Abstract
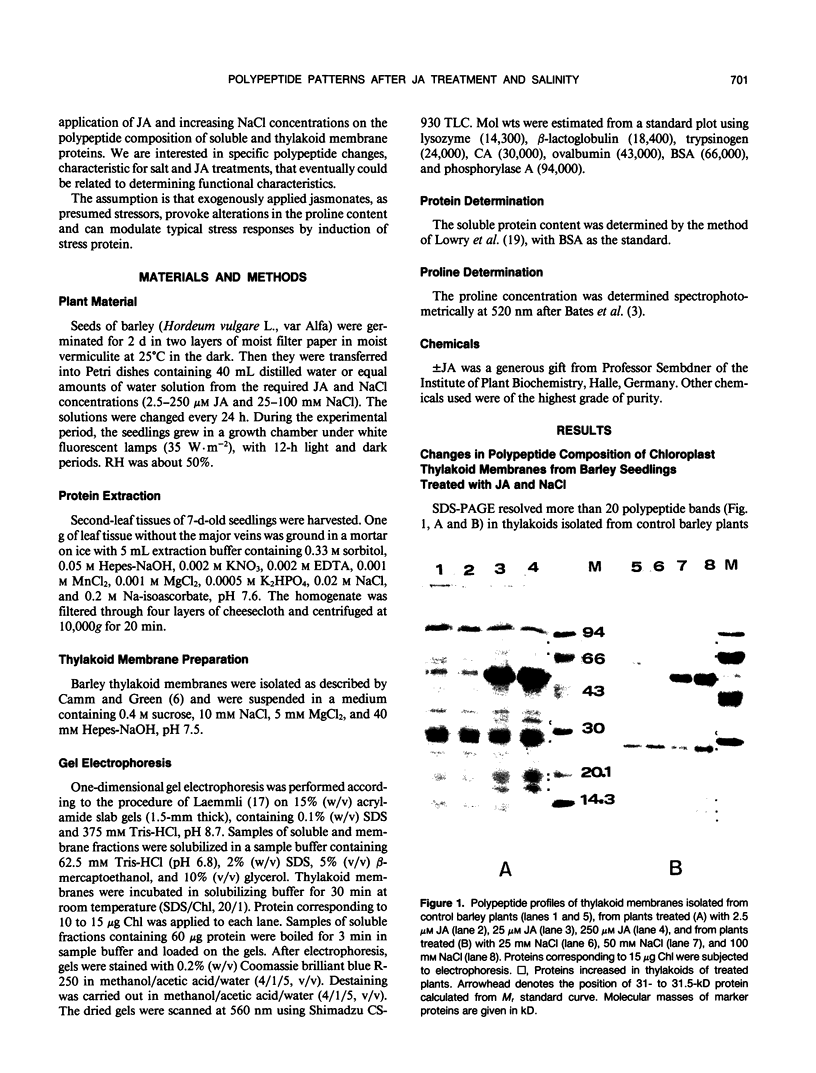
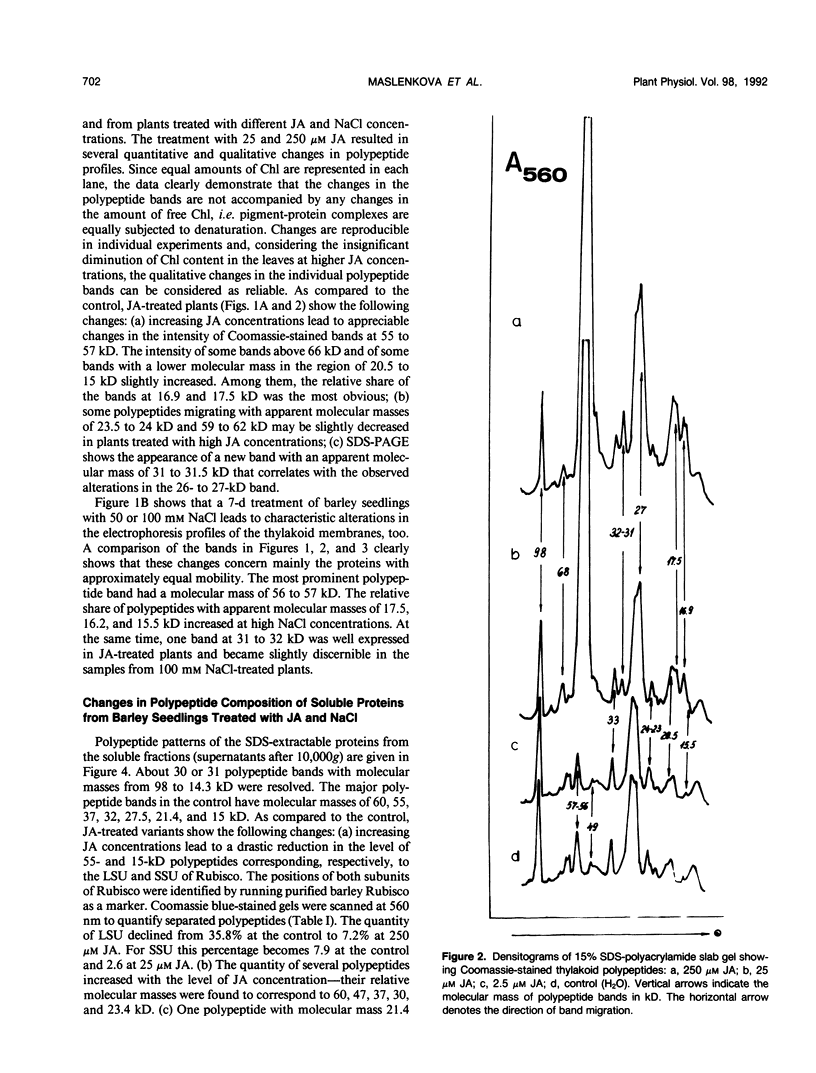
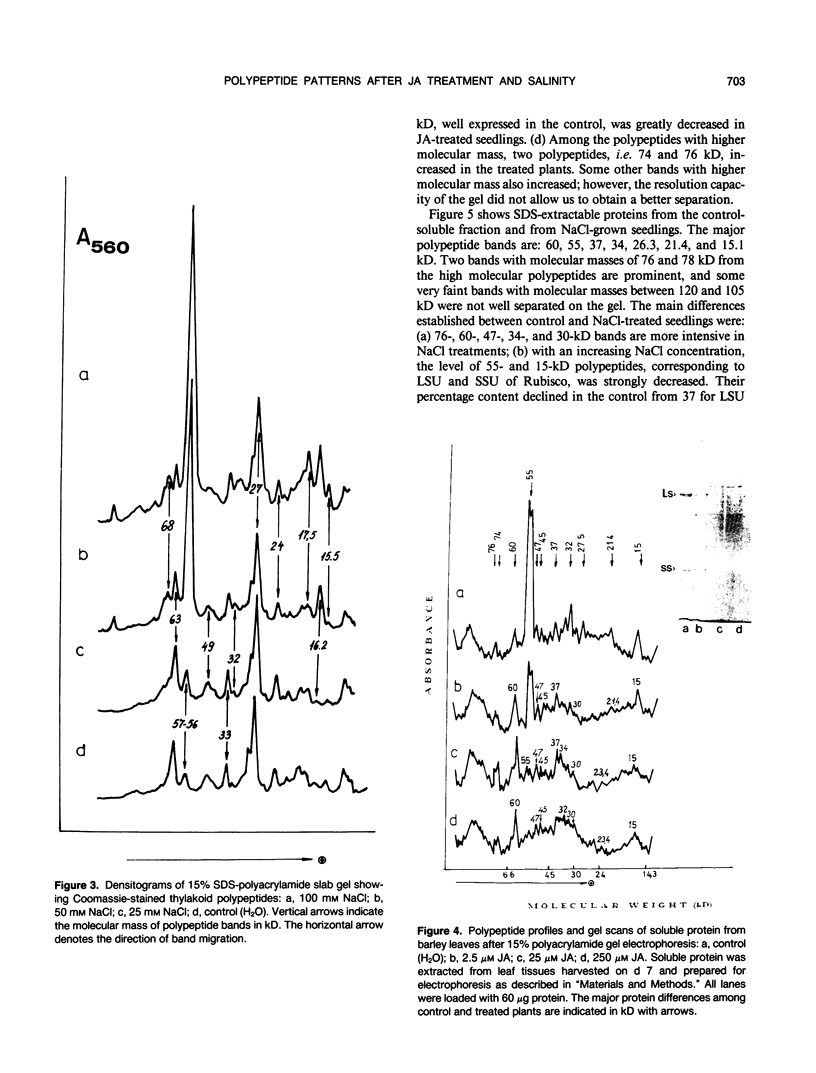
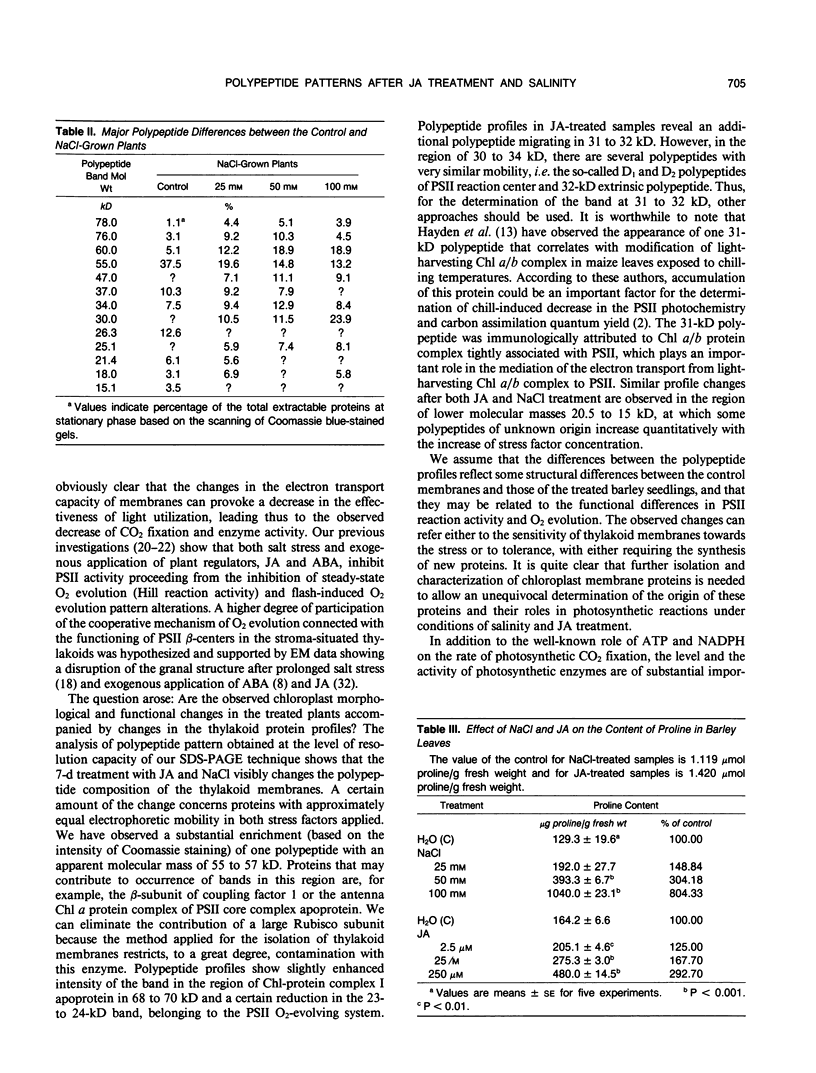
Soluble and thylakoid membrane proteins of jasmonic acid (JA)-treated and salt-stressed barley (Hordeum vulgare L.) seedlings were investigated using 15% sodium dodecyl sulfate-polyacrylamide slab gel electrophoresis. High JA concentrations induced marked quantitative and qualitative changes in polypeptide profiles concerning mainly the proteins with approximately equal mobility, as in NaCl-stressed plants. The most obvious increase in thylakoid polypeptide band intensity was at 55 to 57 kilodaltons (kD). The relative share of some polypeptides with apparent molecular masses above 66 kD and of polypeptides with lower molecular masses in the region of 20.5 to 15 kD was enhanced. At the same time, one new band at 31 to 31.5 kD was well expressed at 25 and 250 micromolar JA concentrations and became discernible in the 100 micromolar NaCl-treated plants. The intensity of some polypeptides of soluble proteins (molecular masses of 60, 47, 37, 30, and 23.4 kD) increased with increasing JA concentration, whereas