Abstract
Study Design
Systematic Review and Meta-analysis.
Objectives
To evaluate the impact of race on post-operative outcomes and complications following elective spine surgery in the United States.
Methods
PUBMED, MEDLINE(R), ERIC, EMBASE, and SCOPUS were searched for studies documenting peri-operative events for White and African American (AA) patients following elective spine surgery. Pooled odds ratios were calculated for each 90-day outcome and meta-analyses were performed for 4 peri-operative events and 7 complication categories. Sub-analyses were performed for each outcome on single institution (SI) studies and works that included <100,000 patients.
Results
53 studies (5,589,069 patients, 9.8% AA) were included. Eleven included >100,000 patients. AA patients had increased rates of 90-day readmission (OR 1.33, P = .0001), non-routine discharge (OR 1.71, P = .0001), and mortality (OR 1.66, P = .0003), but not re-operation (OR 1.16, P = .1354). AA patients were more likely to have wound-related complications (OR 1.47, P = .0001) or medical complications (OR 1.35, P = .0006), specifically cardiovascular (OR 1.33, P = .0126), deep vein thrombosis/pulmonary embolism (DVT/PE) (OR 2.22, P = .0188) and genitourinary events (OR 1.17, P = .0343). SI studies could only detect racial differences in re-admissions and non-routine discharges. Studies with <100,000 patients replicated the above findings but found no differences in cardiovascular complications. Disparities in mortality were only detected when all studies were included.
Conclusions
AA patients faced a greater risk of morbidity across several distinct categories of peri-operative events. SI studies can be underpowered to detect more granular complication types (genitourinary, DVT/PE). Rare events, such as mortality, require larger sample sizes to identify significant racial disparities.
Keywords: disparities, race, complications, spine surgery, outcomes
Introduction
A growing body of evidence has reported on the prevalence of systemic racial and socioeconomic disparities in the United States healthcare system.1,2 Race has been identified as a primary predictor of clinical care outcomes across nearly every field of medicine. 3 Among racial minorities, African American (AA) patients have been specifically shown to experience worse health outcomes, including chronic somatic disease burden and mortality, compared with matched White cohorts.4-6 A growing number of studies have shown that race is a significant predictor of operative outcomes 7 after oncologic, transplant, and orthopedic surgeries.8-10 These racial disparities have been attributed to many factors, including patient-related factors (such as comorbidities and socioeconomic status), engagement with healthcare appointments and treatments, quality of care, and limited health literacy. 11 Racial minorities oftentimes encounter racial bias from the provider, cultural barriers, and a lack of insurance coverage. 12
Multiple cohort studies have described disparities in post-operative complications and outcomes for AA patients following spine surgery. Re-admission rates after elective spine surgery were significantly higher in AA than White patients. 13 AA patients had increased length of stays and operative times for anterior cervical decompression and fusion across different fusion levels. 14 AA patients also had an increased risk of medical complications after lumbar decompression and fusion surgery, in particular cardiac, renal, and respiratory adverse events. 14
Pooled analyses in spine surgery further support the outcomes gap between White and AA patients. A meta-analysis in 2011 by Schoenfeld et al 15 analyzed 11 articles and eight “unfavorable” outcome measures, reporting that non-White patients were more likely to have unfavorable outcomes. 15 A further meta-analysis in 2021 by Khan et al 16 included a more robust analysis of 30 studies and 6 outcome measures, concluding that AA patients had a significantly increased risk of mortality, prolonged length of stay, non-home discharge, and 30-day re-admission compared with non-AA patients. 16
Despite the robust analyses performed in these prior systematic reviews, both works studied a fairly small sample size and identified only a moderate number of outcomes, limiting their conclusions. These prior works also grouped all of their included studies together without considering potential differences in the composition and analysis of single institution (SI) vs national database works. Given the dominance of lower quality, retrospective cohort studies in the current literature, the origin and nature of the included work can considerably influence pooled analyses. The purpose of our review was to use an AI-assisted graphical bibliometric platform to analyze the influence of race on peri-operative outcomes and post-operative complications after elective spine surgery. We sought to characterize and present the types and frequencies of post-operative variables in the literature using an organ system-based hierarchy. We further sought to identify relative differences between works of different sample sizes and sample origin (single vs multi-institutional, low vs high-volume registry). Such findings may allow us to better characterize the landscape of pertinent studies on spine surgery-related health outcomes, correlate differences in outcomes based on sample origin, and more accurately describe relationships between race and adverse post-operative outcomes.
Methods
Literature Search
A comprehensive literature search of the PUBMED, MEDLINE(R), ERIC, EMBASE, and SCOPUS databases was performed on 11/07/2022 using a semi-automated software platform (AutoLit, Nested 17 ). De-duplication was performed automatically. Only original articles in English from 2010 onward were included because the Affordable Care Act was passed in 2010. The Affordable Care Act significantly expanded the eligibility for Medicaid, government insurance targeted to low-income individuals, thereby broadly increasing access to care that included spine surgery.18,19 Analyzing only those studies published after this major transformation would more accurately characterize racial disparities in our current healthcare system. Nested Knowledge provides a semi-automated platform for screening, organizing, and extracting data. This review was performed by 2 authors (IA and NK). A detailed methodology, including our search, screening, and raw data extraction, is publicly available on the Nested Knowledge website (https://nested-knowledge.com/). 17 This study was not registered on PROSPERO.
Study Selection
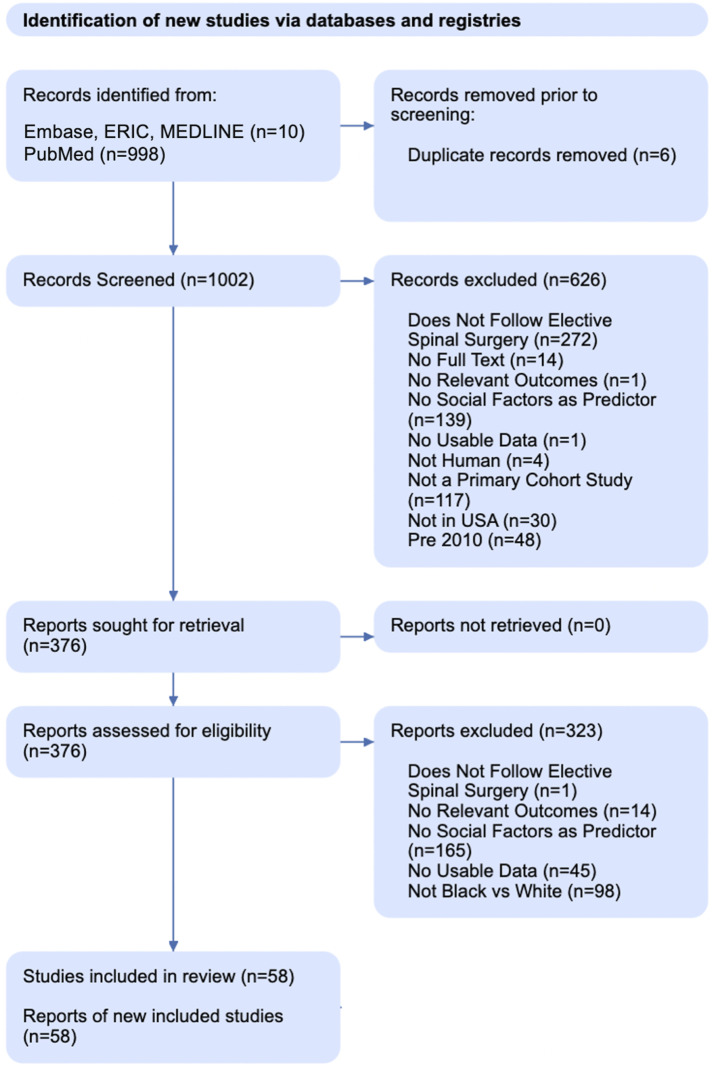
This study sought to examine if AA patients were more likely to experience an adverse outcome following elective spine surgery in the United States than White patients by performing a series of meta-analyses. Inclusion criteria were as follows: (1) patients who underwent elective surgery on any portion of the spine for degenerative disease; (2) studies tracked any peri-operative event, medical complication, or surgical complication as outlined in Table 2; and (3) outcomes were compared between non-Hispanic AA and White patients. Exclusion criteria were: (1) studies that did not utilize patients from the United States (“Not in USA” in Figure 1); (2) publication before 2010 (“Pre 2010” in Figure 1); (3) a lack of available raw incidence data for each cohort (“No Usable Data” in Figure 1); and (4) unavailable full texts (“No Full Text” in Figure 1). We excluded surgeries for tumors, infections, trauma, and spinal cord pathology to decrease heterogeneity, as outcomes for those procedures are more strongly influenced by internal and external factors (“Not Elective Spine Surgery” in Figure 1). A distinction was made between studies that investigated race as an independent variable (RaI) (i.e., studies targeted towards measuring the impact of social factors on outcomes) and studies where race was a recorded as demographic (RaD) data while measuring a post-operative outcome. This distinction is shown in Table 1 under the column header “Variable Type.” Appendix A1 shows a detailed review of our queries and search terms.
