Abstract
The subcellular localization of O-acetyiserine(thiol)lyase (EC 4.2.99.8) in nongreen tissue from higher plants has been studied using purified proplastids, mitochondria, and protoplasts from cauliflower (Brassica oleracea L.) buds as a source of subcellular fractions. O-Acetylserine(thiol)lyase has been detected in both organelles (proplastids and mitochondria) and a cytosolic extract obtained by protoplast fractionation. We confirmed these observations, demonstrating that a form of the enzyme different in global charge and separated from others by anion-exchange chromatography corresponded to each subcellular location. Our observations are consistent with the need for cysteine biosynthesis in each subcellular compartment where the synthesis of proteins occurs.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alban C., Joyard J., Douce R. Preparation and characterization of envelope membranes from nongreen plastids. Plant Physiol. 1988 Nov;88(3):709–717. doi: 10.1104/pp.88.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertagnolli B. L., Wedding R. T. Purification and initial kinetic characterization of different forms of o-acetylserine sulfhydrylase from seedlings of two species of phaseolus. Plant Physiol. 1977 Jul;60(1):115–121. doi: 10.1104/pp.60.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunold C., Schiff J. A. Studies of sulfate utilization of algae: 15. Enzymes of assimilatory sulfate reduction in euglena and their cellular localization. Plant Physiol. 1976 Mar;57(3):430–436. doi: 10.1104/pp.57.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell J. N. Sulfate Assimilation in C(4) Plants: Intercellular and Intracellular Location of ATP Sulfurylase, Cysteine Synthase, and Cystathionine beta-Lyase in Maize Leaves. Plant Physiol. 1984 Jul;75(3):873–875. doi: 10.1104/pp.75.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell J. N., Whatley F. R. Sulphur metabolism in Paracoccus denitrificans. Purification, properties and regulation of serine transacetylase, O-acetylserine sulphydrylase and beta-cystathionase. Biochim Biophys Acta. 1977 Mar 15;481(1):246–265. doi: 10.1016/0005-2744(77)90157-7. [DOI] [PubMed] [Google Scholar]
- Ching T. M., Poklemba C. J., Metzger R. J. Starch synthesis in shriveled and plump triticale seeds. Plant Physiol. 1983 Nov;73(3):652–657. doi: 10.1104/pp.73.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Douce R., Joyard J. Biochemistry and function of the plastid envelope. Annu Rev Cell Biol. 1990;6:173–216. doi: 10.1146/annurev.cb.06.110190.001133. [DOI] [PubMed] [Google Scholar]
