Abstract
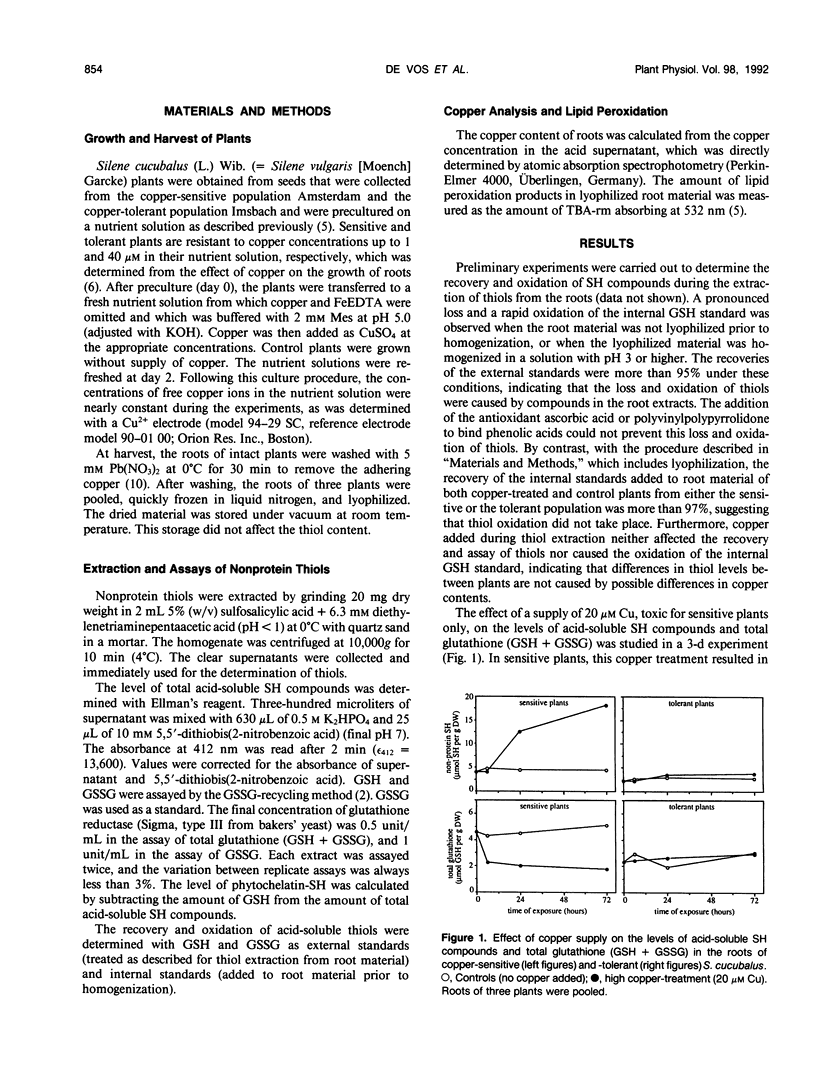
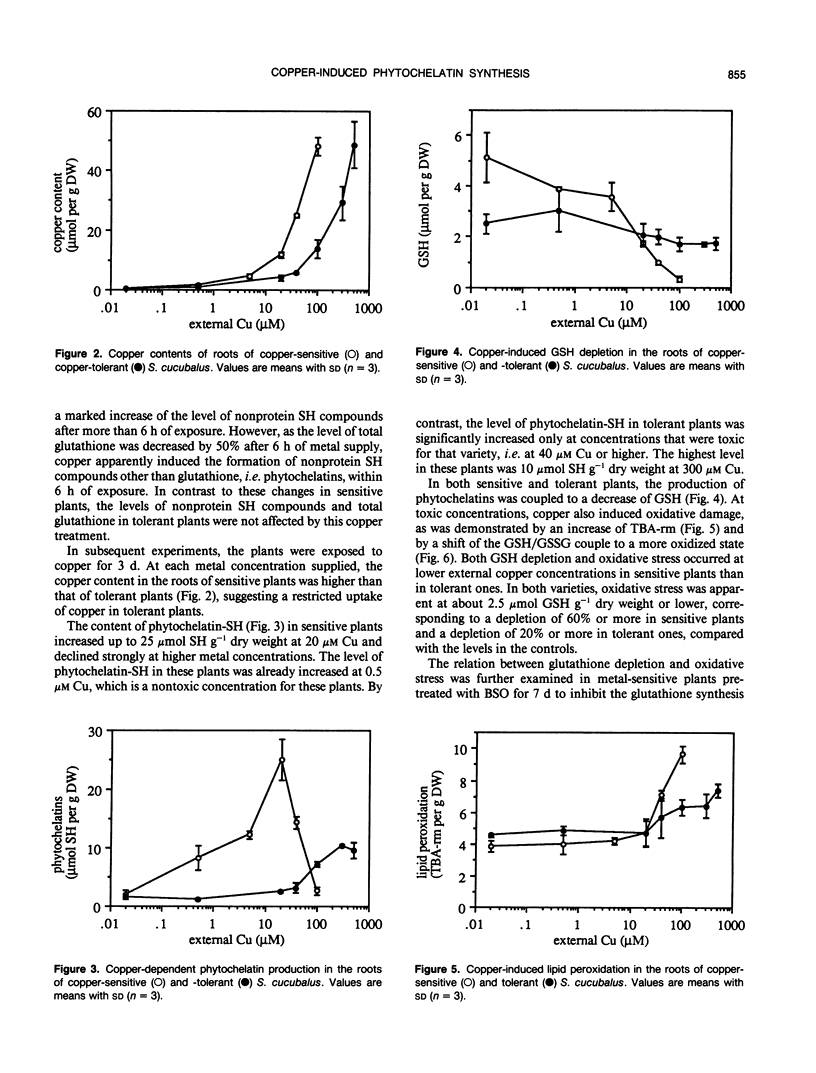
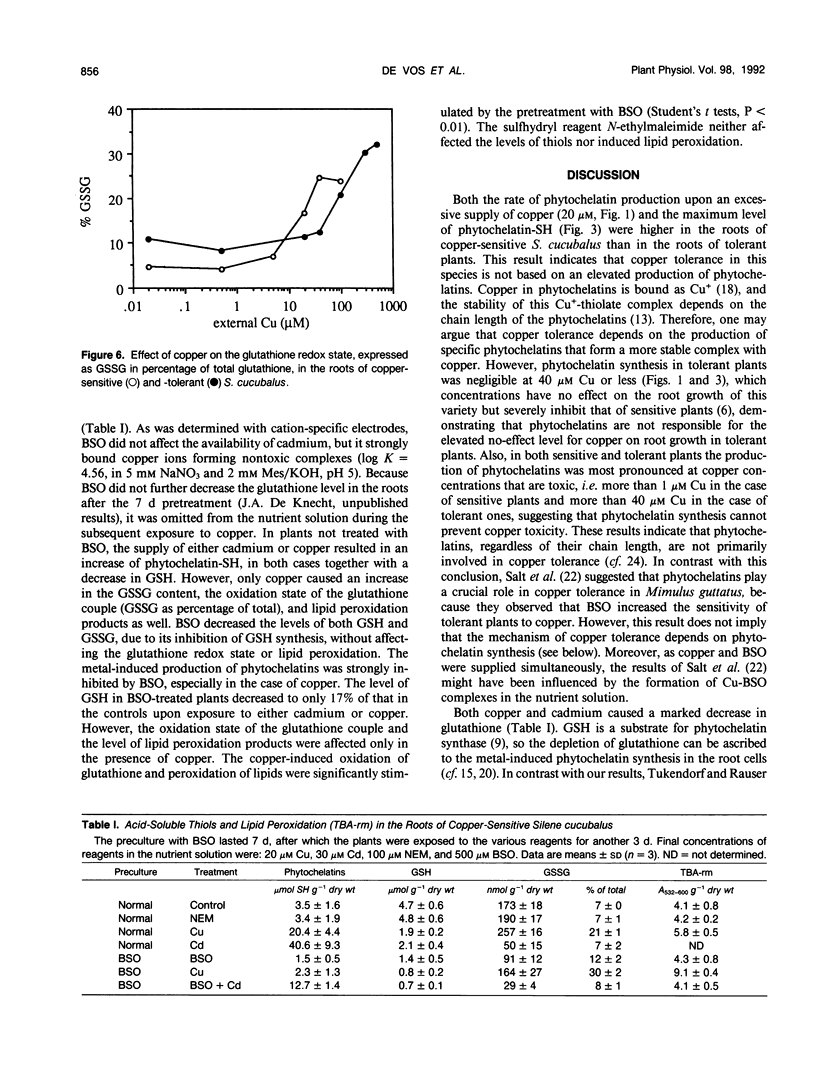
The relation between loss of glutathione due to metal-induced phytochelatin synthesis and oxidative stress was studied in the roots of copper-sensitive and tolerant Silene cucubalus (L.) Wib., resistant to 1 and 40 micromolar Cu, respectively. The amount of nonprotein sulfhydryl compounds other than glutathione was taken as a measure of phytochelatins. At a supply of 20 micromolar Cu, which is toxic for sensitive plants only, phytochelatin synthesis and loss of total glutathione were observed only in sensitive plants within 6 h of exposure. When the plants were exposed to a range of copper concentrations for 3 d, a marked production of phytochelatins in sensitive plants was already observed at 0.5 micromolar Cu, whereas the production in tolerant plants was negligible at 40 micromolar or lower. The highest production in tolerant plants was only 40% of that in sensitive plants. In both varieties, the synthesis of phytochelatins was coupled to a loss of glutathione. Copper at toxic concentrations caused oxidative stress, as was evidenced by both the accumulation of lipid peroxidation products and a shift in the glutathione redox couple to a more oxidized state. Depletion of glutathione by pretreatment with buthionine sulfoximine significantly increased the oxidative damage by copper. At a comparably low glutathione level, cadmium had no effect on either lipid peroxidation or the glutathione redox couple in buthionine sulfoximine-treated plants. These results indicate that copper may specifically cause oxidative stress by depletion of the antioxidant glutathione due to phytochelatin synthesis. We conclude that copper tolerance in S. cucubalus does not depend on the production of phytochelatins but is related to the plant's ability to prevent glutathione depletion resulting from copper-induced phytochelatin production, e.g. by restricting its copper uptake.