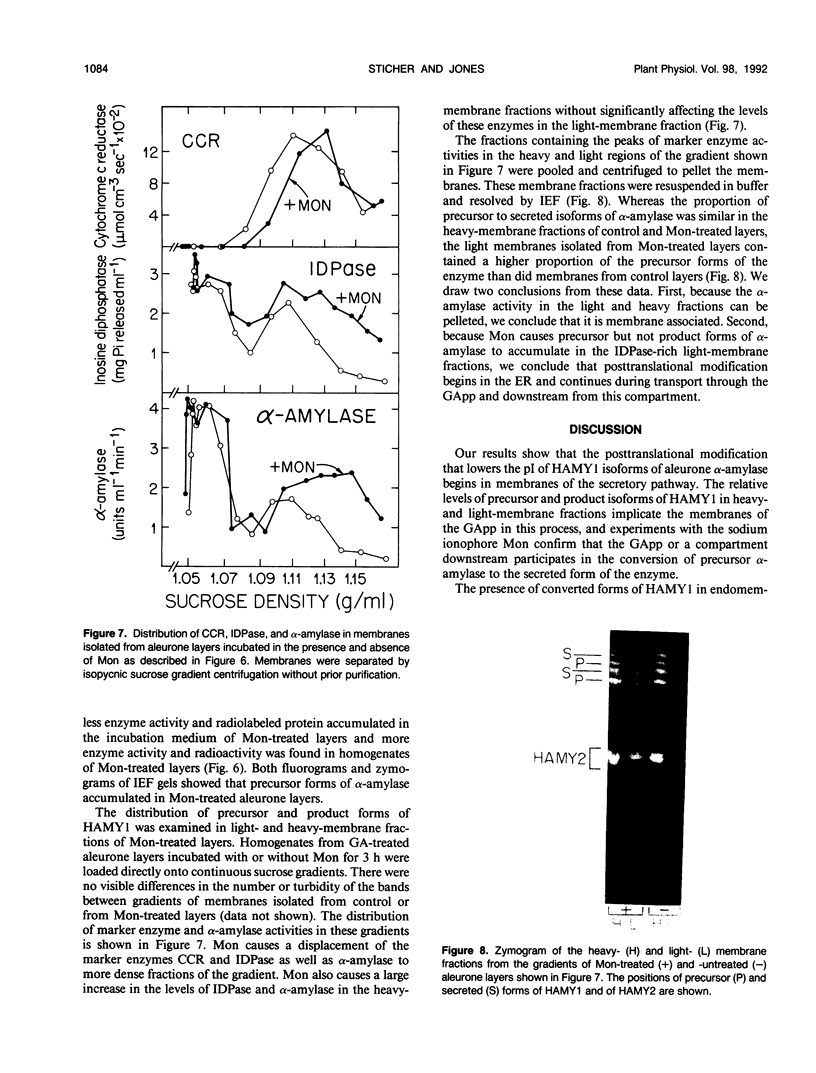
Abstract
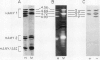
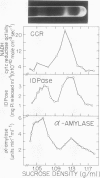
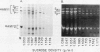
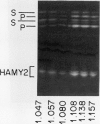
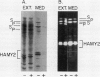
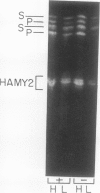
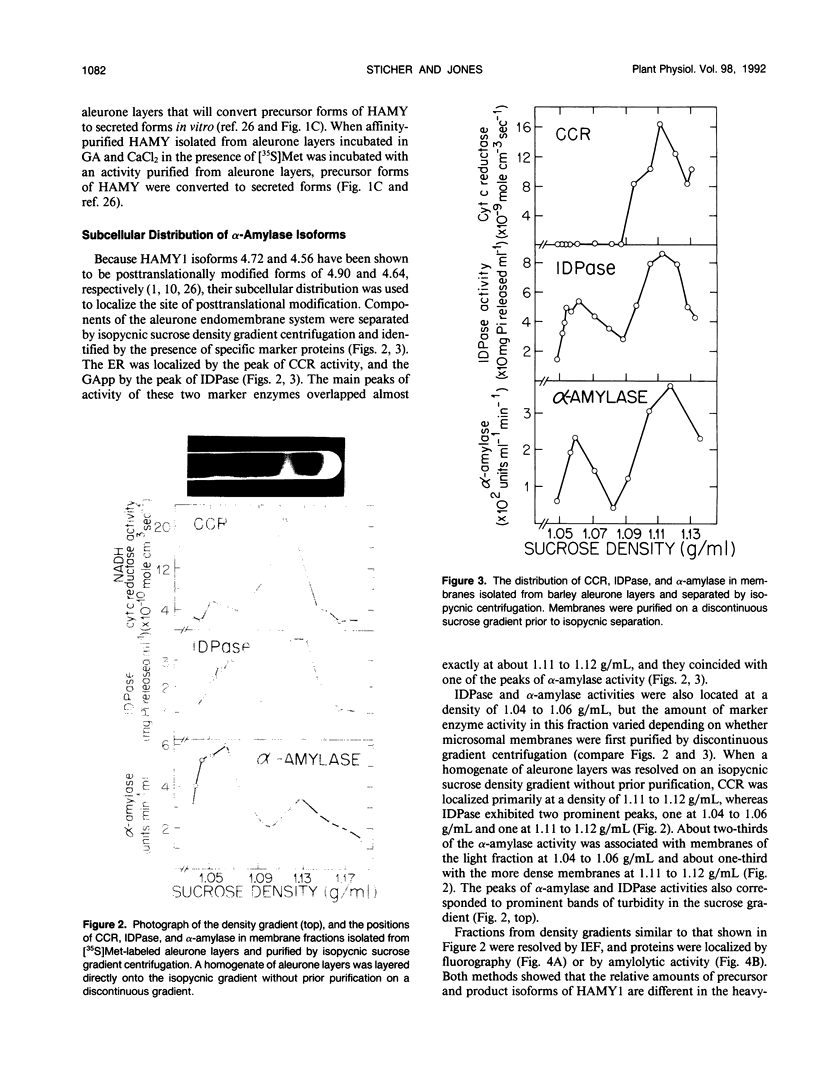
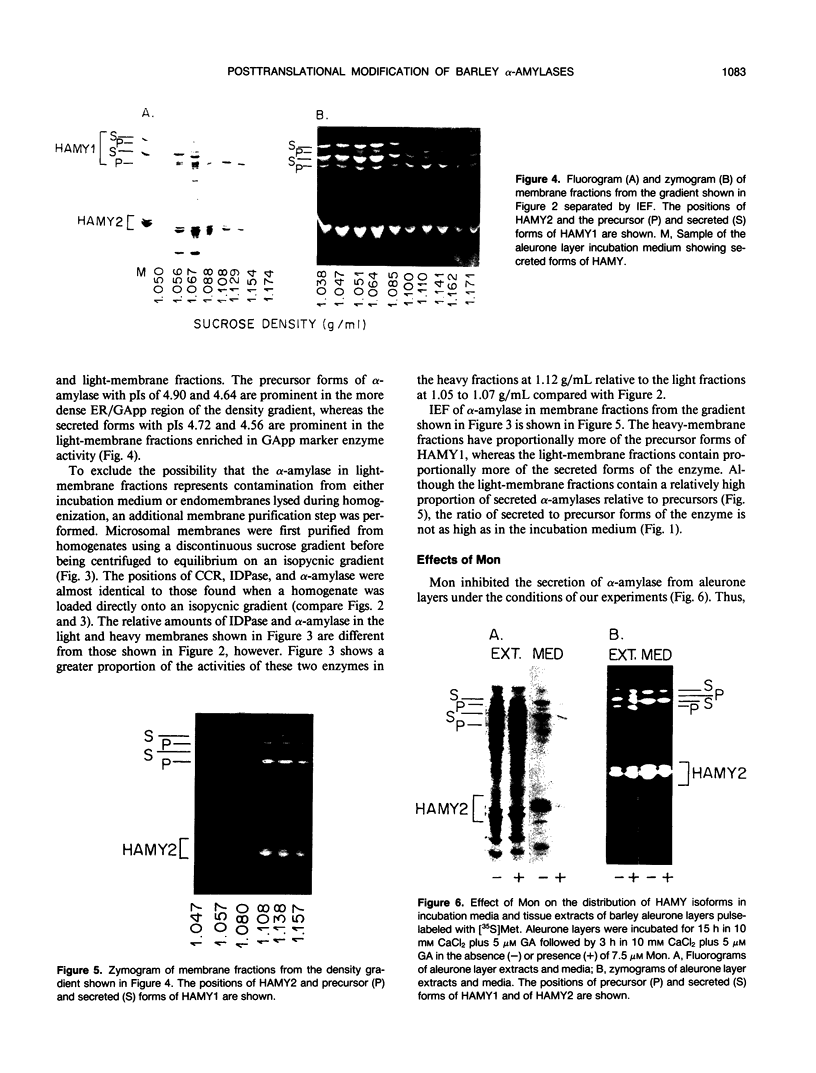
The subcellular site of the posttranslational modification of α-amylase was investigated in aleurone layers of barley (Hordeum vulgare L. cv Himalaya). Aleurone layers of Himalaya barley synthesize and secrete two groups of α-amylase isoforms, referred to as low-isoelectric point (low-pl) or HAMY1 and high-pl or HAMY2, when incubated in gibberellic acid and CaCl2. Whereas homogenates of aleurone layers contain four isoforms of HAMY1 with pls 4.90, 4.72, 4.64, and 4.56, incubation media contain predominantly isoforms 4.72 and 4.56. Microsomal membranes isolated from aleurone layers contain all four isoforms of HAMY1. Microsomal membranes can be resolved into two peaks by isopycnic density gradient centrifugation: a peak of heavy membranes with endoplasmic reticulum and Golgi apparatus (GApp) marker enzyme activities and a peak of light membranes with characteristics of the GApp. The heavy membranes contain proportionally more HAMY1 pl 4.90 and 4.64 isoforms, whereas light membranes contain a higher proportion of pl 4.72 and 4.56 isoforms. Experiments with the ionophore monensin show that membranes of the GApp as well as the endoplasmic reticulum are involved in the posttranslational modification of HAMY1 isoforms. Monensin inhibits the secretion of α-amylase and causes the enzyme to accumulate within the cell. Precursor forms of HAMY1 accumulate in light membranes isolated from monensin-treated aleurone layers indicating that the GApp is involved in the conversion of the precursor to the secreted forms of the enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buisson G., Duée E., Haser R., Payan F. Three dimensional structure of porcine pancreatic alpha-amylase at 2.9 A resolution. Role of calcium in structure and activity. EMBO J. 1987 Dec 20;6(13):3909–3916. doi: 10.1002/j.1460-2075.1987.tb02731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D. S., Sticher L., van Huystee R., Wagner D., Jones R. L. The calcium requirement for stability and enzymatic activity of two isoforms of barley aleurone alpha-amylase. J Biol Chem. 1989 Nov 15;264(32):19392–19398. [PubMed] [Google Scholar]
