Abstract
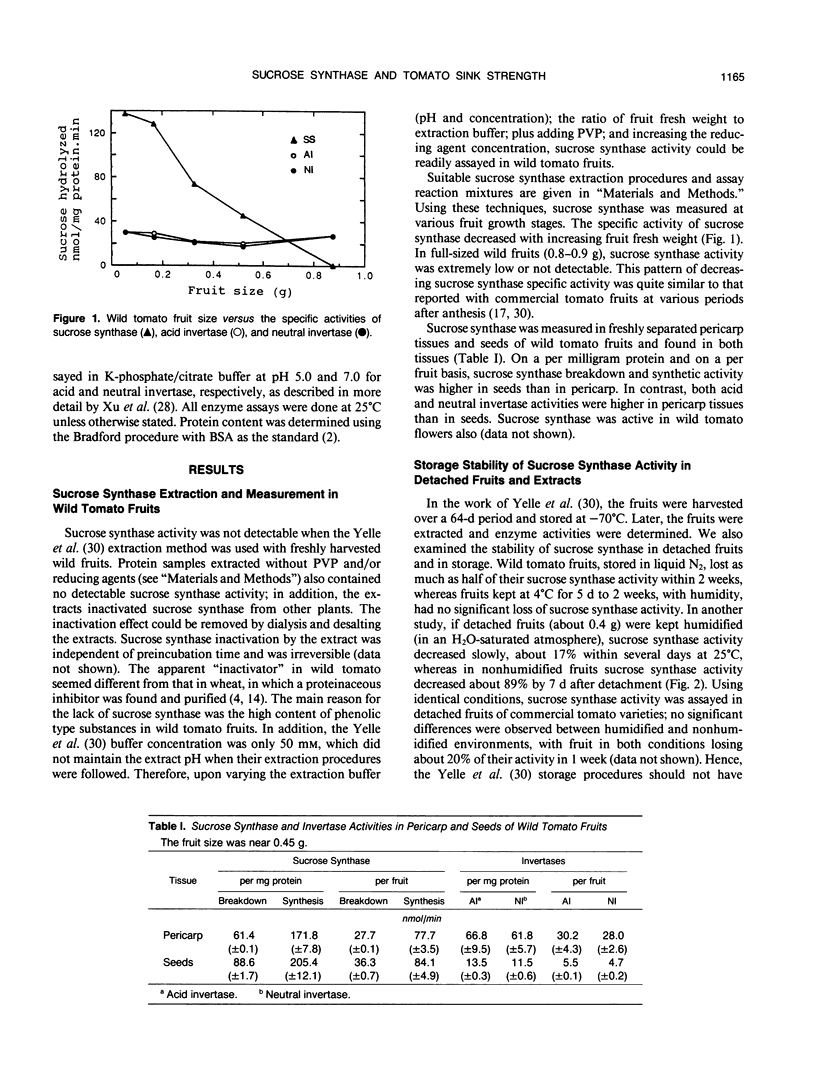
Here it is reported that sucrose synthase can be readily measured in growing wild tomato fruits (Lycopersicon chmielewskii) when suitable methods are adopted during fruit extraction. The enzyme also was present in fruit pericarp tissues, in seeds, and in flowers. To check for novel characteristics, the wild tomato fruit sucrose synthase was purified, by (NH4)2SO4 fraction and chromatography with DE-32, Sephadex G-200, and PBA-60, to one major band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The following characteristics were obtained: native protein relative molecular weight 380,000; subunit relative molecular weight 89,000; Km values with: sucrose 53 millimolar, UDP 18.9 micromolar, UDP-glucose 88 micromolar, fructose 8.4 millimolar; pH optima between 6.2 to 7.3 for sucrose breakdown and 7 to 9 for synthesis; and temperature optima near 50°C. The enzyme exhibited a high affinity and a preference for uridylates. The enzyme showed more sensitivity to divalent cations in the synthesis of sucrose than in its breakdown. Sink strength in tomato fruits also was investigated in regard to sucrose breakdown enzyme activities versus fruit weight gain. Sucrose synthase activity was consistently related to increases in fruit weight (sink strength) in both wild and commercial tomatoes. Acid and neutral invertases were not, because the published invertase activity values were too variable for quantitative analyses regarding the roles of invertases in tomato fruit development. In rapidly growing fruits of both wild and commercially developed tomato plants, the activity of sucrose synthase per growing fruit, i.e. sucrose synthase peak activity X fruit size, was linearly related to final fruit size; and the activity exceeded fruit growth and carbon import rates by at least 10-fold. In mature, nongrowing fruits, sucrose synthase activities approached nil values. Therefore, sucrose synthase can serve as an indicator of sink strength in growing tomato fruits.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. N. The selection of sucrose as the translocate of higher plants. J Theor Biol. 1968 Oct;21(1):13–20. doi: 10.1016/0022-5193(68)90056-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Miron D., Schaffer A. A. Sucrose Phosphate Synthase, Sucrose Synthase, and Invertase Activities in Developing Fruit of Lycopersicon esculentum Mill. and the Sucrose Accumulating Lycopersicon hirsutum Humb. and Bonpl. Plant Physiol. 1991 Feb;95(2):623–627. doi: 10.1104/pp.95.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell M., Copeland L. Sucrose synthase of soybean nodules. Plant Physiol. 1985 May;78(1):149–154. doi: 10.1104/pp.78.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T., Akazawa T. Enzymic mechanism of starch stynthesis in ripening rice grains. VII. Purification and enzymic properties of sucrose synthetase. Arch Biochem Biophys. 1973 Jun;156(2):644–652. doi: 10.1016/0003-9861(73)90316-0. [DOI] [PubMed] [Google Scholar]
