Abstract
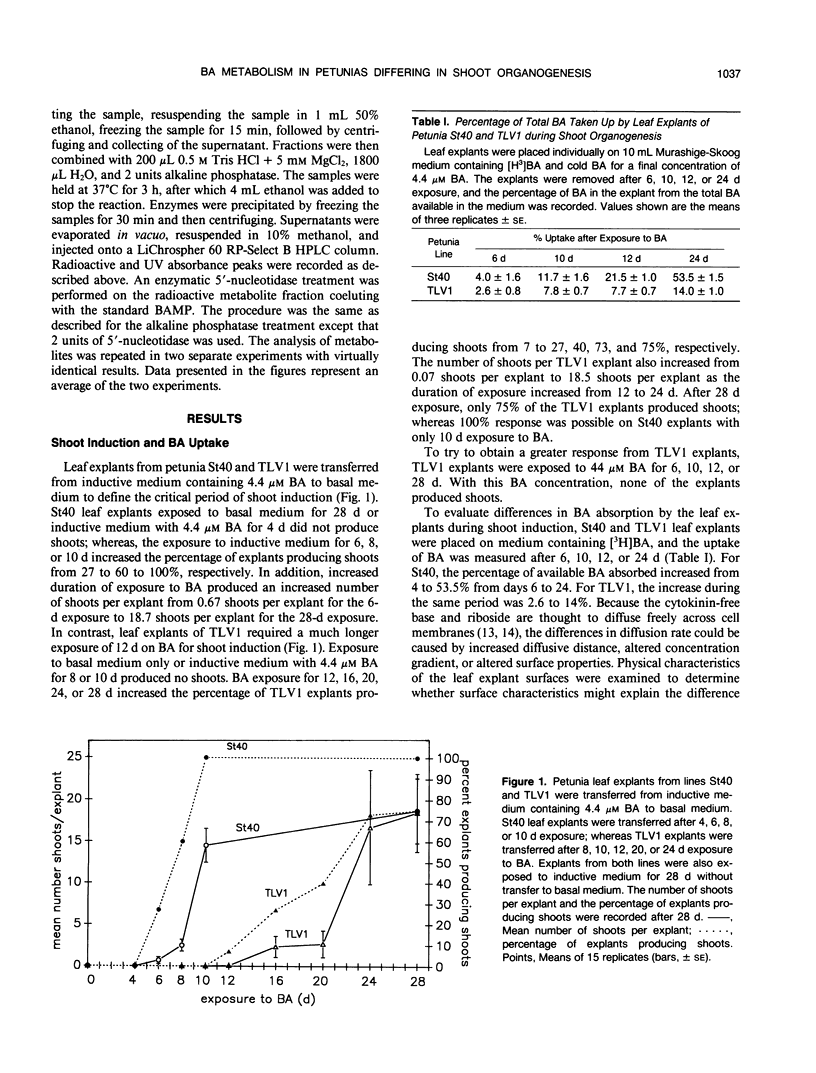
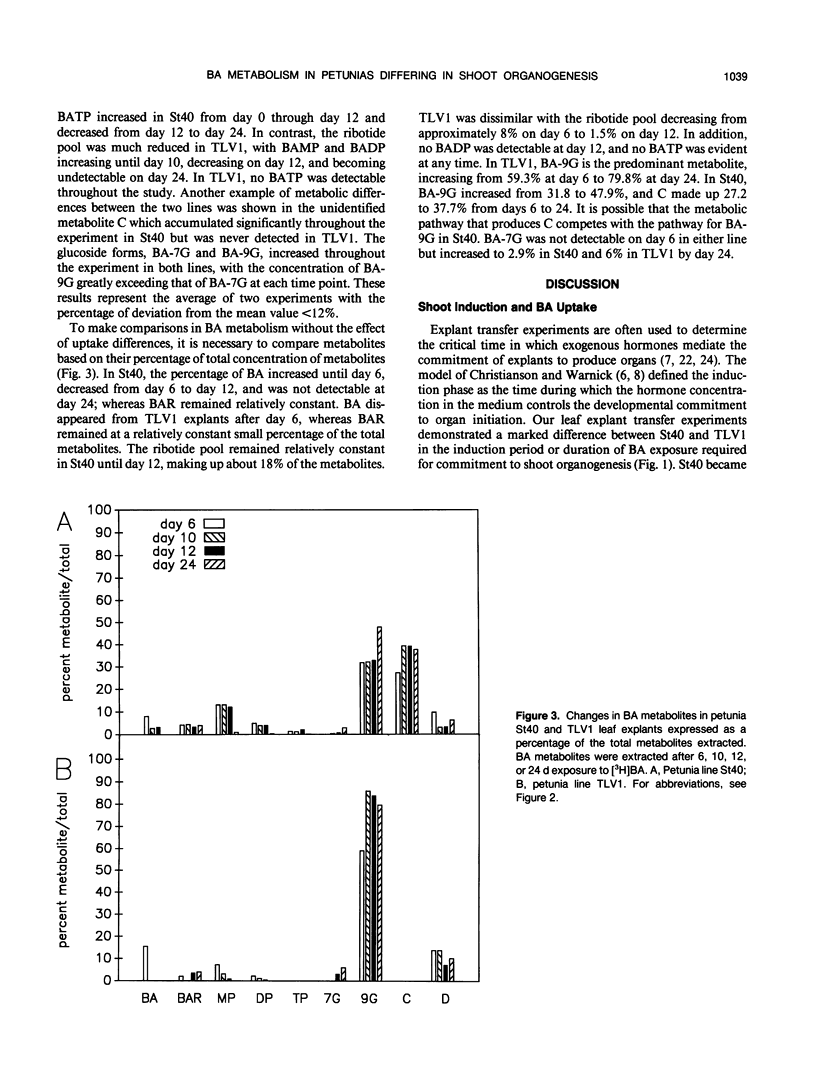
The uptake and metabolism of the cytokinin benzyl adenine (BA) was compared in two lines of Petunia hybrida Vilm. differing in their shoot organogenic response. Leaf transfer experiments using shoot induction medium containing 4.4 micromolar BA showed that leaf explants from petunia line St40 required a shoot induction period of 6 to 10 days for commitment to shoot organogenesis; whereas leaf explants from petunia TLV1 required 12 to 28 days. The short induction period of petunia St40 and the higher organogenic response was positively associated with a threefold higher absorption of BA from the medium, an increased BA ribotide metabolite pool, the presence of BA within the explant during the shoot induction period, and the production of an unidentified metabolite C. However, the study of petunia TLV1 leaf explants showed that neither BA nor metabolite C are required during the shoot induction period for eventual shoot development. The longer shoot induction period of TLV1 was associated with low BA uptake during 24 days, a decreasing ribotide metabolite pool, the absence of benzyl adenosine triphosphate and metabolite C throughout the study, and the absence of BA within the explant during the shoot induction period. Differences in the shoot organogenic response of these related plant lines have been shown to be associated with differences in exogenous cytokinin uptake and the subsequent metabolism of that hormone.