Table 2.
Summarizing the List of Post-Operative Complications Gathered from Included Studies and the Classification Scheme Implemented in this Study.
| Outcome type (total studies) | Outcome subtype tier 1 (total studies) | Outcome subtype tier 2 (total studies) |
|---|---|---|
| General complications (15) | Any complication (15) | — |
| Medical complications (27) | Cardiovascular complication (12) | Myocardial infarction (3) |
| Cardiac arrest (3) | ||
| Acute post-Hemorrhagic Anemia (2) | ||
| Cardiac Arrest or MI (1) | ||
| Hypertension (1) | ||
| Hypotension (1) | ||
| Post-Op shock (1) | ||
| Peripheral vascular complication (1) | ||
| DVT/PE (11) | — | |
| Neurologic complication (10) | Stroke (4) | |
| Delirium (2) | ||
| Sensory deficit (1) | ||
| Altered mental status (1) | ||
| Nervous system complication (1) | ||
| Coma (1) | ||
| Central nervous system complication (1) | ||
| Visual loss (1) | ||
| Genitourinary (10) | UTI (6) | |
| Renal/Urinary (3) | ||
| Urinary Retention (3) | ||
| D/C with Foley (1) | ||
| Respiratory complication (10) | Pneumonia (4) | |
| Unplanned Reintubation (4) | ||
| Prolonged ventilation (2) | ||
| Systemic infection (4) | Sepsis (3) | |
| Other infection (1) | ||
| Other medical (3) | Opioid Overdose (2) | |
| ICU-transfer change (1) | ||
| Gastrointestinal (2) | Ileus (1) | |
| Surgical complications (16) | Wound complication (16) | Wound infection (9) |
| Hematoma (7) | ||
| Superficial surgical site infection (4) | ||
| Deep surgical site infection (3) | ||
| Surgical site infection (3) | ||
| Infectious complication (3) | ||
| Organ space surgical site infection (2) | ||
| Post-Op infection (2) | ||
| Wound dehiscence (2) | ||
| Wound disruption (2) | ||
| Cellulitis (1) | ||
| Wound drainage (1) | ||
| Post-Op bleeding (1) | ||
| Operative wound (1) | ||
| Post-Op outcomes (43) | Re-admission (20) | — |
| Discharge disposition (18) | — | |
| Mortality (9) | — | |
| Re-operation (8) | — |
(n) reflects the number of studies each complication was included in. For categories of complications (i.e., Cardiovascular), the (n) denotes the number of studies in which the complication category or any of the sub-complications in that category was included.
Figure 1.
PRISMA diagram illustrating literature search and screening method.
Table 1.
Summary Characteristics for the 53 Included Studies.
| Journal article | Sample size White/Black (White:Black) |
Database | Surgery type | Variable type | Medical complications | Surgical complications | Post-Op outcomes |
|---|---|---|---|---|---|---|---|
| Adogwa et al. 2016 | 458/142 (3.2:1) | SI | Spine surgery | RaI | Cardiovascular, DVT/PE, GU, respiratory, systemic infection | Wound infection | LOS, Re-Ad |
| Aladdin et al. 2020 | 208,088/17,326 (12:1) | HCUP | Lumbar fusion | RaI | — | — | NRD, LOS, Re-Ad |
| Arena et al. 2020 | 38,249/2545 (15:1) | Optum EHR | Posterior lumbar fusion | RaD | Neurologic | — | — |
| Baek et al. 2019 | 906/193 (4.7:1) | NSQIP | Posterior spinal fusion | RaD | — | — | NRD |
| Cook et al. 2018 | 232,525/18,048 (12.9:1) | Medicare Claims | Lumbar fusion | RaD | — | — | NRD |
| De la Garza-Ramos et al. 2016 | 27,834/7531 (3.7:1) | NIS | Spinal fusion | RaD | Neurologic | — | — |
| Dial et al. 2020 | 1463/305 (4.8:1) | SI | ACDF | RaD | — | — | Re-Ad |
| Doherty et al. 2020 | 3176/200 (15.9:1) | HCUP | Lumbar fusion | RaD | Opioid Overdose | — | — |
| Drazin et al. 2017 a | 513/513 (1:1) | Medicare Claims | Lumbar laminectomy/Fusion | RaI | — | Wound infection | NRD, LOS, mortality, Re-Ad, Re-Op |
| Elsamadicy et al. 2016 | 32/28 (1.1:1) | SI | ACDF | RaI | DVT/PE, GU, respiratory | Wound infection | Re-Ad |
| Elsamadicy et al. 2017 | 438/52 (8.4:1) | SI | Complex spinal fusion | RaI | Cardiovascular, DVT/PE, GI, GU, neurologic, systemic infection | Cellulitis, Hematoma, wound drainage, wound infection | LOS, Re-Ad |
| Elsamadicy et al. 2018 | 292/53 (5.5:1) | SI | Lumbar spine surgery | RaI | DVT/PE, GU | Hematoma, wound infection | LOS, Re-Ad |
| Elsamadicy et al. 2020 | 13,250/2150 (6.2:1) | NIS | ACDF | RaI | Cardiovascular, DVT/PE, neurologic | Hematoma, wound disruption | NRD, LOS |
| (a) Elsamadicy et al. 2021 | 2096/552 (3.8:1) | KID | Posterior spinal fusion | RaI | Cardiovascular, DVT/PE, GI, GU, neurologic, respiratory | Hematoma, infection complication, wound dehiscence | NRD, LOS |
| (b) Elsamadicy et al. 2021 | 4505/383 (11.8:1) | NSQIP | Lumbar decompression/Fusion | RaD | — | — | NRD |
| Engler et al. 2022 | 376,385/22,878 (16.5:1) | Medicare Claims | Spine surgery | RaI | DVT/PE, neurologic, respiratory | — | Mortality, Re-Ad |
| Feng et al. 2018 | 57,717/10,060 (5.7:1) | SPARCS | Cervical spinal fusion | RaD | Systemic infection | Post-Op bleeding, infection complication | Mortality |
| Fineberg et al. 2013 | 486,181/35,757 (13.6:1) | NIS | Lumbar decompression/Fusion | RaD | Neurologic | — | — |
| Gephart et al. 2012 | 5311/368 (14.4:1) | NIS | Spinal fusion | RaD | DVT/PE | — | — |
| Ghenbot et al. 2022 | 307/63 (4.9:1) | SI | Spine surgery | RaD | — | — | Re-Op |
| Guan et al. 2018 | 201/4 (50.3:1) | QOD | Lumbar fusion | RaD | — | — | NRD |
| Hardman et al. 2022 | 40,041/4959 (8.1:1) | NIS | ACDF | RaD | Respiratory | — | — |
| Kashkoush et al. 2019 | 263,765/28,601 (9.2:1) | NIS | ACDF | RaD | — | — | Mortality |
| Kerezoudis et al. 2019 | 150,635/16,112 (9.4:1) | NSQIP | Cranial and spine surgery | RaD | — | — | Re-Op |
| Kim et al. 2018 | 4703/441 (10.7:1) | NSQIP | Spinal fusion | RaD | Cardiovascular, DVT/PE | Wound complication | Mortality |
| Knusel et al. 2020 | 4676/1577 (2.9:1) | NSQIP | Posterior lumbar decompression | RaD | — | Hematoma | — |
| Kohls et al. 2018 | 307/36 (8.5:1) | SI | Lumbar discectomy | RaD | — | — | Re-Ad |
| Lad et al. 2013 | 1052/336 (3.1:1) | Medicaid dataset | Lumbar laminectomy/Fusion | RaI | Cardiovascular, DVT/PE, GU, neurologic, respiratory | Wound complication, infection complication | LOS, Re-Op |
| Lee et al. 2017 | 4709/442 (10.7:1) | NSQIP | Spinal fusion | RaD | — | Wound complication | — |
| Lee et al. 2018 | 2008/163 (12.3:1) | NSQIP | Posterior lumbar fusion | RaD | — | — | Re-Ad |
| Macki et al. 2021 | 16,788/1436 (11.7:1) | SSID | Lumbar spine surgery | RaI | GU | — | NRD, LOS, Re-Ad |
| Malik et al. 2018 | 19,620/1914 (10.3:1) | NSQIP | Posterior lumbar fusion | RaD | — | — | NRD |
| Marquez-Lara et al. 2014 | 147,671/15,309 (9.7:1) | NIS | ACF | RaD | Respiratory | — | — |
| Mohanty et al. 2022 | 2024/596 (3.4:1) | SI | Spine surgery | RaD | — | — | Re-Ad |
| Mummaneni et al. 2021 | 852/160 (5.3:1) | QOD | Cervical spine surgery | RaD | — | — | NRD |
| Murphy et al. 2017 | 7192/487 (14.8:1) | NSQIP | Lumbar decompression | RaD | — | — | NRD |
| Nandyala et al. 2014 | 6923/847 (8.2:1) | NSQIP | Cervical spine surgery | RaD | Respiratory | — | — |
| Ogura et al. 2020 | 1307/147 (8.9:1) | SI | Lumbar fusion | RaD | — | — | NRD |
| Park et al. 2018 | 6378/628 (10.2:1) | SSID | Lumbar fusion | RaD | — | — | Re-Ad |
| (a)Passias et al. 2018 | 1743/88 (19.8:1) | NSQIP | Thoracic lumbar surgery | RaD | — | — | NRD |
| (b)Passias et al. 2018 | 51,895/4325 (12:1) | NSQIP | Spine surgery | RaD | Cardiovascular | — | — |
| Passias et al. 2022 | 179,802/13,374 (13.4:1) | NSQIP | Spine surgery | RaD | MI | — | — |
| Phan et al. 2017 | 1252/153 (8.2:1) | NSQIP | ACDF | RaD | — | — | Re-Ad |
| (a)Poorman et al. 2018 | 220,082/25,991 (8.5:1) | NIS | Cervical spine surgery | RaD | — | — | Mortality |
| (b)Poorman et al. 2018 | 529,044/39,988 (13.2:1) | NIS | Lumbar spine surgery | RaD | — | — | Mortality |
