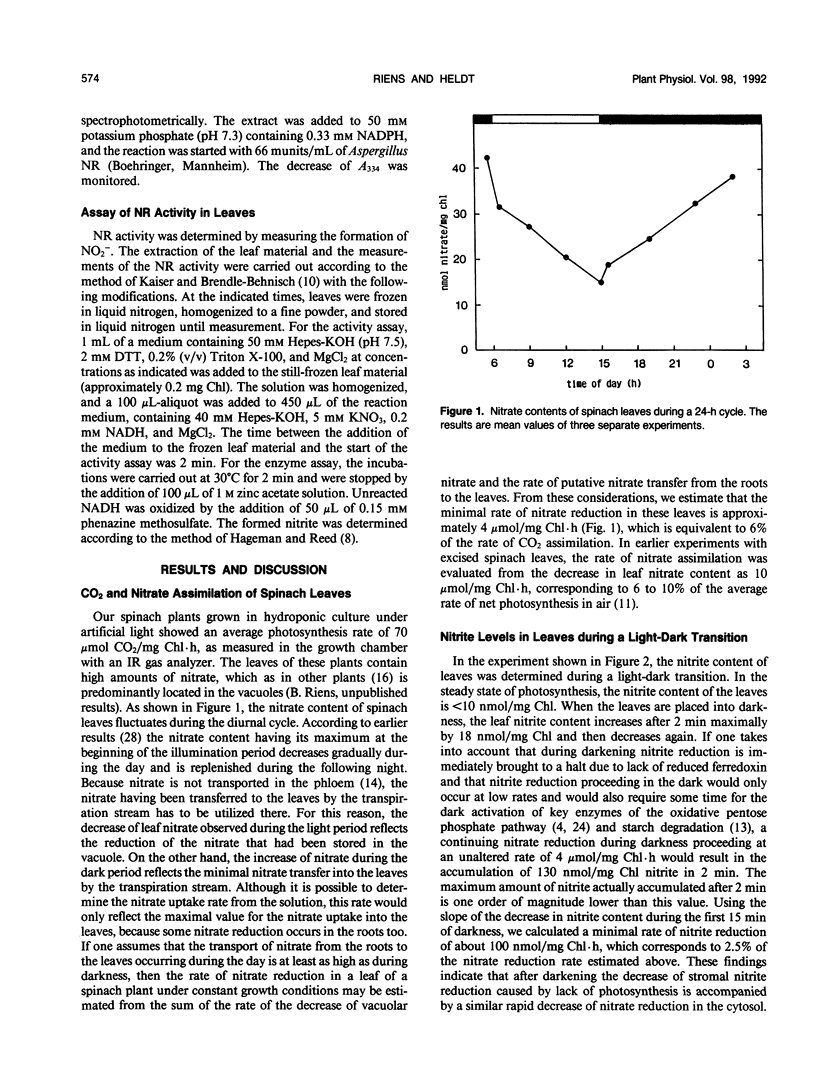
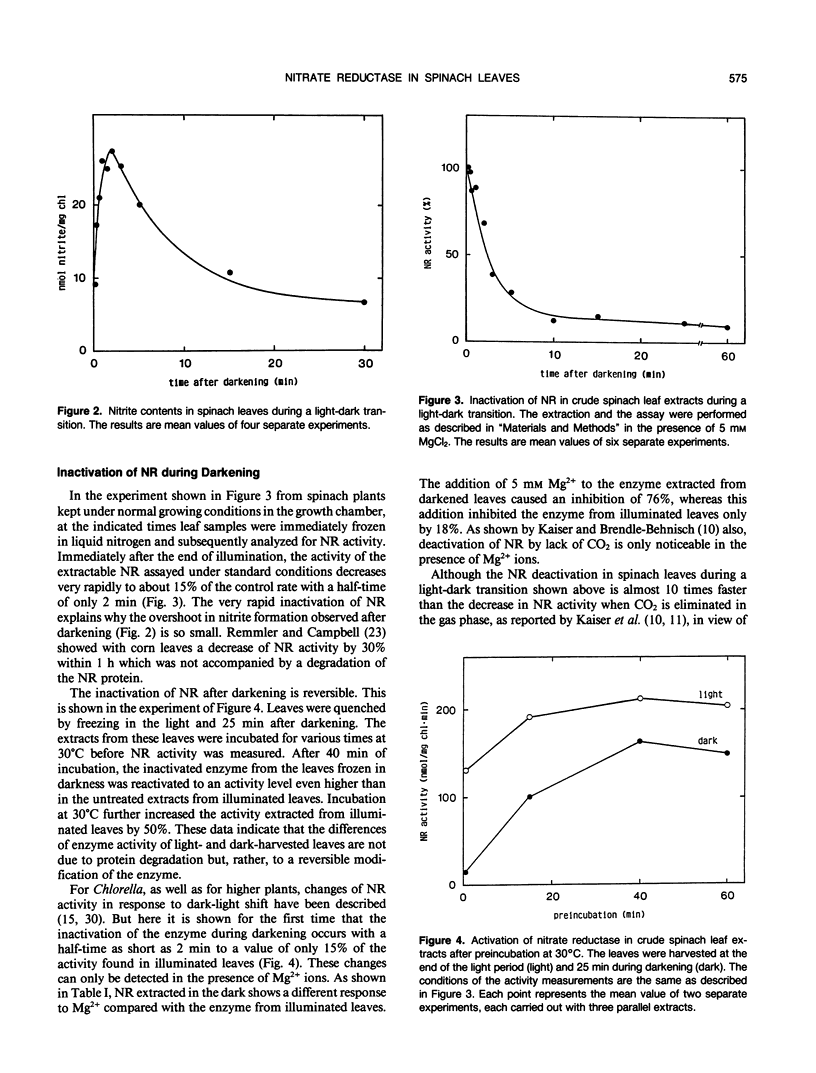
In leaves of spinach plants (Spinacia oleracea L.) performing CO2 and NO3− assimilation, at the time of sudden darkening, which eliminates photosystem I-dependent nitrite reduction, only a minor temporary increase of the leaf nitrite content is observed. Because nitrate reduction does not depend on redox equivalents generated by photosystem I activity, a continuation of nitrate reduction after darkening would result in a large accumulation of nitrite in the leaves within a very short time, which is not observed. Measurements of the extractable nitrate reductase activity from spinach leaves assayed under standard conditions showed that in these leaves the nitrate reductase activity decreased during darkening to 15% of the control value with a half-time of only 2 minutes. Apparently, in these leaves nitrate reductase is very rapidly inactivated at sudden darkness avoiding an accumulation of the toxic nitrite in the cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M., Huffaker R. C., Rains D. W., Rao K. P. Influence of light and ambient carbon dioxide concentration on nitrate assimilation by intact barley seedlings. Plant Physiol. 1979 Jun;63(6):1205–1209. doi: 10.1104/pp.63.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunswick P., Cresswell C. F. Nitrite Uptake into Intact Pea Chloroplasts : II. Influence of Electron Transport Regulators, Uncouplers, ATPase and Anion Uptake Inhibitors and Protein Binding Reagents. Plant Physiol. 1988 Feb;86(2):384–389. doi: 10.1104/pp.86.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineke D., Riens B., Grosse H., Hoferichter P., Peter U., Flügge U. I., Heldt H. W. Redox Transfer across the Inner Chloroplast Envelope Membrane. Plant Physiol. 1991 Apr;95(4):1131–1137. doi: 10.1104/pp.95.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. M., Brendle-Behnisch E. Rapid Modulation of Spinach Leaf Nitrate Reductase Activity by Photosynthesis : I. Modulation in Vivo by CO(2) Availability. Plant Physiol. 1991 Jun;96(2):363–367. doi: 10.1104/pp.96.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. M., Förster J. Low CO(2) Prevents Nitrate Reduction in Leaves. Plant Physiol. 1989 Nov;91(3):970–974. doi: 10.1104/pp.91.3.