Abstract
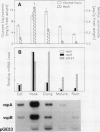
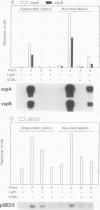
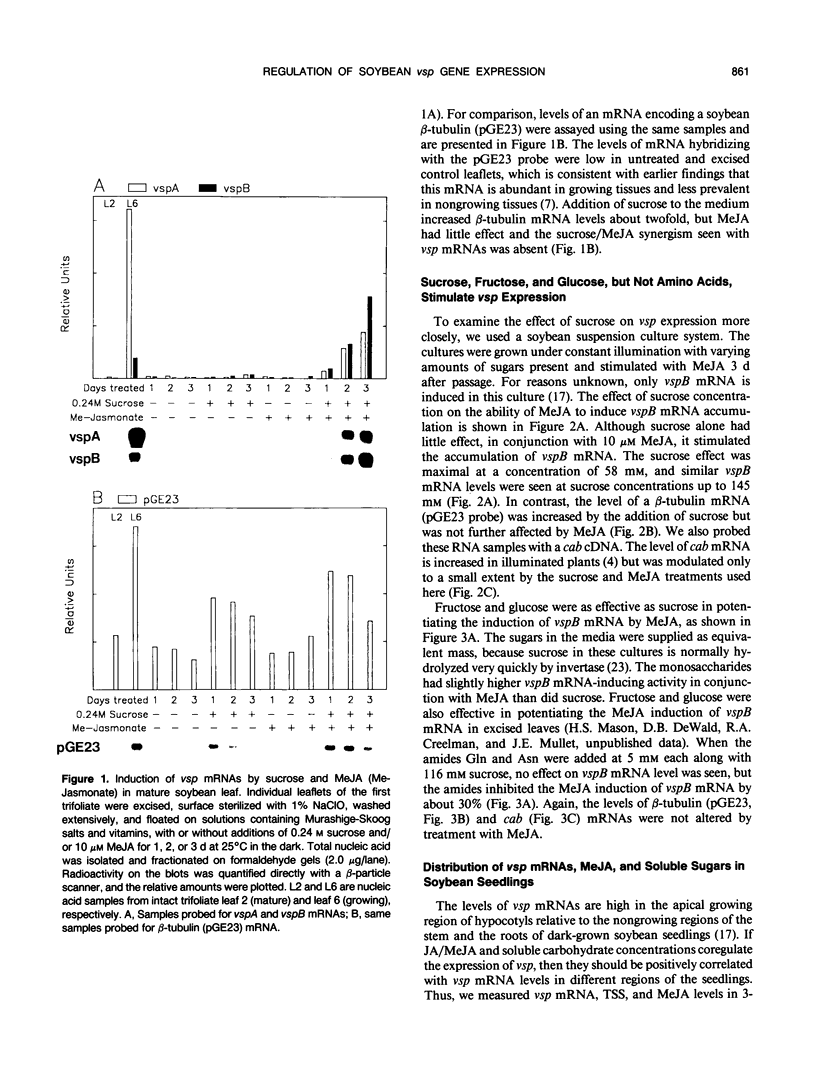
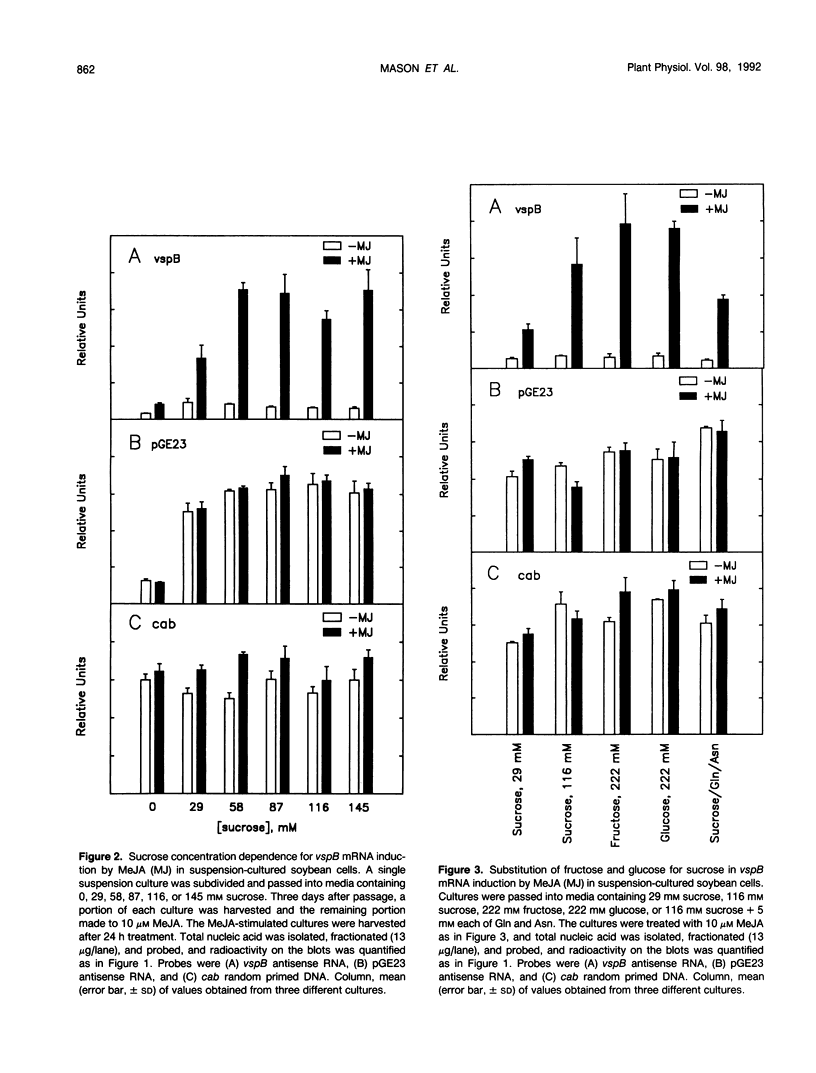
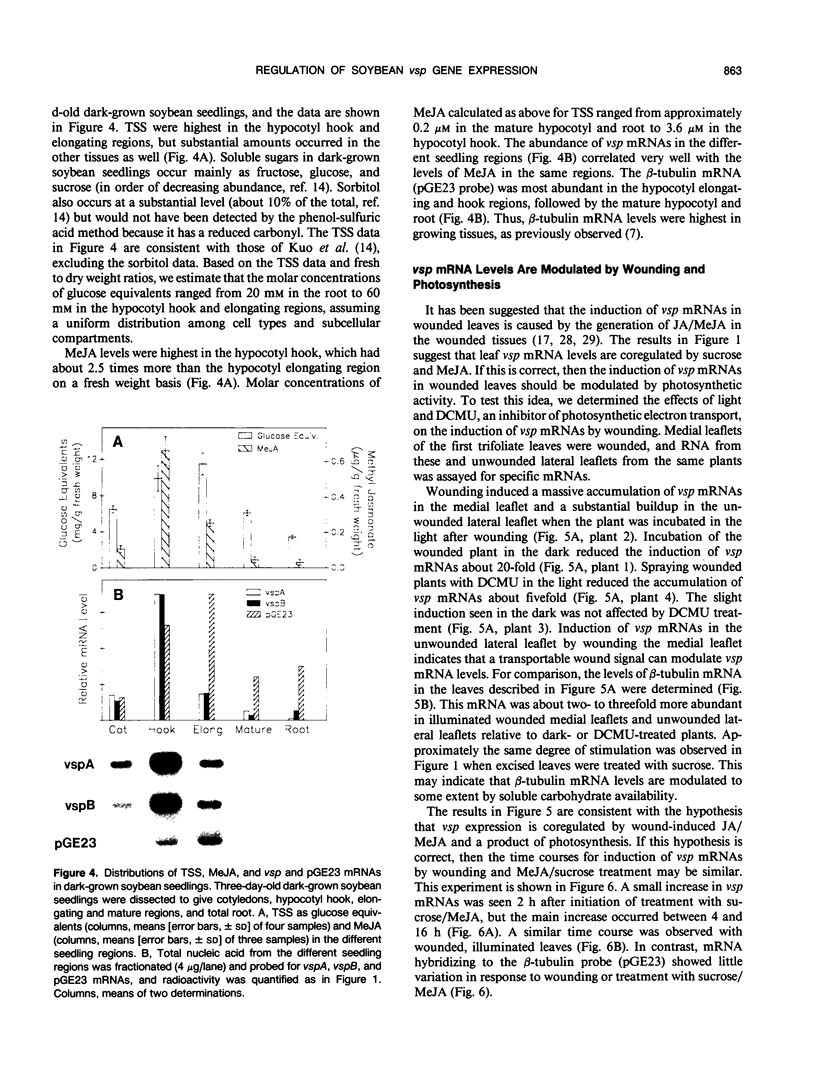
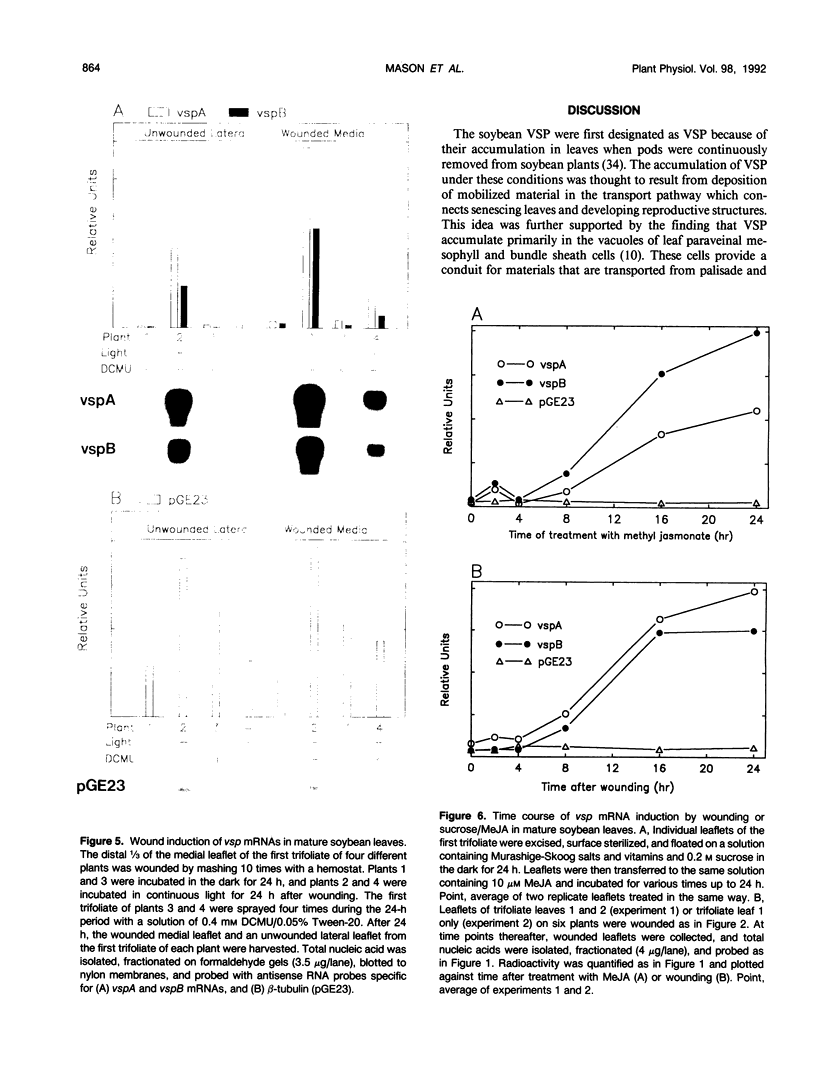
The soybean vegetative storage protein genes vspA and vspB are highly expressed in developing leaves, stems, flowers, and pods as compared with roots, seeds, and mature leaves and stems. In this paper, we report that physiological levels of methyl jasmonate (MeJA) and soluble sugars synergistically stimulate accumulation of vsp mRNAs. Treatment of excised mature soybean (Glycine max Merr. cv Williams) leaves with 0.2 molar sucrose and 10 micromolar MeJA caused a large accumulation of vsp mRNAs, whereas little accumulation occurred when these compounds were supplied separately. In soybean cell suspension cultures, the synergistic effect of sucrose and MeJA on the accumulation of vspB mRNA was maximal at 58 millimolar sucrose and was observed with fructose or glucose substituted for sucrose. In dark-grown soybean seedlings, the highest levels of vsp mRNAs occurred in the hypocotyl hook, which also contained high levels of MeJA and soluble sugars. Lower levels of vsp mRNAs, MeJA, and soluble sugars were found in the cotyledons, roots, and nongrowing regions of the stem. Wounding of mature soybean leaves induced a large accumulation of vsp mRNAs when wounded plants were incubated in the light. Wounded plants kept in the dark or illuminated plants sprayed with dichlorophenyldimethylurea, an inhibitor of photosynthetic electron transport, showed a greatly reduced accumulation of vsp mRNAs. The time courses for the accumulation of vsp mRNAs induced by wounding or sucrose/MeJA treatment were similar. These results strongly suggest that vsp expression is coregulated by endogenous levels of MeJA (or jasmonic acid) and soluble carbohydrate during normal vegetative development and in wounded leaves.