| Pugely et al. 2014 | 13,141/912 (14.4:1) | NSQIP | Lumbar spine surgery | RaD | — | — | Re-Ad |
| Quinn et al. 2017 b | 51,397/5072 (10.1:1) | NSQIP | Cranial and spinal surgery | RaD | Cardiovascular | — | — |
| Sanford et al. 2019 | 4106/522 (7.9:1) | NSQIP | Spine surgery | RaI | Cardiovascular, DVT/PE, GU, neurologic, respiratory | Wound dehiscence, wound infection | LOS, Re-Ad, Re-Op |
| Schoenfeld et al. 2012 | 1367/101 (13.5:1) | SPORT | Spine surgery | RaI | — | Wound infection, Hematoma | LOS, mortality, Re-Op |
| Seicean et al. 2017 a | 3489/3489 (1:1) | NSQIP | Laminectomy/Fusion | RaI | Cardiovascular, DVT/PE, GU, neurologic, respiratory | Wound disruption, wound infection | NRD, LOS, mortality, Re-Ad |
| Sivaganesan et al. 2019 | 29,876/2277 (13.1:1) | QOD | Lumbar spine surgery | RaD | — | — | Re-Ad |
| Skolasky et al. 2014 | 851,641/86,541 (9.8:1) | NIS | Cervical spine surgery | RaI | Cardiovascular, neurologic, respiratory | Hematoma, operative wound, wound infection | Mortality |
| Snyder et al. 2019 | 6633/1117 (5.94:1) | NSQIP | PCDF | RaD | — | — | NRD |
| Thirumala et al. 2017 | 1,142,902/125,547 (9.1:1) | NIS | Spinal fusion | RaD | Neurologic | — | — |
| Wick et al. 2022 | 6007/646 (9.3:1) | Vizient | Cervical spine surgery | RaI | — | — | LOS, Re-Ad |
| Woodard et al. 2022 | 199/79 (2.5:1) | SI | ACDF | RaI | — | — | NRD, LOS, Re-Ad, Re-Op |
| Ye et al. 2018 | 2242/376 (5.9:1) | NSQIP | Posterior cervical fusion | RaD | — | — | NRD |
| Zakaria et al. 2019 | 4582/602 (7.6:1) | SSID | Cervical spine surgery | RaD | GU | — | Re-Ad |
aMatched data was extracted for analysis.
bStudy reports on both cranial and spinal surgery, cranial data was not collected
GI = Gastrointestinal, GU = Genitourinary, HCUP = Healthcare Cost and Utilization Project, KID = Kids Inpatient Database, NIS = National Inpatient Database, NRD = Non-Routine Discharge, NSQIP = ACS NSQIP, Optum EHR = Optum Electronic Health Records, QOD = Quality Outcomes Database, Re-Ad = Re-Admission, Re-Op = Re-Operation, SI = Single Institution, SSID = Michigan Spine Surgery Improvement Collaborative.
Outcome Measures and Categorization
Predefined data, including study size, data source, surgery type, peri-operative outcomes, and complications, were extracted independently by 2 authors (IA and NK), with disagreements settled by the senior author (Table 1).
Four key peri-operative outcomes were chosen and recorded from the selected studies: re-admission, non-routine or non-home discharge (NRD), re-operation, and mortality. These outcomes were chosen because they are well-known and understood metrics for assessing the outcomes of major surgeries. 20 Clinical/economic significance, racial heterogeneity, and abundance of reporting in the literature were also considered. Data were collected if events occurred within 90 days of surgery, which was chosen based on the peri-operative period defined by Medicare.
Due to the inconsistent methods used to report complications in the available literature, a systematic survey of all included studies was performed. All complications reported within 90 days of surgery were gathered using the most granular data provided by the authors. We recorded each complication as it was described by the original authors. Complications were excluded if they were (1) not a medically relevant complication as determined by the senior author (MSF); or (2) iatrogenic complications (i.e., dural tears or hardware malposition). Included and excluded complications and their categorizations are summarized in Tables 2 and 3, respectively. The remaining complications were grouped into 8 medical (Cardiovascular, DVT/PE, Gastrointestinal, Genitourinary, Systemic Infection, Neurologic, Respiratory, Other) sub-categories and one surgical sub-category (Wound Complications). If a study reported more than one complication in a single sub-category, the incidence of all associated complications was ascribed to that category. If studies presented both matched and un-matched cohorts, matched data was preferentially extracted. For studies published by the same author in the same year, (a) and (b) in the author’s name was used to distinguish between studies. Appendix A2 shows cases where exceptions in data extraction occurred.
Table 3.
Summarizing the Post-Operative Complications that were Reported in Included Studies but were not Extracted for the Purposes of this Review.
| Removed outcome type (total studies) | Removed outcome subtype (total studies) |
|---|---|
| Medical complications (5) | Extended LOS (3) |
| Fever (1) | |
| Weakness (1) | |
| Pain (1) | |
| Anesthesia-related (1) | |
| Neuro-dural injury (7) | |
| Surgical complications (11) | Durotomy (2) |
| Nerve root injury (3) | |
| Dural injury (1) | |
| Nerve cord injury (1) | |
| Spinal fluid Leak or dural tear (3) | |
| Peripheral nerve injury (1) | |
| Spinal cord injured (1) | |
| Hardware related (4) | |
| Device complication (2) | |
| Hardware failure (1) | |
| Displaced fixation device (1) | |
| Prosthesis failure (1) | |
| Other surgical (6) | |
| Dysphagia (4) | |
| Vascular injury (1) | |
| Disc Herniation or Listhesis (1) | |
| Carotid or vertebral injury (1) | |
| Adjacent level disease (1) | |
| Pseudoarthrosis (2) | |
| Fusion rate (1) | |
| Radicular finding (1) |
(n) reflects the number of studies each complication was included in. For categories of complications (i.e., Medical), the (n) denotes the number of studies in which the complication category or any of the sub-complications in that category was included.
Quality Assessment and Strength of Evidence
The Newcastle-Ottawa Scale was used to evaluate retrospective cohort studies (Appendix A3). Authors IA and NK independently judged the quality of all eligible studies, with disagreements settled by the senior author (MSF).
Statistical Analysis
Statistical analyses were performed using RStudio 4.1.2. 21 Pooled outcomes and complication rates were calculated for AA and White cohorts. Eleven comparative meta-analyses were performed based on the categories of peri-operative outcomes and post-operative complications detailed above.
Odds ratios (OR) and 95% confidence intervals (CI) were calculated as pooled metrics using the Mantel-Haenszel method. For medical complications, one analysis was performed each for cardiovascular, DVT/PE, genitourinary, neurologic, and respiratory complications. A general analysis was done for all medical complications, which included the above and gastrointestinal, systemic infectious, and other complications that had too few member studies to merit their own sub-category analysis. An additional sub-analysis was performed for wound complications.
Heterogeneity was assessed using I2 statistics. If there was no evidence of substantial heterogeneity (I2 ≤ 50%), a fixed-effect model was used. The risk of publication bias was evaluated using a funnel plot analysis on the 4 peri-operative events, all medical complications, and wound complications. (Appendix B1).
Due to the significant variability in the sample sizes of the included works, which ranged from hundreds to hundreds of thousands of patients, each analysis was run in 3 different ways: (1) all studies, (2) studies with <100,000 patients, and (3) studies sourced from a single institution (SI). This ensured that large cohort sizes did not drown out the pooled effects of comparatively smaller studies and that the inter-reliability of different data sources could be investigated. Forest plots showing “all studies” were used as our primary finding and are presented in the text. Forest plots for analyses with <100,000 patients and SI subsets of data are shown in Appendix B2 and B3.