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. M., Spill D. Rapid Modulation of Spinach Leaf Nitrate Reductase by Photosynthesis : II. In Vitro Modulation by ATP and AMP. Plant Physiol. 1991 Jun;96(2):368–375. doi: 10.1104/pp.96.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow Y. W., Erbes D. L., Gibbs M. Chloroplast Respiration : A MEANS OF SUPPLYING OXIDIZED PYRIDINE NUCLEOTIDE FOR DARK CHLOROPLASTIC METABOLISM. Plant Physiol. 1982 Feb;69(2):442–447. doi: 10.1104/pp.69.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks A., Aslam M., Boesel I. Ammonium and amino acids as regulators of nitrate reductase in corn roots. Plant Physiol. 1977 Mar;59(3):391–394. doi: 10.1104/pp.59.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oji Y., Ryoma Y., Wakiuchi N., Okamoto S. Effect of Inorganic Orthophosphate on in Vitro Activity of NADH-Nitrate Reductase Isolated from 2-Row Barley Leaves. Plant Physiol. 1987 Mar;83(3):472–474. doi: 10.1104/pp.83.3.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall P. J., Bouma D. Zinc deficiency, carbonic anhydrase, and photosynthesis in leaves of spinach. Plant Physiol. 1973 Sep;52(3):229–232. doi: 10.1104/pp.52.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmler J. L., Campbell W. H. Regulation of Corn Leaf Nitrate Reductase : II. Synthesis and Turnover of the Enzyme's Activity and Protein. Plant Physiol. 1986 Feb;80(2):442–447. doi: 10.1104/pp.80.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M. Carbon dioxide and nitrite photoassimilatory processes do not intercompete for reducing equivalents in spinach and soybean leaf chloroplasts. Plant Physiol. 1986 Mar;80(3):676–684. doi: 10.1104/pp.80.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J., Heldt H. W. On the regulation of spinach nitrate reductase. Plant Physiol. 1990 Mar;92(3):684–689. doi: 10.1104/pp.92.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Gerhardt R., Kürzel B., Heldt H. W. A role for fructose 2,6-bisphosphate in the regulation of sucrose synthesis in spinach leaves. Plant Physiol. 1983 Aug;72(4):1139–1141. doi: 10.1104/pp.72.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischner R. Light-mediated Activation of Nitrate Reductase in Synchronous Chlorella. Plant Physiol. 1978 Aug;62(2):284–286. doi: 10.1104/pp.62.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]