- Pressey R. Potato sucrose synthetase: purification, properties, and changes in activity associated with maturation. Plant Physiol. 1969 May;44(5):759–764. doi: 10.1104/pp.44.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N. L., Hewitt J. D., Bennett A. B. Sink metabolism in tomato fruit : I. Developmental changes in carbohydrate metabolizing enzymes. Plant Physiol. 1988 Jul;87(3):727–730. doi: 10.1104/pp.87.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla R. N., Sanwal G. G. Studies on UDP-glucose: D-fructose 2-glucosyltransferase from tapioca tuber. Arch Biochem Biophys. 1971 Jan;142(1):303–309. doi: 10.1016/0003-9861(71)90288-8. [DOI] [PubMed] [Google Scholar]
- Sobell L. C., Sobell M. B. Validity of self-reports in three populations of alcoholics. J Consult Clin Psychol. 1978 Oct;46(5):901–907. doi: 10.1037//0022-006x.46.5.901. [DOI] [PubMed] [Google Scholar]
- Su J. C., Preiss J. Purification and properties of sucrose synthase from maize kernels. Plant Physiol. 1978 Mar;61(3):389–393. doi: 10.1104/pp.61.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. J., Xu D. P., Black C. C. Identification of actively filling sucrose sinks. Plant Physiol. 1989 Apr;89(4):1117–1121. doi: 10.1104/pp.89.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. S., Kormanik P. P., Xu D. P., Black C. C. Sucrose metabolic pathways in sweetgum and pecan seedlings. Tree Physiol. 1989 Mar;5(1):39–52. doi: 10.1093/treephys/5.1.39. [DOI] [PubMed] [Google Scholar]
- Victorio R. G., Moreno U., Black C. C. Growth, partitioning, and harvest index of tuber-bearing solanum genotypes grown in two contrasting peruvian environments. Plant Physiol. 1986 Sep;82(1):103–108. doi: 10.1104/pp.82.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. W., Morrison J. F. The kinetics of reversible tight-binding inhibition. Methods Enzymol. 1979;63:437–467. doi: 10.1016/0076-6879(79)63019-7. [DOI] [PubMed] [Google Scholar]
- Xu D. P., Sung S. J., Black C. C. Sucrose metabolism in lima bean seeds. Plant Physiol. 1989 Apr;89(4):1106–1116. doi: 10.1104/pp.89.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelle S., Chetelat R. T., Dorais M., Deverna J. W., Bennett A. B. Sink Metabolism in Tomato Fruit : IV. Genetic and Biochemical Analysis of Sucrose Accumulation. Plant Physiol. 1991 Apr;95(4):1026–1035. doi: 10.1104/pp.95.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelle S., Hewitt J. D., Robinson N. L., Damon S., Bennett A. B. Sink Metabolism in Tomato Fruit : III. Analysis of Carbohydrate Assimilation in a Wild Species. Plant Physiol. 1988 Jul;87(3):737–740. doi: 10.1104/pp.87.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]