Results
Search Results
Database queries retrieved a total of 1023 results. No further studies were identified through other sources or via direct reference list review. After abstract and full-text screening, a total of 59 studies and 5,746,520 patients (9.6% AA) were included in the present review (Table 1). Figure 1 details our PRISMA screening process. All studies were retrospective cohort studies, 10 were SI, and 12 had more than 100,000 patients. Only 3 studies provided propensity-matched data.22-24
Overview of Findings
Table 4 shows a summary of the OR values, P-values, number of studies (k), and heterogeneity (I2) from each of the 3 data source subsets across the 11 meta-analyses that were performed. When comparing across studies with different sample sizes, SI studies had less heterogeneity, with all 11 meta-analyses employing a fixed effects model (I2 ≤ 50%) compared with just 3/11 meta-analyses that included all studies. All meta-analyses (and subset analyses) showed OR values favoring White patients except for All Medical (SI), DVT/PE (SI), Neurologic (SI), and Respiratory (SI). The smallest and largest significant OR values were 1.16 (GU) and 2.2 (DVT/PE). The Forest plots of the subset analyses for the <100,000 patient and SI subsets are shown in Appendix B2 and B3.
Table 4.
Summary of OR Values, P-values, Number of Studies (k), and Heterogeneity (I2) for 11 Outcomes and Across 3 Subsets of Included Studies: All studies, <100,000, and Single Institution (SI).
| OR | P-value | k (studies) | I2 (%) | |
|---|---|---|---|---|
| All/<100,000/SI | ||||
| Peri-Op events | ||||
| Re-admission | 1.27/1.42/1.77 | 0/0/0 | 21/19/8 | 47/27/13 |
| Non-routine discharge | 1.71/1.8/1.69 | 0/0/0.01 | 18/16/2 | 92/69/45 |
| Re-operation | 1.16/1.08/1.08 | .17/0.35/0.76 | 8/7/2 | 72/50/0 |
| Post-Op complications | ||||
| Mortality | 1.56/1.24/— | 0/0.08/— | 10/5/— | 82/26/- |
| All medical | 1.38/1.38/0.95 | 0/0/0.80 | 27/21/4 | 94/69/35 |
| Cardiovascular | 2.11/1.48/1.31 | .01/0.05/0.61 | 12/10/4 | 64/65/0 |
| DVT/PE | 1.44/2.2/0.39 | .01/0.02/0.07 | 12/11/2 | 60/66/0 |
| Genitourinary | 1.16/1.16/1.31 | .04/0.04/0.61 | 10/10/4 | 0/0/0 |
| Neurologic | 1.36/1.48/0.65 | .21/0.32/0.35 | 11/7/1 | 97/81/- |
| Respiratory | 1.38/1.33/0.41 | .04/0.25/0.20 | 11/8/2 | 77/70/0 |
| Wound | 1.47/1.38/1.35 | 0/0/0.34 | 16/15/4 | 0/0/0 |
OR values with significance (P < .05) are bolded.
Re-Admission
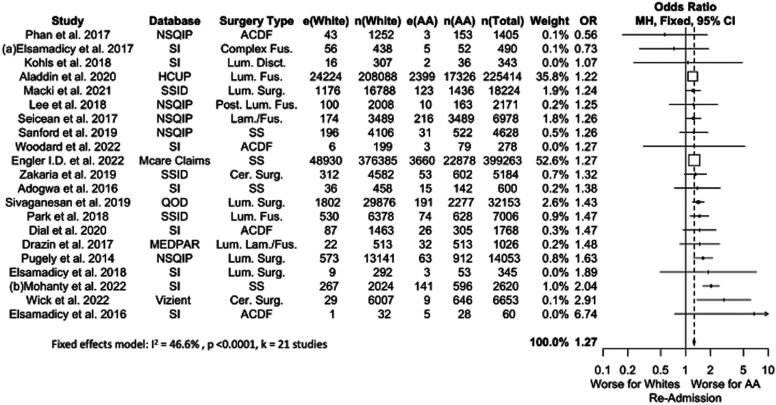
Twenty-one studies comprising 331,399 patients were included in our meta-analysis for 90-day re-admission, of whom 9.0% were AA (Figure 2). Pooled analysis showed that AA patients were more likely to have a 90-day re-admission than White patients (OR 1.27, P < .0001), a difference that persisted across all subset-analyses. OR values ranged from 1.27 to 1.77, with smaller sample size studies reporting greater OR values.
Figure 2.
Meta-analysis with a fixed effects model of all studies reporting re-admission complications for AA versus White cohorts. OR=odds ratio. e(AA) = number of adverse events in AA patients. n(AA) = sample size of AA patients. e(White) = number of adverse events in White patients. n(White) = sample size of white patients. N(Total) = total sample size in study. Cer. = Cervical, Fus. = Fusion, Lam. = Laminectomy, Lum. = Lumbar, Post. = Posterior, SS = Spine Surgery, Surg. = Surgery.
Non-Routine Discharge
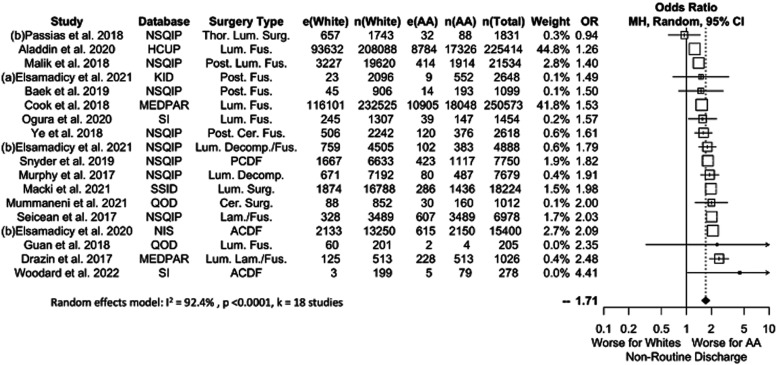
Eighteen studies comprising 570,611 patients (8.5% AA) were considered when pooling results for NRD (Figure 3). Results showed a significant difference favoring Whites (OR 1.71, P = .0001). This difference persisted across all data subsets, with OR values ranging between 1.69 and 1.80.
Figure 3.
Meta-analysis with a fixed effects model of all studies reporting non-routine discharge complications for AA versus White cohorts. OR=odds ratio. e(AA) = number of adverse events in AA patients. n(AA) = sample size of AA patients. e(White) = number of adverse events in White patients. n(White) = sample size of white patients. N(Total) = total sample size in study. Cer. = Cervical, Decomp. = Decompression, Fus. = Fusion, Lam. = Laminectomy, Lum. = Lumbar, Post. = Posterior, SS = Spine Surgery, Surg. = Surgery, Thor. = Thoracic.
Re-Operation
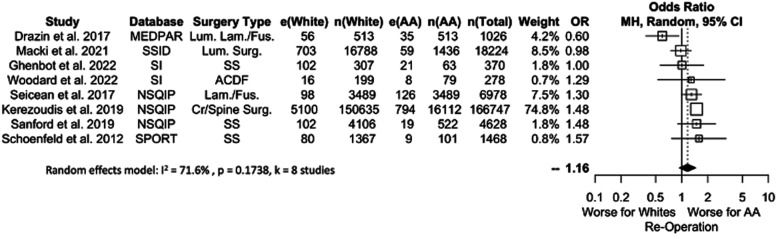
A pooled analysis of re-operation rates is shown in Figure 4. Eight studies were included, with data from 201,107 patients (11.3% AA). No significant differences were detected between AA and White patients (OR 1.16, P = .1354), an effect that persisted when considering different data subsets (OR range: 1.08-1.16).
Figure 4.
Meta-analysis with a fixed effects model of all studies reporting re-operation complications for AA versus White cohorts. OR=odds ratio. e(AA) = number of adverse events in AA patients. n(AA) = sample size of AA patients. e(White) = number of adverse events in White patients. n(White) = sample size of white patients. N(Total) = total sample size in study. Cr. = Cranial, Fus. = Fusion, Lam. = Laminectomy, Lum. = Lumbar, SS = Spine Surgery, Surg. = Surgery.
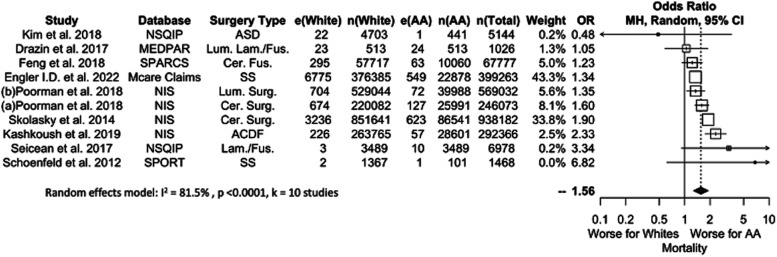
Mortality
Ten studies comprising 1,559,014 patients (10.0% AA) considered mortality (Figure 5). AA patients were 1.56 times more likely to die following elective spine surgery (P < .0001). This finding was not significant when considering the 5 studies that included <100,000 patients. No analysis of SI findings could be performed due to a lack of SI studies. OR values ranged from 1.24 to 1.56.