- Dupont F. M. Effect of temperature on the plasma membrane and tonoplast ATPases of barley roots : comparison of results obtained with acridine orange and quinacrine. Plant Physiol. 1989 Apr;89(4):1401–1412. doi: 10.1104/pp.89.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. V., Bush D. S., Sticher L., Jones R. L. Evidence for precursor forms of the low isoelectric point alpha-amylase isozymes secreted by barley aleurone cells. Plant Physiol. 1988 Dec;88(4):1168–1174. doi: 10.1104/pp.88.4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Herman E. M., Chrispeels M. J. An abundant, highly conserved tonoplast protein in seeds. Plant Physiol. 1989 Nov;91(3):1006–1013. doi: 10.1104/pp.91.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Bush D. S. Gibberellic Acid Regulates the Level of a BiP Cognate in the Endoplasmic Reticulum of Barley Aleurone Cells. Plant Physiol. 1991 Sep;97(1):456–459. doi: 10.1104/pp.97.1.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Jacobsen J. V. Regulation of synthesis and transport of secreted proteins in cereal aleurone. Int Rev Cytol. 1991;126:49–88. doi: 10.1016/s0074-7696(08)60682-8. [DOI] [PubMed] [Google Scholar]
- Karn R. C., Rosenblum B. B., Ward J. C., Merritt A. D., Shulkin J. D. Immunological relationships and post-translational modification of human salivary amylase (Amy) and pancreatic amylase (Amy) isozymes. Biochem Genet. 1974 Dec;12(6):485–499. doi: 10.1007/BF00486066. [DOI] [PubMed] [Google Scholar]
- Kelly R. B. Cell biology. Tracking an elusive receptor. Nature. 1990 Jun 7;345(6275):480–481. doi: 10.1038/345480a0. [DOI] [PubMed] [Google Scholar]