- Fankhauser H., Brunold C., Erismann K. H. Subcellular localization of O-acetylserine sulfhydrylase in spinach leaves. Experientia. 1976 Dec 15;32(12):1494–1497. doi: 10.1007/BF01924412. [DOI] [PubMed] [Google Scholar]
- Gaitonde M. K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J. 1967 Aug;104(2):627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H. Sulfuration of O-acetylhomoserine and O-acetylserine by two enzyme fractions from spinach. Biochem Biophys Res Commun. 1968 Apr 19;31(2):275–280. doi: 10.1016/0006-291x(68)90742-0. [DOI] [PubMed] [Google Scholar]
- Journet E. P., Douce R. Enzymic capacities of purified cauliflower bud plastids for lipid synthesis and carbohydrate metabolism. Plant Physiol. 1985 Oct;79(2):458–467. doi: 10.1104/pp.79.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J., Billecocq A., Bartlett S. G., Block M. A., Chua N. H., Douce R. Localization of polypeptides to the cytosolic side of the outer envelope membrane of spinach chloroplasts. J Biol Chem. 1983 Aug 25;258(16):10000–10006. [PubMed] [Google Scholar]
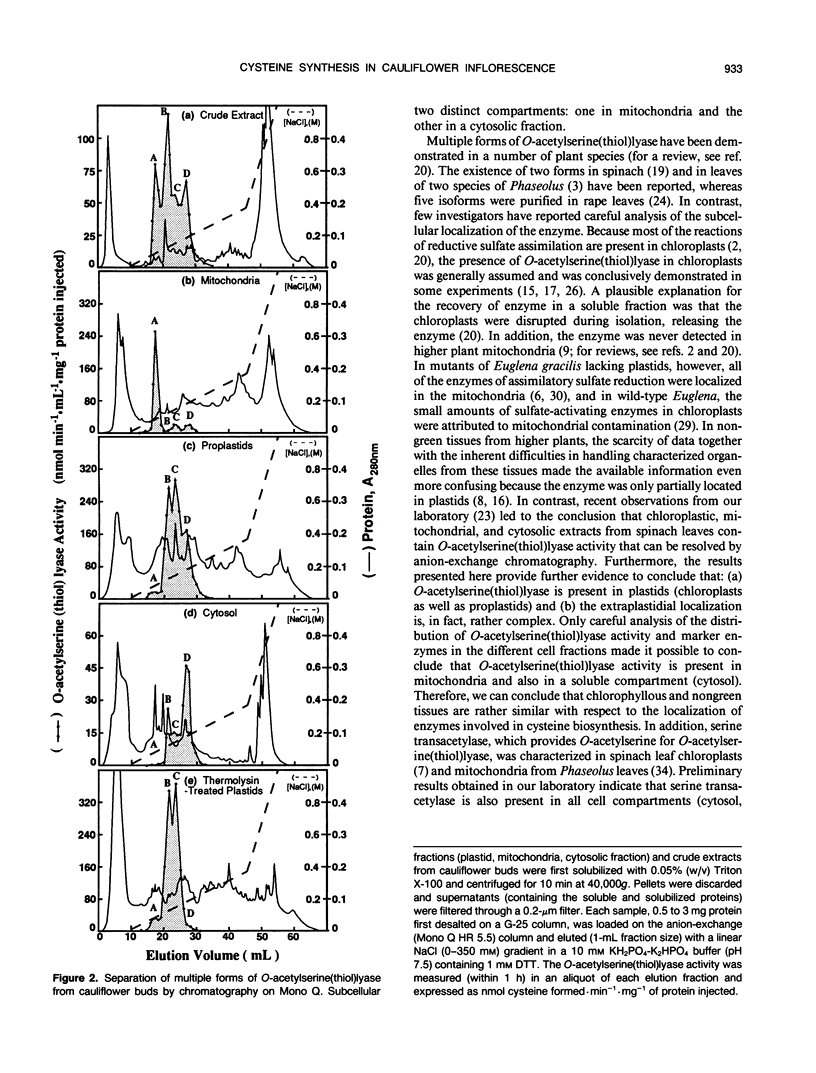
- Lunn J. E., Droux M., Martin J., Douce R. Localization of ATP Sulfurylase and O-Acetylserine(thiol)lyase in Spinach Leaves. Plant Physiol. 1990 Nov;94(3):1345–1352. doi: 10.1104/pp.94.3.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuburger M., Journet E. P., Bligny R., Carde J. P., Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch Biochem Biophys. 1982 Aug;217(1):312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Rocha V., Ting I. P. Preparation of cellular plant organelles from spinach leaves. Arch Biochem Biophys. 1970 Oct;140(2):398–407. doi: 10.1016/0003-9861(70)90081-0. [DOI] [PubMed] [Google Scholar]
- Saidha T., Na S. Q., Li J. Y., Schiff J. A. A sulphate metabolizing centre in Euglena mitochondria. Biochem J. 1988 Jul 15;253(2):533–539. doi: 10.1042/bj2530533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidha T., Stern A. I., Lee D. H., Schiff J. A. Localization of a sulphate-activating system within Euglena mitochondria. Biochem J. 1985 Dec 1;232(2):357–365. doi: 10.1042/bj2320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. K. Studies of l-Cysteine Biosynthetic Enzymes in Phaseolus vulgaris L. Plant Physiol. 1972 Oct;50(4):477–479. doi: 10.1104/pp.50.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]