Figure 5.
Meta-analysis with a fixed effects model of all studies reporting mortality complications for AA versus White cohorts. OR=odds ratio. e(AA) = number of adverse events in AA patients. n(AA) = sample size of AA patients. e(White) = number of adverse events in White patients. n(White) = sample size of white patients. N(Total) = total sample size in study. ASD = Surgery for Adult Spinal Deformity, Cer. = Cervical, Fus. = Fusion, Lam. = Laminectomy, Lum. = Lumbar, SS = Spine Surgery, Surg. = Surgery.
Post-Operative Complications
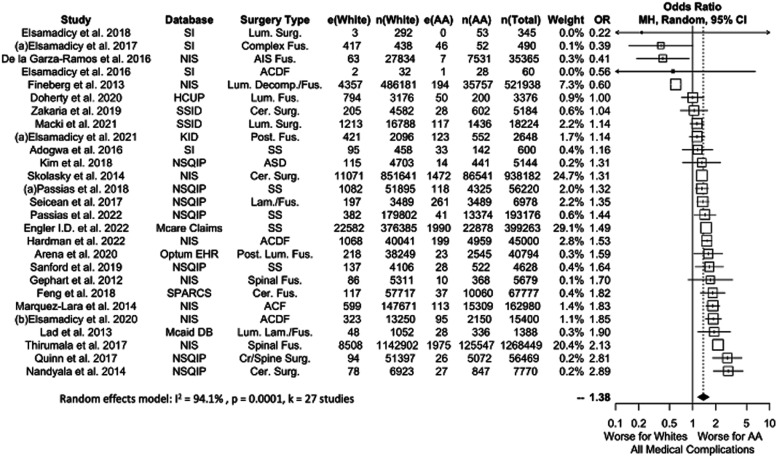
Medical Complications
The pooled analysis for patients experiencing a medical complication is shown in Figure 6. Twenty-seven studies were included for a total of 3,863,527 patients (9.8% AA). Medical complications were significantly more common in AA vs White patients (OR 1.38, P = .0001).
Figure 6.
Meta-analysis with a fixed effects model of all studies reporting all medical complications for AA versus White cohorts. OR=odds ratio. e(AA) = number of adverse events in AA patients. n(AA) = sample size of AA patients. e(White) = number of adverse events in White patients. n(White) = sample size of white patients. N(Total) = total sample size in study. ASD = Surgery for Adult Spinal Deformity, Cer. = Cervical, Cr. = Cranial, Decomp. = Decompression, Fus. = Fusion, Lam. = Laminectomy, Lum. = Lumbar, Post. = Posterior, SS = Spine Surgery, Surg. = Surgery.
A complication sub-analysis identified significant differences between AA and White patients in the incidence of Cardiovascular (12 studies, OR 1.44, P = .0126), DVT/PE (12 studies, OR 2.11, P = .0103), Genitourinary (10 studies, OR 1.16, P = .0399), and Respiratory (11 studies, OR 1.38, P = .0407) complications. Neurologic (11 studies, OR 1.36, P = .2081) complications failed to show a difference between AA and White patients. Forest plots of individual medical complications are shown in Appendix B2 and B3.
When studies with <100,000 patients were considered alone, only DVT/PE and GU complications were significantly different between White and AA patients. The non-significance in Cardiovascular and Respiratory complications in this sub-analysis is likely because both analyses included small sample size studies with OR values favoring AA patients.13,25-27 (See Appendix B2). Neurologic and Respiratory complications were not significant regardless of data source.
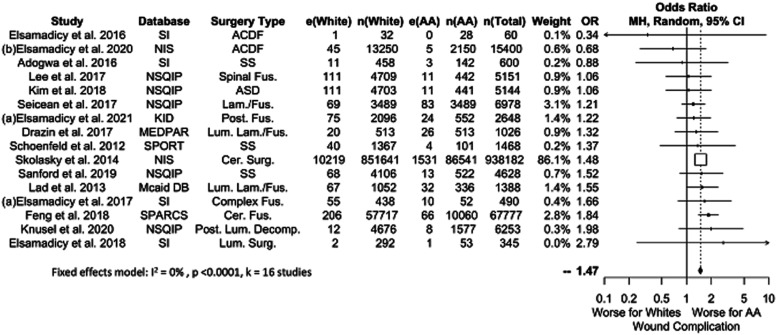
Surgical Complications
Sixteen studies reported surgical complications related to wound healing, comprising a total of 1,057,538 patients (9.2% AA, Figure 7). Results favored White patients (OR 1.47, P = .0001), and this significance persisted across all data sources except for SI. This was likely due to the small number of SI studies (k = 4). OR values ranged from 1.38-1.47 across significant results.
Figure 7.
Meta-analysis with a fixed effects model of all studies reporting would related complications for AA versus White cohorts. OR=odds ratio. e(AA) = number of adverse events in AA patients. n(AA) = sample size of AA patients. e(White) = number of adverse events in White patients. n(White) = sample size of white patients. N(Total) = total sample size in study. Cer. = Cervical, Decomp. = Decompression, Fus. = Fusion, Lam. = Laminectomy, Lum. = Lumbar, Post. = Posterior, SS = Spine Surgery, Surg. = Surgery.
Discussion
The goal of this review was to quantify the relative risk of peri-operative events and post-operative complications in AA patients compared with White patients after elective spine surgery in the United States. Our systematic review and meta-analysis showed that AA patients were more likely to experience a re-admission, a non-routine discharge, and mortality during the perioperative period. AA patients were also more likely to have medical (All Medical, Cardiovascular, DVT/PE, Genitourinary, and Respiratory) and surgical (Wound) complications. Our analyses did not show any significant difference in neurologic complications, nor did the increased rate of wound complications translate into an increased rate of re-operation. Based on funnel plot analysis, our results indicate minimal publication bias.
It was our goal to determine whether race is a vulnerability factor in the setting of spine surgery in the AA community. The meta-analysis by Schoenfeld et al 15 reported that AA patients had a higher likelihood of having an unfavorable outcome than White patients, which is consistent with the findings of the present work. We found that the greatest differences in complication rates between White and AA patients were for those complications that are likely attributable to aspects of post-operative management that are most affected by socioeconomic factors. Non-routine discharge can be the result of an inability to afford or be covered by insurance for adequate at-home care, or of the lack of family and community support during the postoperative period. 28 Wound complications can arise from improper home-management and have been associated with minority race. 29 DVT/PE has been associated with reduced mobility and poor adherence to post-operative physical therapy, 30 which has been shown correlate with socioeconomic factors, namely AA vs White race. 31 DVT/PE complications were particularly notable in our analysis, with AA patients 2.11 times more likely to experience a thromboembolic event than White patients. Socially associated risk factors, such as hypertension and diabetes, were more incident in minority patients and have been shown to delay wound healing and increase the risk of a DVT/PE. 32 Re-admission can also result from an inability to manage post-operative care at home, which may be a challenge in lower-income households due to the lack of community resources. 33 Patients sent to rehabilitation centers are also more likely to be sent to the ER for complications that could have otherwise been managed at home with close observation (such as transient fevers without hemodynamic instability).
Despite differences in re-admission and wound complication rates, it is notable that re-operation rates between AA vs White patients were equivalent despite higher adverse event rates in the former. This finding was echoed by Lad et al and Khan et al,16,34 although our finding persisted even when iatrogenic complications (such as dural tears and hardware malposition) were omitted from our analysis. This work’s exclusion of iatrogenic peri-operative re-operations we believe makes it more reflective of patient socioeconomic support and peri-operative optimization.
Differences between AA and White patients after elective spine surgery are more reflective of the social disparities that exist between these groups rather than any genetic predisposition towards complication. Haider et al 7 found that contributing factors to racial disparities included socioeconomic status, insurance status, provider factors, access to care, hospital volume, and hospital patient population. Feng et al 35 and Schoenfeld et al 36 have shown that AA patients were more likely to undergo spine surgery at low-volume centers, which have been consistently shown to be associated with worse outcomes compared with high volume centers due to factors that likely include limited resources, fewer specialists, and slower adaptation of new technology.37-39 Jancuska et al 40 demonstrated that AA patients face significant barriers to being treated at larger and higher-quality centers, highlighting a systematic pitfall in our current healthcare system.