- Koehler S. M., Ho T. H. Hormonal regulation, processing, and secretion of cysteine proteinases in barley aleurone layers. Plant Cell. 1990 Aug;2(8):769–783. doi: 10.1105/tpc.2.8.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtshtein D., Dunlop K., Kaback H. R., Blume A. J. Mechanism of monensin-induced hyperpolarization of neuroblastoma-glioma hybrid NG108-15. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2580–2584. doi: 10.1073/pnas.76.6.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M., Kosaki G., Matsuura K., Fujimoto K. I., Minamiura N., Yamamoto T., Kikuchi M. Modification of human pancreatic amylase isozymes by peptidoglutaminase I and II. Clin Chim Acta. 1978 Jul 1;87(1):17–21. doi: 10.1016/0009-8981(78)90051-7. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Sambrook J. F. The involvement of calcium in transport of secretory proteins from the endoplasmic reticulum. Cell. 1990 Apr 20;61(2):197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- Sticher L., Biswas A. K., Bush D. S., Jones R. L. Heat Shock Inhibits alpha-Amylase Synthesis in Barley Aleurone without Inhibiting the Activity of Endoplasmic Reticulum Marker Enzymes. Plant Physiol. 1990 Feb;92(2):506–513. doi: 10.1104/pp.92.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticher L., Jones R. L. Isolation and Partial Characterization of a Factor from Barley Aleurone that Modifies alpha-Amylase in Vitro. Plant Physiol. 1991 Nov;97(3):936–942. doi: 10.1104/pp.97.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard M., Olsen F. L., Svensson B. C-terminal processing of barley alpha-amylase 1 in malt, aleurone protoplasts, and yeast. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8140–8144. doi: 10.1073/pnas.88.18.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of the structure and function of the Golgi complex by monovalent carboxylic ionophores. Methods Enzymol. 1983;98:47–59. doi: 10.1016/0076-6879(83)98138-7. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Plasma cell immunoglobulin secretion: arrest is accompanied by alterations of the golgi complex. J Exp Med. 1977 Nov 1;146(5):1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weselake R. J., Macgregor A. W., Hill R. D. An endogenous alpha-amylase inhibitor in barley kernels. Plant Physiol. 1983 Jul;72(3):809–812. doi: 10.1104/pp.72.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]