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bozarth C. S., Mullet J. E., Boyer J. S. Cell wall proteins at low water potentials. Plant Physiol. 1987 Sep;85(1):261–267. doi: 10.1104/pp.85.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglie R., Bellemare G., Bartlett S. G., Chua N. H., Cashmore A. R. Cloned DNA sequences complementary to mRNAs encoding precursors to the small subunit of ribulose-1,5-bisphosphate carboxylase and a chlorophyll a/b binding polypeptide. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7304–7308. doi: 10.1073/pnas.78.12.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton N. J., Silvius J. E. Photosynthate Partitioning into Starch in Soybean Leaves: I. Effects of Photoperiod versus Photosynthetic Period Duration. Plant Physiol. 1979 Nov;64(5):749–753. doi: 10.1104/pp.64.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Mason H. S., Bensen R. J., Boyer J. S., Mullet J. E. Water Deficit and Abscisic Acid Cause Differential Inhibition of Shoot versus Root Growth in Soybean Seedlings : Analysis of Growth, Sugar Accumulation, and Gene Expression. Plant Physiol. 1990 Jan;92(1):205–214. doi: 10.1104/pp.92.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Mullet J. E. Water deficit modulates gene expression in growing zones of soybean seedlings. Analysis of differentially expressed cDNAs, a new beta-tubulin gene, and expression of genes encoding cell wall proteins. Plant Mol Biol. 1991 Oct;17(4):591–608. doi: 10.1007/BF00037046. [DOI] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi V. R., Wittenbach V. A., Giaquinta R. T. Paraveinal Mesophyll of Soybean Leaves in Relation to Assimilate Transfer and Compartmentation : III. Immunohistochemical Localization of Specific Glycopeptides in the Vacuole after Depodding. Plant Physiol. 1983 Jun;72(2):586–589. doi: 10.1104/pp.72.