AA patients are more likely to be either uninsured 41 or on Medicaid in the United States. 42 Safety-net hospitals predominantly treat these patients and have been shown to have worse post-operative outcomes. 43 Aladdin et al 33 illustrated this association, reporting that AA patients were more likely to have undergone surgery at safety-net hospitals and had higher odds of 30-day and 90-day re-admissions. These differences are likely the result of a combination of factors that impact the quality of safety-net hospital care, which include limited resources, staff shortages, a higher incidence of failure to rescue, and a general lack of specialized coordinated care.44,45
Patient-specific factors are important contributors to outcomes after spine surgery. Obese patients have been shown to have an increased incidence of complications after spine surgery (DVT and surgical site infection),46,47 as have people with diabetes.48,49 Drazin et al 22 reported a higher incidence of both of these comorbidities among AA patients undergoing spine surgery, and Aladdin et al 33 similarly showed a higher comorbidity burden among minority groups.
Such disparities may also influence how minority racial groups interact with the health care system. 50 Arega et al 51 reported that AA patients are less likely to choose operative management compared with other racial groups. Although the ratio of White to AA people in the United States is 5.74:1, 52 most of the studies in this present review had a lower subset of AA patients (Table 1). There is thus a concern that AA patients experience greater delays in obtaining treatment, which in the setting of degenerative spine disease can lead to a higher risk of disability.53,54
Forest plot analyses utilizing studies stratified by data origin and sample size largely yielded consistent results with small deviations in effect size. However, SI studies were not able to replicate the same significance seen when database-derived papers were also included. This was likely because (1) relatively fewer studies were included when only SI studies were considered, making them less likely to achieve significance, and (2) SI studies are likely to be underpowered for reliably detecting rare complications. This is evidenced by the fact that many outcomes and complication categories had an incidence of 0 in SI studies. Our findings on mortality in particular highlighted this discrepancy. When larger sample size studies were included (all studies), the data showed a significant difference between White and AA patients in agreement with prior evidence in the field.55-58 Smaller sample studies (<100,000) failed to show this difference.
The studies included in our analysis do not control for the socioeconomic determinants examined above or other systemic parameters such as area deprivation, which is associated with negative outcomes following spine surgery.59,60 One study showed that the effect of race on outcomes following spine surgery is negligible after controlling for systemic factors such as social vulnerability. 61 This suggests that different post-operative outcomes between White and AA patients are rooted in structural differences in access and barriers to healthcare. Changes in policy, education and representation of healthcare staff, and childhood development are among the systemic changes that are required to address these spine surgery outcome differences.62,63 Surgical care changes that can also be implemented to improve this outcome disparity include enhanced recovery after surgery (ERAS) protocols. ERAS is a multidimensional approach for promoting recovery after surgery, and included counseling and optimization during the pre-admission period, avoidance of prolonged fasting, pre-operative multimodal analgesia, prevention of hypothermia, appropriate fluid management, antimicrobial prophylaxis, blood conservation during surgery, early oral nutrition, thromboembolism prophylaxis, and early post-operative mobilization. 64 ERAS principles have only recently been applied to spine surgery due to barriers such as cultural and institutional reluctance to change and increased demands on workforces and resources. 65 Introductory studies demonstrate that spine ERAS protocols can reduce lengths of stay, reduce post-operative complications, accelerate return of function, minimize post-operative pain, and save money. 66 Patients who had greater compliance with ERAS items had fewer post-operative complications, regardless of whether or not the center had an established ERAS protocol. 67
Beyond ERAS, increased patient engagement through patient portals, mobile health applications, and chatbots can provide post-operative benefits. 68 A study by Eastwood et al. found that a single 2-hour educational session prior to spinal fusion surgery can reduce emergency room utilization, improve patient satisfaction, and alleviate back pain 69 Furthermore, text messages or digital applications designed to facilitate smoking cessation, modify physical activity, and better manage hypertension and diabetes can optimize the preoperative physical readiness, which is a major determinant of post-operative outcome. Activity trackers and wearable devices (e.g., pedometers, pulse oximeter, blood pressure monitors) may also be useful for monitoring early mobilization, and electronic checklists can be used to reinforce compliance with early recovery protocol elements. 70
Limitations
It should primarily be noted that this study is based on a meta-analysis of studies from a single nation (the United States of America). Racial data is unlikely to be generalizable to other nations around the globe for myriad historical, social, economic, and political reasons. An additional key limitation of this review comes from the source of the underlying data, which was all collected retrospectively. Most studies were judged to be “good” by the Newcastle-Ottawa grading scale. All included studies were retrospective in design, which creates a susceptibility to residual confounding and allocation bias. Our results also show significant heterogeneity in most measures. This finding is likely a result of the scope of the review, which included all elective spine surgeries for degenerative disorders, which include a wide range of pathologies. Second, we did not perform sub-analyses for elective surgeries of differing intensity or in different spinal regions. This is another confounder because certain procedures might incur a higher risk of a poor outcome compared with others. Additionally, we did not select for studies focusing on adults only. Given that adolescents are susceptible to different complication risks, this created further heterogeneity in our pooled analyses. However, only 6 studies included adolescent patients.71-76 A further weakness is that we included studies with various sample sizes, which likely had differing levels of controlled variables, assuming that a single institution is exposed to fewer confounding variables than a million patient database. Many studies also drew data from the same databases, for example NSQIP was used in 20 studies. Although each paper had different criteria for patient selection, it is likely that the same patients were counted more than once in our pooled analysis. Finally, other racial groups were not included in our analysis. Although several studies have reported disparities in Hispanic, Asian, Native American, and other racial groups, the available data was not consistent and did not allow for a robust analysis, precluding effective pooling. Our results are therefore not generalizable to other racial minorities. Patients in “Other” or “Not Reported” racial groups might introduce a selection bias, as race is a self-reported metric and some patients might have chosen not to identify themselves.77,78
Supplemental Material
Supplemental Material for Racial Differences in Perioperative Complications, Readmissions, and Mortalities After Elective Spine Surgery in the United States: A Systematic Review Using AI-Assisted Bibliometric Analysis by Izzet Akosman, Neerav Kumar, Richard Mortenson, Amanda Lans, Rafael De La Garza Ramos, Ananth Eleswarapu, Reza Yassari, and Mitchell S. Fourman in Global Spine Journal
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Izzet Akosman https://orcid.org/0000-0002-1433-9078
Neerav Kumar https://orcid.org/0000-0002-2824-6059
Amanda Lans https://orcid.org/0000-0002-4959-6517
Rafael De La Garza Ramos https://orcid.org/0000-0002-5536-2514
Mitchell S. Fourman https://orcid.org/0000-0001-5886-546X
References
- 1.Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in quality: Addressing socioeconomic, racial, and ethnic disparities in health care. JAMA. 2000;283(19):2579-2584. doi: 10.1001/JAMA.283.19.2579. [DOI] [PubMed] [Google Scholar]
- 2.Hall WJ, Chapman Mv, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: A systematic review. Am J Public Health. 2015;105(12):e60-e76. doi: 10.2105/AJPH.2015.302903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson A. Unequal treatment: Confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94(8):666-668. [PMC free article] [PubMed] [Google Scholar]
- 4.Quiñones AR, Botoseneanu A, Markwardt S, et al. Racial/ethnic differences in multimorbidity development and chronic disease accumulation for middle-aged adults. PLoS One. 2019;14(6). doi: 10.1371/JOURNAL.PONE.0218462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zandberg DP, Liu S, Goloubeva O, et al. Oropharyngeal cancer as a driver of racial outcome disparities in squamous cell carcinoma of the head and neck: 10-year experience at the university of Maryland Greenebaum cancer center. Head Neck. 2016;38(4):564-572. doi: 10.1002/HED.23933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chornokur G, Dalton K, Borysova ME, Kumar NB. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate. 2011;71(9):985-997. doi: 10.1002/PROS.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: A comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482-492.e12. doi: 10.1016/J.JAMCOLLSURG.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savitch SL, Grenda TR, Scott W, et al. Racial disparities in rates of surgery for esophageal cancer: A study from the national cancer database. J Gastrointest Surg. 2020;25(3):581-592. doi: 10.1007/S11605-020-04653-Z. [DOI] [PubMed] [Google Scholar]
- 9.Kilic A, Higgins RSD, Whitson BA, Kilic A. Racial disparities in outcomes of adult heart transplantation. Circulation. 2015;131(10):882-889. doi: 10.1161/CIRCULATIONAHA.114.011676. [DOI] [PubMed] [Google Scholar]
- 10.Schoenfeld AJ, Tipirneni R, Nelson JH, Carpenter JE, Iwashyna TJ. The influence of race and ethnicity on complications and mortality after orthopedic surgery: A systematic review of the literature. Med Care. 2014;52(9):842-851. doi: 10.1097/MLR.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 11.Lans A, Bales JR, Tobert DG, Rossi LP, Verlaan JJ, Schwab JH. Prevalence of- and factors associated with limited health literacy in spine patients. Spine J. 2022;11. doi: 10.1016/J.SPINEE.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Williams DR, Sternthal M. Understanding racial-ethnic disparities in health: Sociological contributions. J Health Soc Behav. 2010;51(1_suppl):S15-S27. doi: 10.1177/0022146510383838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adogwa O, Elsamadicy AA, Mehta AI, Cheng J, Bagley CA, Karikari IO. Racial disparities in 30-day readmission rates after elective spine surgery a single institutional experience. Spine (Phila Pa 1976). 2016;41(21):1677-1682. doi: 10.1097/BRS.0000000000001616. [DOI] [PubMed] [Google Scholar]
- 14.Woodard TK, Cortese BD, Gupta S, Mohanty S, Casper DS, Saifi C. Racial differences in patients undergoing anterior cervical discectomy and fusion: A multi-site study. Clin Spine Surg. 2022;35(4):176-180. doi: 10.1097/BSD.0000000000001312. [DOI] [PubMed] [Google Scholar]
- 15.Schoenfeld AJ, Sieg RN, Li G, Bader JO, Belmont PJ, Bono CM. Outcomes after spine surgery among racial/ethnic minorities: A meta-analysis of the literature. Spine J. 2011;11(5):381-388. doi: 10.1016/J.SPINEE.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Khan IS, Huang E, Maeder-York W, et al. Racial disparities in outcomes after spine surgery: A systematic review and meta-analysis. World Neurosurg. 2022;157:e232-e244. doi: 10.1016/J.WNEU.2021.09.140. [DOI] [PubMed] [Google Scholar]
- 17.Nested Knowledge RMU. Autolit: Spine disparities. Published online 2022. https://nested-knowledge.com/gather/1456. Accessed January 31, 2022.