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Delhaize E., Jackson P. J., Lujan L. D., Robinson N. J. Poly(gamma-glutamylcysteinyl)glycine Synthesis in Datura innoxia and Binding with Cadmium : Role in Cadmium Tolerance. Plant Physiol. 1989 Feb;89(2):700–706. doi: 10.1104/pp.89.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Monte D., Bellomo G., Thor H., Nicotera P., Orrenius S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch Biochem Biophys. 1984 Dec;235(2):343–350. doi: 10.1016/0003-9861(84)90207-8. [DOI] [PubMed] [Google Scholar]
- Grill E., Löffler S., Winnacker E. L., Zenk M. H. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific gamma-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc Natl Acad Sci U S A. 1989 Sep;86(18):6838–6842. doi: 10.1073/pnas.86.18.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E., Winnacker E. L., Zenk M. H. Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science. 1985 Nov 8;230(4726):674–676. doi: 10.1126/science.230.4726.674. [DOI] [PubMed] [Google Scholar]
- Hochstein P., Kumar K. S., Forman S. J. Lipid peroxidation and the cytotoxicity of copper. Ann N Y Acad Sci. 1980;355:240–248. doi: 10.1111/j.1749-6632.1980.tb21342.x. [DOI] [PubMed] [Google Scholar]
- Jackson P. J., Unkefer C. J., Doolen J. A., Watt K., Robinson N. J. Poly(gamma-glutamylcysteinyl)glycine: its role in cadmium resistance in plant cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6619–6623. doi: 10.1073/pnas.84.19.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra R. K., Winge D. R. Cu(I) binding to the Schizosaccharomyces pombe gamma-glutamyl peptides varying in chain lengths. Arch Biochem Biophys. 1988 Sep;265(2):381–389. doi: 10.1016/0003-9861(88)90141-5. [DOI] [PubMed] [Google Scholar]