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T. R., Ryan C. A. Wound-induced Proteinase Inhibitor in Tomato Leaves: Some Effects of Light and Temperature on the Wound Response. Plant Physiol. 1973 Jan;51(1):19–21. doi: 10.1104/pp.51.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Ryan C. A. Wound-inducible potato inhibitor II genes: enhancement of expression by sucrose. Plant Mol Biol. 1990 Apr;14(4):527–536. doi: 10.1007/BF00027498. [DOI] [PubMed] [Google Scholar]
- Kuo T. M., Doehlert D. C., Crawford C. G. Sugar metabolism in germinating soybean seeds: evidence for the sorbitol pathway in soybean axes. Plant Physiol. 1990 Aug;93(4):1514–1520. doi: 10.1104/pp.93.4.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas C., Schaal S., Werr W. A feedback control element near the transcription start site of the maize Shrunken gene determines promoter activity. EMBO J. 1990 Nov;9(11):3447–3452. doi: 10.1002/j.1460-2075.1990.tb07552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason H. S., Mullet J. E. Expression of two soybean vegetative storage protein genes during development and in response to water deficit, wounding, and jasmonic acid. Plant Cell. 1990 Jun;2(6):569–579. doi: 10.1105/tpc.2.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Metabolic repression of transcription in higher plants. Plant Cell. 1990 Oct;2(10):1027–1038. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilatro S. R., Anderson J. M. Carbohydrate Metabolism and Activity of Pyrophosphate: Fructose-6-Phosphate Phosphotransferase in Photosynthetic Soybean (Glycine max, Merr.) Suspension Cells. Plant Physiol. 1988 Nov;88(3):862–868. doi: 10.1104/pp.88.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilatro S. R., Anderson J. M. Characterization of a soybean leaf protein that is related to the seed lectin and is increased with pod removal. Plant Physiol. 