- 18.Greenberg JK, Brown DS, Olsen MA, Ray WZ. Association of medicaid expansion under the affordable care act with access to elective spine surgical care. J Neurosurg Spine. 2021;36(2):336-344. doi: 10.3171/2021.3.SPINE2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DC, Liang H, Shi L. The convergence of racial and income disparities in health insurance coverage in the United States. Int J Equity Health. 2021;20(1):1-8. doi: 10.1186/S12939-021-01436-Z/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn JE. Why measure outcomes? Instr Course Lect. 2016;65:583-586. https://pubmed.ncbi.nlm.nih.gov/27049223/. Accessed December 26, 2022. [PubMed] [Google Scholar]
- 21.RStudio Team . RStudio: Integrated Development for R. Boston, MA: RStudio, 2020. [Google Scholar]
- 22.Drazin D, Shweikeh F, Lagman C, Ugiliweneza B, Boakye M. Racial disparities in elderly patients receiving lumbar spinal stenosis surgery. Global Spine J. 2017;7(2). doi: 10.1177/2192568217694012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seicean A, Seicean S, Neuhauser D, Benzel EC, Weil RJ. The influence of race on short-term outcomes after laminectomy and/or fusion spine surgery. Spine (Phila Pa 1976). 2017;42(1). doi: 10.1097/brs.0000000000001657. [DOI] [PubMed] [Google Scholar]
- 24.Meade MA, Cifu DX, Seel RT, McKinley WO, Kreutzer JS. Medical procedures, complications, and outcomes for patients with spinal cord injury: A multicenter investigation comparing African Americans and whites. Arch Phys Med Rehabil. 2004;85(3). doi: 10.1016/j.apmr.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Elsamadicy AA, Koo AB, David WB, et al. Impact of race on outcomes and healthcare utilization following spinal fusion for adolescent idiopathic scoliosis. Clin Neurol Neurosurg. 2021;206(None). doi: 10.1016/j.clineuro.2021.106634. [DOI] [PubMed] [Google Scholar]
- 26.Sanford Z, Taylor H, Fiorentino A, et al. Racial disparities in surgical outcomes after spine surgery: An ACS-NSQIP analysis. Global Spine J. 2019;9(6). doi: 10.1177/2192568218811633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elsamadicy A, Adogwa O, Reiser E, Fatemi P, Cheng J, Bagley C. The effect of patient race on extent of functional improvement after cervical spine surgery. Spine (Phila Pa. 1976). 2016;41(9). doi: 10.1097/brs.0000000000001346. [DOI] [PubMed] [Google Scholar]
- 28.Hung B, Pennington Z, Hersh AM, et al. Impact of race on nonroutine discharge, length of stay, and postoperative complications after surgery for spinal metastases. J Neurosurg Spine. 2021;36(4):678-685. doi: 10.3171/2021.7.SPINE21287. [DOI] [PubMed] [Google Scholar]
- 29.Brooks Carthon JM, Jarrín O, Sloane D, Kutney-Lee A. Variations in postoperative complications across race, ethnicity and sex among older adults. J Am Geriatr Soc. 2013;61(9):1499. doi: 10.1111/JGS.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatsis V, Visintini S. Early mobilization for patients with venous thromboembolism: A review of clinical effectiveness and guidelines. 2018. Published online January. https://www.ncbi.nlm.nih.gov/books/NBK531715/. Accessed November 7, 2022. [PubMed]
- 31.Bove AM, Hausmann LRM, Piva SR, Brach JS, Lewis A, Fitzgerald GK. Race differences in postacute physical therapy utilization and patient-reported function after total knee arthroplasty. Arthritis Care Res. 2022;74(1):79-88. doi: 10.1002/ACR.24792. [DOI] [PubMed] [Google Scholar]
- 32.Black patients significantly less likely to live independently after surgery. https://www.asahq.org/about-asa/newsroom/news-releases/2021/10/black-patients-significantly-less-likely-to-live-independently-after-surgery. Accessed November 7, 2022.
- 33.Aladdin DEH, Tangel V, Lui B, Pryor KO, Witkin LR, White RS. Black race as a social determinant of health and outcomes after lumbar spinal fusion surgery: A multistate analysis, 2007 to 2014. Spine. 2020;45(10):701-711. doi: 10.1097/BRS.0000000000003367. [DOI] [PubMed] [Google Scholar]
- 34.Lad SP, Bagley JH, Kenney KT, et al. Racial disparities in outcomes of spinal surgery for lumbar stenosis. Spine (Phila Pa 1976). 2013;38(11):927-935. doi: 10.1097/BRS.0B013E31828165F9. [DOI] [PubMed] [Google Scholar]
- 35.Feng R, Finkelstein M, Bilal K, Oermann EK, Palese M, Caridi J. Trends and disparities in cervical spine fusion procedures utilization in the New York State. Spine (Phila Pa 1976). 2018;43(10):E601-E606. doi: 10.1097/BRS.0000000000002438. [DOI] [PubMed] [Google Scholar]
- 36.Schoenfeld AJ, Lurie JD, Zhao W, Bono CM. The effect of race on outcomes of surgical or non-surgical treatment of patients in the spine patient outcomes research trial (SPORT). Spine (Phila Pa 1976). 2012;37(17):1505. doi: 10.1097/BRS.0B013E318251CC78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groeneveld PW, Laufer SB, Garber AM. Technology diffusion, hospital variation, and racial disparities among elderly medicare beneficiaries 1989-2000. Med Care. 2005;43(4):320-329. doi: 10.1097/01.MLR.0000156849.15166.EC. [DOI] [PubMed] [Google Scholar]
- 38.Li HZ, Lin Z, Li ZZ, et al. Relationship between surgeon volume and outcomes in spine surgery: A dose-response meta-analysis. Ann Transl Med. 2018;6(22):441-441. doi: 10.21037/ATM.2018.10.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farjoodi P, Skolasky RL, Riley LH. The effects of hospital and surgeon volume on postoperative complications after lumbar spine surgery. Spine. 2011;36(24):2069-2075. doi: 10.1097/BRS.0B013E318202AC56. [DOI] [PubMed] [Google Scholar]
- 40.Jancuska JM, Hutzler L, Protopsaltis TS, Bendo JA, Bosco J. Utilization of lumbar spinal fusion in New York state trends and disparities. Spine (Phila Pa 1976). 2016;41(19):1508-1514. doi: 10.1097/BRS.0000000000001567. [DOI] [PubMed] [Google Scholar]
- 41.Uninsured rates for the nonelderly by race/ethnicity | KFF. https://www.kff.org/uninsured/state-indicator/nonelderly-uninsured-rate-by-raceethnicity/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed November 7, 2022.
- 42.Medicaid coverage rates for the nonelderly by race/ethnicity KFF. https://www.kff.org/medicaid/state-indicator/nonelderly-medicaid-rate-by-raceethnicity/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed November 7, 2022.