the intensities of other polypeptide bands (55, 21.4, and 15 kD) decreased. Enhanced levels of 60-, 47-, 34-, and 30-kD polypeptides and reduced levels of 55- and 15-kD polypeptides were present in NaCl-treated plants. The appearance of one new polypeptide, of 25.1 kD, was observed only in NaCl-treated plants. At 100 millimolar NaCl, an eightfold increase in proline content was observed while at 250 micromolar JA, the proline content was threefold over the control. It is hypothesized that exogenously applied jasmonates act as stress agents. As such, they provoke alterations in the proline content and they can modulate typical stress responses by induction of stress proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buhl M. B., Stewart C. R. Effects of NaCl on Proline Synthesis and Utilization in Excised Barley Leaves. Plant Physiol. 1983 Jul;72(3):664–667. doi: 10.1104/pp.72.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camm E. L., Green B. R. Fractionation of Thylakoid Membranes with the Nonionic Detergent Octyl-beta-d-glucopyranoside: RESOLUTION OF CHLOROPHYLL-PROTEIN COMPLEX II INTO TWO CHLOROPHYLL-PROTEIN COMPLEXES. Plant Physiol. 1980 Sep;66(3):428–432. doi: 10.1104/pp.66.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier Y. Changes in the Electrophoretic Patterns of the Soluble Proteins of Winter Wheat and Rye following Cold Acclimation and Desiccation Stress. Plant Physiol. 1983 Feb;71(2):400–403. doi: 10.1104/pp.71.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daie J., Campbell W. F. Response of Tomato Plants to Stressful Temperatures : INCREASE IN ABSCISIC ACID CONCENTRATIONS. Plant Physiol. 1981 Jan;67(1):26–29. doi: 10.1104/pp.67.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M. C., Alfinito S. H. Proteins Produced during Salt Stress in Tobacco Cell Culture. Plant Physiol. 1984 Mar;74(3):506–509. doi: 10.1104/pp.74.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurkman W. J., Tanaka C. K. The effects of salt on the pattern of protein synthesis in barley roots. Plant Physiol. 1987 Mar;83(3):517–524. doi: 10.1104/pp.83.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Lin C. Y., Chen Y. M. Heat shock proteins of higher plants. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3526–3530. doi: 10.1073/pnas.78.6.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maslenkova L. T., Zanev Y., Popova L. P. Oxygen-evolving activity of thylakoids from barley plants cultivated on different concentrations of jasmonic Acid. Plant Physiol. 1990 Aug;93(4):1316–1320. doi: 10.1104/pp.93.4.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova L. P., Tsonev T. D., Vaklinova S. G. A possible role for abscisic Acid in regulation of photosynthetic and photorespiratory carbon metabolism in barley leaves. Plant Physiol. 1987 Apr;83(4):820–824. doi: 10.1104/pp.83.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Stewart C. R. The Mechanism of Abscisic Acid-induced Proline Accumulation in Barley Leaves. Plant Physiol. 1980 Aug;66(2):230–233. doi: 10.1104/pp.66.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R., Voetberg G. Relationship between Stress-Induced ABA and Proline Accumulations and ABA-Induced Proline Accumulation in Excised Barley Leaves. Plant Physiol. 1985 Sep;79(1):24–27. doi: 10.1104/pp.79.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling E., Key J. L. Ribulose 1,5-Bisphosphate Carboxylase Synthesis during Heat Shock. Plant Physiol. 1985 May;78(1):155–162. doi: 10.1104/pp.78.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]