Abstract
A cell suspension culture of tobacco (Nicotiana tabacum L.) was used as a model to study injury to cells during biolistic transformation. Lawns of cells were bombarded with tungsten particles that were coated with a plasmid containing the β-glucuronidase and the neomycin phosphotransferase II genes. When a gunpowder-driven biolistic device was used, numerous transiently expressing cells were focused around the epicenter of the blast which was manifested by a hole blown in the filter paper supporting the cells. However, transformed cells nearest the blast epicenter were injured and could not be recovered as stable transformants. The injury was primarily caused by physical trauma to the cells from gas blast and acoustic shock generated by the device. Postlaunch baffles or meshes placed in the gunpowder device reduced cell injury and increased the recovery of kanamycin-resistant colonies 3.5- and 2.5-fold, respectively. A newly developed helium-driven device was more gentle to the cells and also increased the number of transformants. Cell injury could be further moderated by using a mesh and a prelaunch baffle in the helium device. Toxicity of the tungsten microprojectiles also contributed to cell injury. Gold microprojectiles were not toxic and resulted in fourfold more kanamycin-resistant colonies than when similar quantities of similarly sized tungsten particles were used.
Full text
PDF

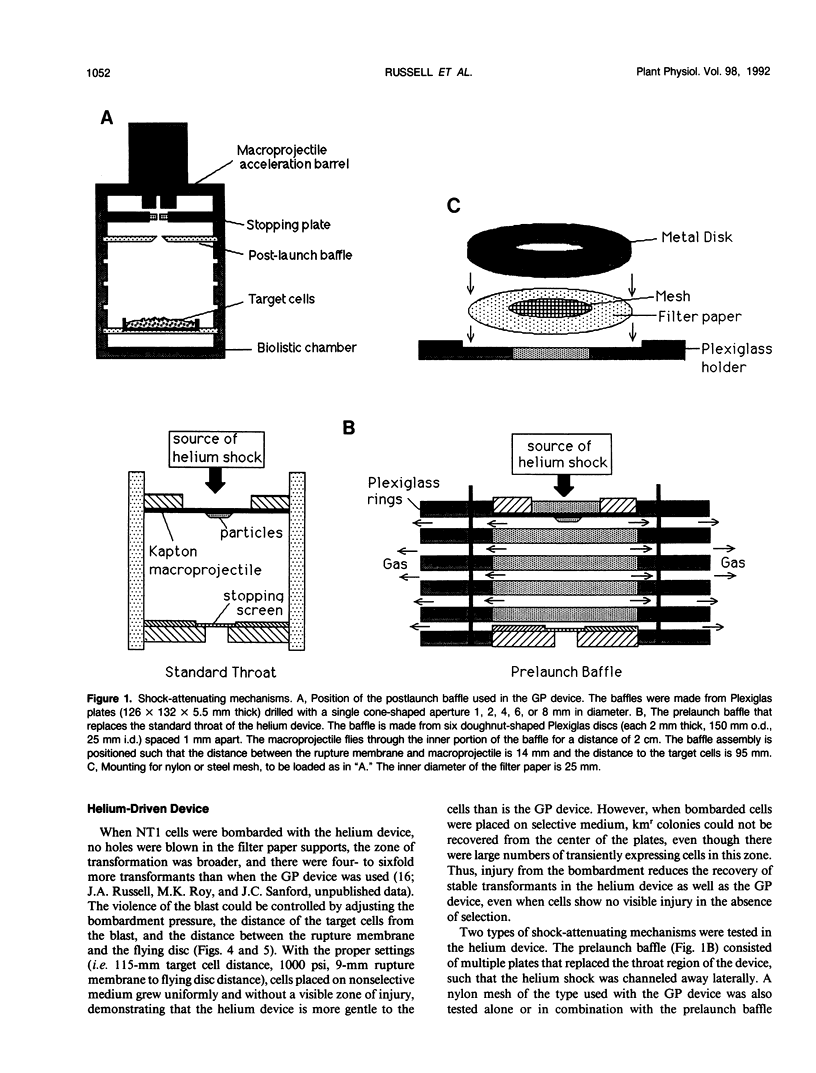
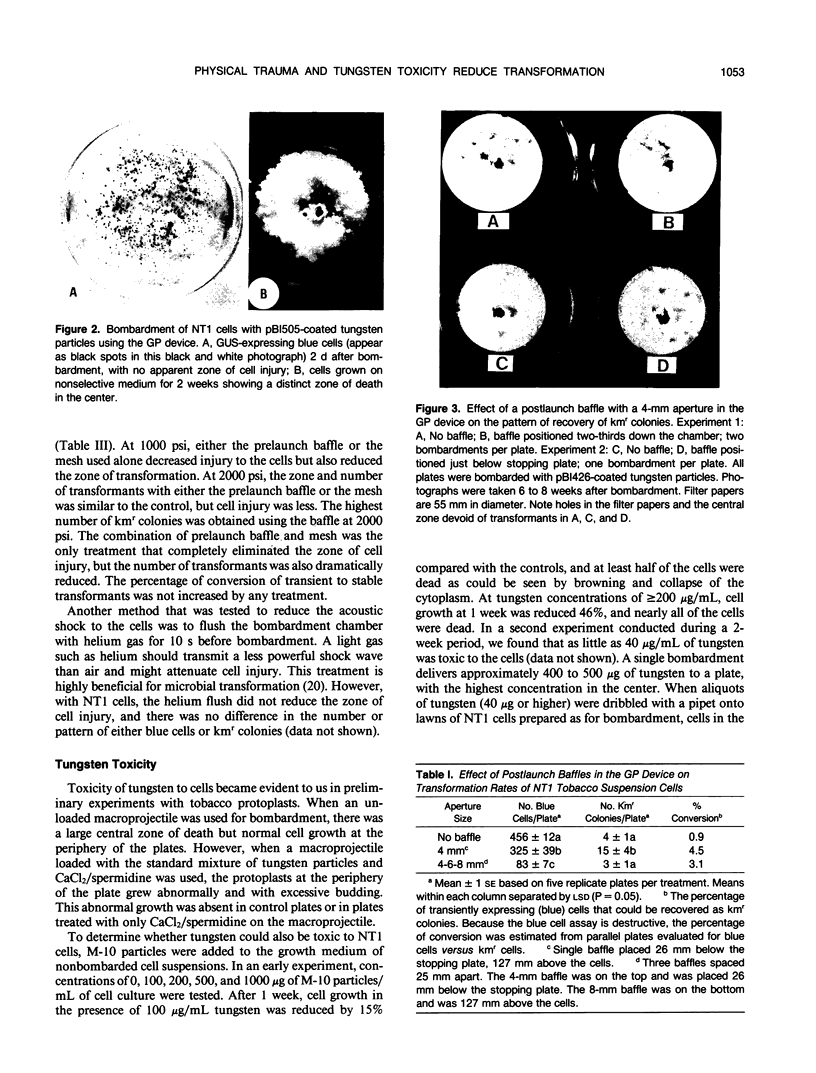
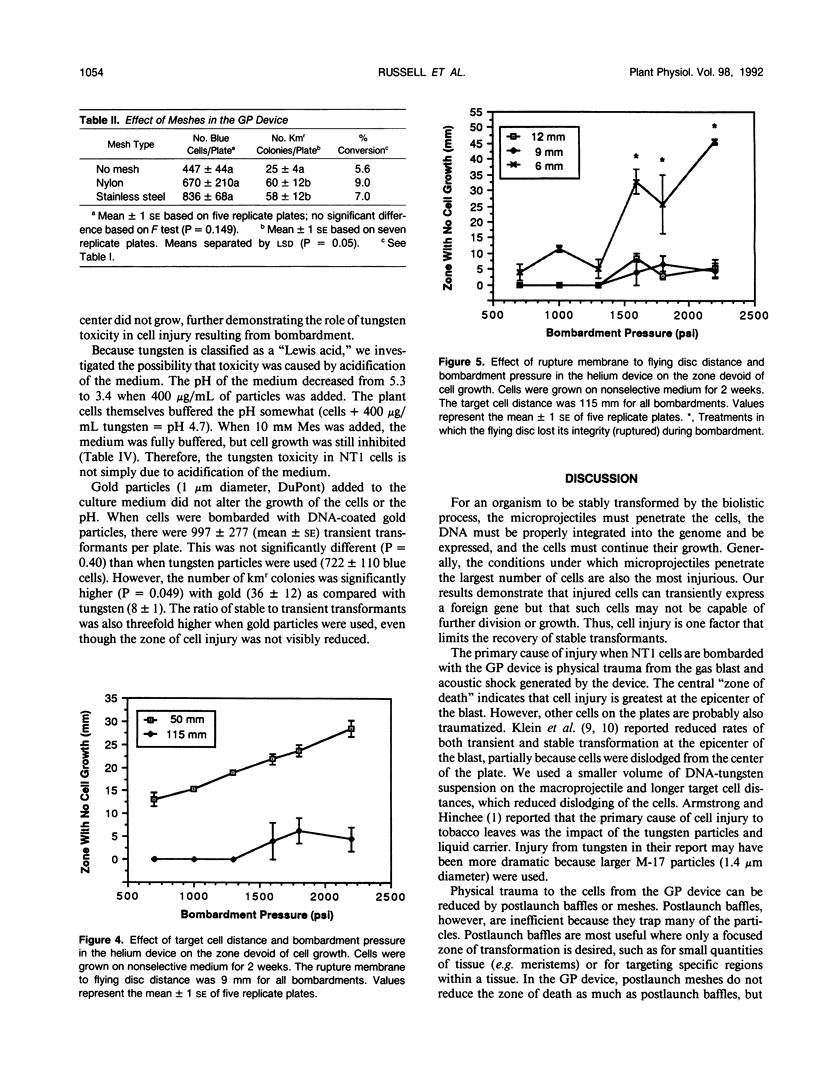

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blowers A. D., Bogorad L., Shark K. B., Sanford J. C. Studies on Chlamydomonas chloroplast transformation: foreign DNA can be stably maintained in the chromosome. Plant Cell. 1989 Jan;1(1):123–132. doi: 10.1105/tpc.1.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Harris E. H., Hosler J. P., Johnson A. M., Jones A. R., Randolph-Anderson B. L., Robertson D., Klein T. M., Shark K. B. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988 Jun 10;240(4858):1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- Daniell H., Vivekananda J., Nielsen B. L., Ye G. N., Tewari K. K., Sanford J. C. Transient foreign gene expression in chloroplasts of cultured tobacco cells after biolistic delivery of chloroplast vectors. Proc Natl Acad Sci U S A. 1990 Jan;87(1):88–92. doi: 10.1073/pnas.87.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M. E., Morrish F., Armstrong C., Williams R., Thomas J., Klein T. M. Inheritance and expression of chimeric genes in the progeny of transgenic maize plants. Biotechnology (N Y) 1990 Sep;8(9):833–839. doi: 10.1038/nbt0990-833. [DOI] [PubMed] [Google Scholar]