1989 Aug;90(4):1387–1393. doi: 10.1104/pp.90.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Developmental regulation and the influence of plant sinks on vegetative storage protein gene expression in soybean leaves. Plant Physiol. 1989 Jan;89(1):309–315. doi: 10.1104/pp.89.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E., Huang J. F., Rhee Y. Nitrogen and methyl jasmonate induction of soybean vegetative storage protein genes. Plant Physiol. 1991 May;96(1):130–136. doi: 10.1104/pp.96.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Novel Regulation of Vegetative Storage Protein Genes. Plant Cell. 1990 Jan;2(1):1–6. doi: 10.1105/tpc.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Preferential Loss of an Abundant Storage Protein from Soybean Pods during Seed Development. Plant Physiol. 1989 Aug;90(4):1252–1255. doi: 10.1104/pp.90.4.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Soybean vegetative storage protein structure and gene expression. Plant Physiol. 1988 May;87(1):250–254. doi: 10.1104/pp.87.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surowy T. K., Boyer J. S. Low water potentials affect expression of genes encoding vegetative storage proteins and plasma membrane proton ATPase in soybean. Plant Mol Biol. 1991 Feb;16(2):251–262. doi: 10.1007/BF00020556. [DOI] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. Biosynthesis of jasmonic Acid by several plant species. Plant Physiol. 1984 Jun;75(2):458–461. doi: 10.1104/pp.75.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. Levels of oxygenated Fatty acids in young corn and sunflower plants. Plant Physiol. 1982 May;69(5):1103–1108. doi: 10.1104/pp.69.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Purification and characterization of a soybean leaf storage glycoprotein. Plant Physiol. 1983 Sep;73(1):125–129. doi: 10.1104/pp.73.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]