- Miller D. M., Buettner G. R., Aust S. D. Transition metals as catalysts of "autoxidation" reactions. Free Radic Biol Med. 1990;8(1):95–108. doi: 10.1016/0891-5849(90)90148-c. [DOI] [PubMed] [Google Scholar]
- Reese R. N., Mehra R. K., Tarbet E. B., Winge D. R. Studies on the gamma-glutamyl Cu-binding peptide from Schizosaccharomyces pombe. J Biol Chem. 1988 Mar 25;263(9):4186–4192. [PubMed] [Google Scholar]
- Reese R. N., Wagner G. J. Effects of buthionine sulfoximine on cd-binding Peptide levels in suspension-cultured tobacco cells treated with cd, zn, or cu. Plant Physiol. 1987 Jul;84(3):574–577. doi: 10.1104/pp.84.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegsegger A., Schmutz D., Brunold C. Regulation of Glutathione Synthesis by Cadmium in Pisum sativum L. Plant Physiol. 1990 Aug;93(4):1579–1584. doi: 10.1104/pp.93.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhany J. M., Swanson J. C., Cordes K. A., Gaines S. B., Gaines K. C. Evidence suggesting direct oxidation of human erythrocyte membrane sulfhydryls by copper. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1294–1299. doi: 10.1016/0006-291x(78)90328-5. [DOI] [PubMed] [Google Scholar]
- Scheller H. V., Huang B., Hatch E., Goldsbrough P. B. Phytochelatin synthesis and glutathione levels in response to heavy metals in tomato cells. Plant Physiol. 1987 Dec;85(4):1031–1035. doi: 10.1104/pp.85.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens J. C., Hunt D. F., Williams B. G. Accumulation of non-protein metal-binding polypeptides (gamma-glutamyl-cysteinyl)n-glycine in selected cadmium-resistant tomato cells. J Biol Chem. 1986 Oct 25;261(30):13879–13882. [PubMed] [Google Scholar]
- Vögeli-Lange R., Wagner G. J. Subcellular localization of cadmium and cadmium-binding peptides in tobacco leaves : implication of a transport function for cadmium-binding peptides. Plant Physiol. 1990 Apr;92(4):1086–1093. doi: 10.1104/pp.92.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]