- 43.Hoehn RS, Wima K, Vestal MA, et al. Effect of hospital safety-net burden on cost and outcomes after surgery. JAMA Surg. 2016;151(2):120-128. doi: 10.1001/JAMASURG.2015.3209. [DOI] [PubMed] [Google Scholar]
- 44.Wakeam E, Hevelone ND, Maine R, et al. Failure to rescue in safety-net hospitals: Availability of hospital resources and differences in performance. JAMA Surg. 2014;149(3):229-235. doi: 10.1001/JAMASURG.2013.3566. [DOI] [PubMed] [Google Scholar]
- 45.Nurse Staffing Effects on Patient Outcomes . Safety-net and non-safety-net hospitals on JSTOR. https://www.jstor.org/stable/41103933#metadata_info_tab_contents. Accessed November 7, 2022. [DOI] [PubMed]
- 46.Epstein N. More risks and complications for elective spine surgery in morbidly obese patients. Surg Neurol Int. 2017;8(1). doi: 10.4103/SNI.SNI_49_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson KL, Devine JG. The effects of obesity on spine surgery: A systematic review of the literature. Global Spine J. 2016;6(4):394-400. doi: 10.1055/S-0035-1570750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo W, Sun RX, Jiang H, Ma XL. The effect of diabetes on perioperative complications following spinal surgery: A meta-analysis. Ther Clin Risk Manag. 2018;14:2415-2423. doi: 10.2147/TCRM.S185221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Epstein N. Predominantly negative impact of diabetes on spinal surgery: A review and recommendation for better preoperative screening. Surg Neurol Int. 2017;8(1):107. doi: 10.4103/SNI.SNI_101_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Penner LA, Blair Iv, Albrecht TL, Dovidio JF. Reducing racial health care disparities: A social psychological analysis. Policy Insights Behav Brain Sci. 2014;1(1):204-212. doi: 10.1177/2372732214548430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arega A, Birkmeyer NJO, Lurie JDN, et al. Racial variation in treatment preferences and willingness to randomize in the spine patient outcomes research trial (SPORT). Spine. 2006;31(19):2263-2269. doi: 10.1097/01.BRS.0000232708.66608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.U.S. Census Bureau . QuickFacts: United States. https://www.census.gov/quickfacts/fact/table/US/PST045221. Accessed November 7, 2022.
- 53.Elsamadicy AA, Adogwa O, Fialkoff J, et al. Race as an independent predictor of temporal delay in time to diagnosis and treatment in patients with cervical stenosis: A study of 133 patients with anterior cervical discectomy and fusion. World Neurosurg. 2016;96:107-110. doi: 10.1016/J.WNEU.2016.08.070. [DOI] [PubMed] [Google Scholar]
- 54.Pope DH, Mowforth OD, Davies BM, Kotter MRN. Diagnostic delays lead to greater disability in degenerative cervical myelopathy and represent a health inequality. Spine. 2020;45(6):368-377. doi: 10.1097/BRS.0000000000003305. [DOI] [PubMed] [Google Scholar]
- 55.Lucas FL, Stukel TA, Morris AM, Siewers AE, Birkmeyer JD. Race and surgical mortality in the United States. Ann Surg. 2006;243(2):281-286. doi: 10.1097/01.SLA.0000197560.92456.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rangrass G, Ghaferi AA, Dimick JB. Explaining racial disparities in outcomes after cardiac surgery: The role of hospital quality. JAMA Surg. 2014;149(3):223-227. doi: 10.1001/JAMASURG.2013.4041. [DOI] [PubMed] [Google Scholar]
- 57.Morris AM, Rhoads KF, Stain SC, Birkmeyer JD. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211(1):105-113. doi: 10.1016/J.JAMCOLLSURG.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 58.Osborne NH, Upchurch GR, Mathur AK, Dimick JB. Explaining racial disparities in mortality after abdominal aortic aneurysm repair. J Vasc Surg. 2009;50(4):709-713. doi: 10.1016/J.JVS.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 59.Zhang JK, Greenberg JK, Javeed S, et al. Association between neighborhood-level socioeconomic disadvantage and patient-reported outcomes in lumbar spine surgery. Neurosurgery. 2023;92(1):92-101. doi: 10.1227/NEU.0000000000002181. [DOI] [PubMed] [Google Scholar]
- 60.Hagan MJ, Sastry RA, Feler J, et al. Neighborhood-level socioeconomic status predicts extended length of stay after elective anterior cervical spine surgery. World Neurosurg. 2022;163:e341-e348. doi: 10.1016/J.WNEU.2022.03.124. [DOI] [PubMed] [Google Scholar]
- 61.de La Garza Ramos R, Javed K, Ryvlin J, Gelfand Y, Murthy S, Yassari R. Are there racial or socioeconomic disparities in ambulatory outcome or survival after oncologic spine surgery for metastatic cancer? Results from a medically underserved center. Clin Orthop Relat Res. 2023;481(2):301-307. doi: 10.1097/CORR.0000000000002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams DR, Cooper LA. Reducing racial inequities in health: Using what we already know to take action. Int J Environ Res Public Health. 2019;16(4). doi: 10.3390/IJERPH16040606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacobs L. Addressing racial inequity in surgery: Reflections on a career in medicine by a surgeon. J Law Med Ethics. 2021;49(2):174-180. doi: 10.1017/JME.2021.27. [DOI] [PubMed] [Google Scholar]
- 64.Choi YS, Kim TW, Chang MJ, Kang SB, Chang CB. Enhanced recovery after surgery for major orthopedic surgery: A narrative review. Knee Surg Relat Res. 2022;34(1):1-12. doi: 10.1186/S43019-022-00137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maessen J, Dejong CHC, Hausel J, et al. A protocol is not enough to implement an enhanced recovery programme for colorectal resection. Br J Surg. 2007;94(2):224-231. doi: 10.1002/BJS.5468. [DOI] [PubMed] [Google Scholar]
- 66.Elsarrag M, Soldozy S, Patel P, et al. Enhanced recovery after spine surgery: A systematic review. Neurosurg Focus. 2019;46(4):E3. doi: 10.3171/2019.1.FOCUS18700. [DOI] [PubMed] [Google Scholar]
- 67.Ripollés-Melchor J, Abad-Motos A, Díez-Remesal Y, et al. Association between use of enhanced recovery after surgery protocol and postoperative complications in total hip and knee arthroplasty in the postoperative outcomes within enhanced recovery after surgery protocol in elective total hip and knee arthroplasty study (POWER2). JAMA Surg. 2020;155(4):e196024. doi: 10.1001/JAMASURG.2019.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Campbell K, Louie P, Levine B, Gililland J. Using patient engagement platforms in the postoperative management of patients. Curr Rev Musculoskelet Med. 2020;13(4):479. doi: 10.1007/S12178-020-09638-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eastwood D, Manson N, Bigney E, et al. Improving postoperative patient reported benefits and satisfaction following spinal fusion with a single preoperative education session. Spine J. 2019;19(5):840-845. doi: 10.1016/J.SPINEE.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 70.Michard F, Gan TJ, Kehlet H. Digital innovations and emerging technologies for enhanced recovery programmes. Br J Anaesth. 2017;119(1):31-39. doi: 10.1093/BJA/AEX140. [DOI] [PubMed] [Google Scholar]
- 71.de la Garza-Ramos R, Samdani AF, Sponseller PD, et al. Visual loss after corrective surgery for pediatric scoliosis: incidence and risk factors from a nationwide database. Spine J. 2016;16(4). doi: 10.1016/j.spinee.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 72.Elsamadicy AA, Adogwa O, Sergesketter A, et al. Impact of race on 30-day complication rates after elective complex spinal fusion (≥5 Levels): A single institutional study of 446 patients. World Neurosurg. 2017;99(None). doi: 10.1016/j.wneu.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 73.Thirumala P, Zhou J, Natarajan P, et al. Perioperative neurologic complications during spinal fusion surgery: Incidence and trends. Spine J. 2017;17(11). doi: 10.1016/j.spinee.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 74.Baek J, Malik AT, Tamer R, Yu E, Kim J, Khan SN. Non-home discharge disposition after posterior spinal fusion in neuromuscular scoliosis-an analysis of the American college of surgeons national surgical quality improvement program (ACS-NSQIP) pediatric database. J Spine Surg. 2019;5(1). doi: 10.21037/jss.2019.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poorman GW, Moon JY, Horn SR, et al. Rates of mortality in cervical spine surgical procedures and factors associated with its occurrence over a 10-year period: A study of 342 477 patients on the nationwide inpatient sample. Int J Spine Surg. 2018;12(2). doi: 10.14444/5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poorman GW, Moon JY, Wang C, et al. Rates of mortality in lumbar spine surgery and factors associated with its occurrence over a 10-year period: A study of 803,949 patients in the nationwide inpatient sample. Int J Spine Surg. 2018;12(5). doi: 10.14444/5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wendler D, Kington R, Madans J, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3(2):0201-0210. doi: 10.1371/JOURNAL.PMED.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flanagin A, Frey T, Christiansen SL, Bauchner H. The reporting of race and ethnicity in medical and science journals: comments invited. JAMA. 2021;325(11):1049-1052. doi: 10.1001/JAMA.2021.2104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Racial Differences in Perioperative Complications, Readmissions, and Mortalities After Elective Spine Surgery in the United States: A Systematic Review Using AI-Assisted Bibliometric Analysis by Izzet Akosman, Neerav Kumar, Richard Mortenson, Amanda Lans, Rafael De La Garza Ramos, Ananth Eleswarapu, Reza Yassari, and Mitchell S. Fourman in Global Spine Journal