- Gordon-Kamm W. J., Spencer T. M., Mangano M. L., Adams T. R., Daines R. J., Start W. G., O'Brien J. V., Chambers S. A., Adams W. R., Jr, Willetts N. G. Transformation of Maize Cells and Regeneration of Fertile Transgenic Plants. Plant Cell. 1990 Jul;2(7):603–618. doi: 10.1105/tpc.2.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. M., Harper E. C., Svab Z., Sanford J. C., Fromm M. E., Maliga P. Stable genetic transformation of intact Nicotiana cells by the particle bombardment process. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8502–8505. doi: 10.1073/pnas.85.22.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren G., Lau A., Klein J., Golas C., Bologa-Campeanu M., Soldin S., MacLeod S. M., Prober C. Pharmacokinetics and adverse effects of amphotericin B in infants and children. J Pediatr. 1988 Sep;113(3):559–563. doi: 10.1016/s0022-3476(88)80653-x. [DOI] [PubMed] [Google Scholar]
- Shark K. B., Smith F. D., Harpending P. R., Rasmussen J. L., Sanford J. C. Biolistic transformation of a procaryote, Bacillus megaterium. Appl Environ Microbiol. 1991 Feb;57(2):480–485. doi: 10.1128/aem.57.2.480-485.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svab Z., Hajdukiewicz P., Maliga P. Stable transformation of plastids in higher plants. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8526–8530. doi: 10.1073/pnas.87.21.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Ye G. N., Daniell H., Sanford J. C. Optimization of delivery of foreign DNA into higher-plant chloroplasts. Plant Mol Biol. 1990 Dec;15(6):809–819. doi: 10.1007/BF00039421. [DOI] [PubMed] [Google Scholar]