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bialek K., Meudt W. J., Cohen J. D. Indole-3-acetic Acid (IAA) and IAA Conjugates Applied to Bean Stem Sections: IAA Content and the Growth Response. Plant Physiol. 1983 Sep;73(1):130–134. doi: 10.1104/pp.73.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson M. L., Warnick D. A. Competence and determination in the process of in vitro shoot organogenesis. Dev Biol. 1983 Feb;95(2):288–293. doi: 10.1016/0012-1606(83)90029-5. [DOI] [PubMed] [Google Scholar]
- King P. J. Plant hormone mutants. Trends Genet. 1988 Jun;4(6):157–162. doi: 10.1016/0168-9525(88)90021-2. [DOI] [PubMed] [Google Scholar]
- SKOOG F., MILLER C. O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–130. [PubMed] [Google Scholar]
- Smulders M. J., Croes A. F., Wullems G. J. Polar transport of 1-naphthaleneacetic Acid determines the distribution of flower buds on explants of tobacco. Plant Physiol. 1988 Nov;88(3):752–756. doi: 10.1104/pp.88.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Krieken W. M., Croes A. F., Smulders M. J., Wullems G. J. Cytokinins and flower bud formation in vitro in tobacco: role of the metabolites. Plant Physiol. 1990 Mar;92(3):565–569. doi: 10.1104/pp.92.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]


