Abstract
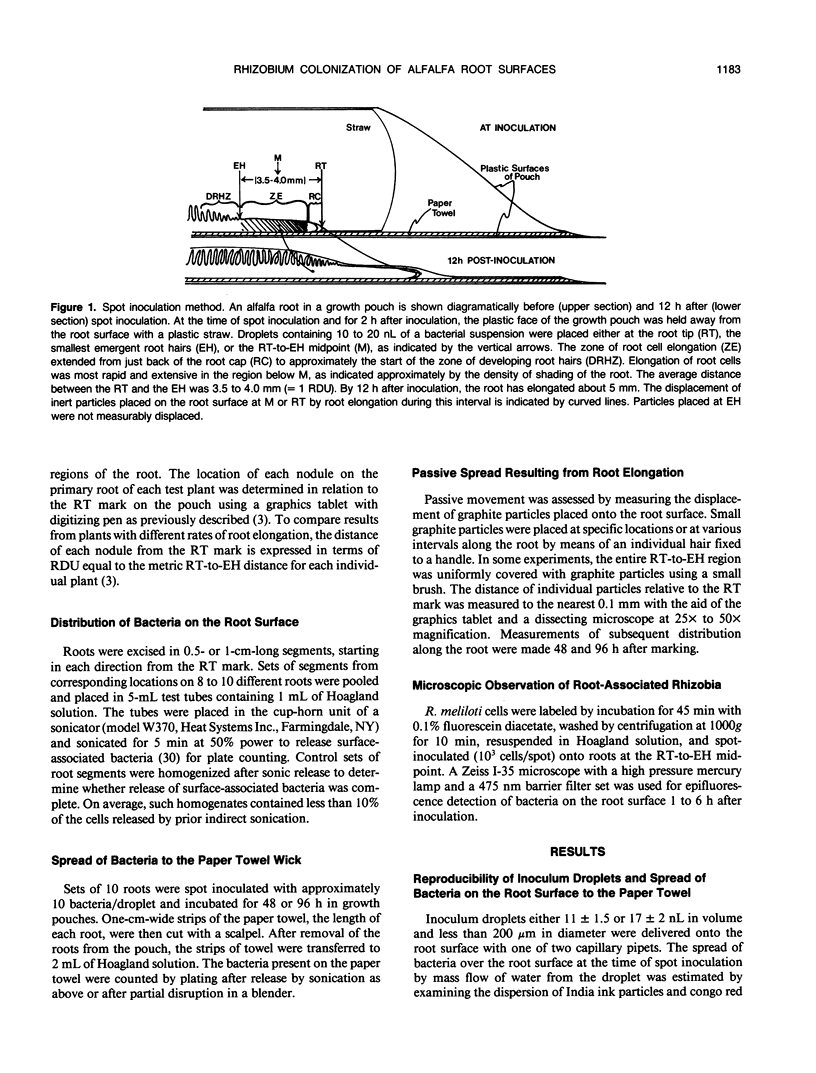
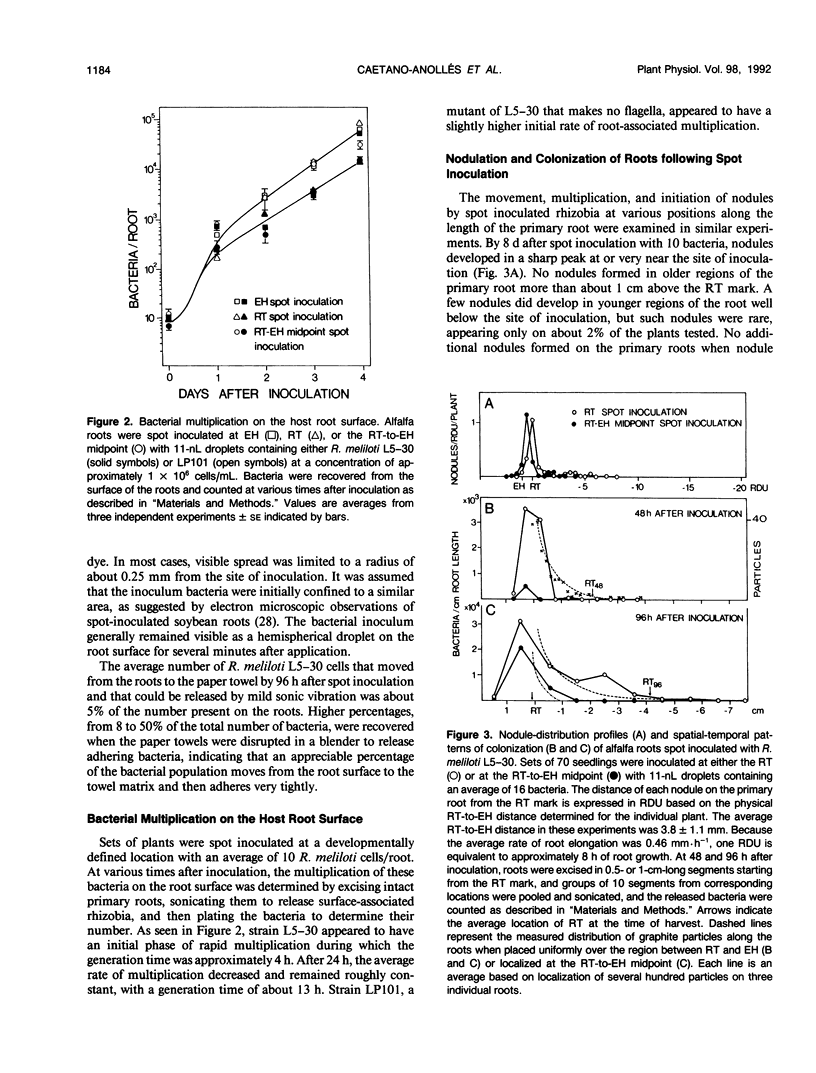
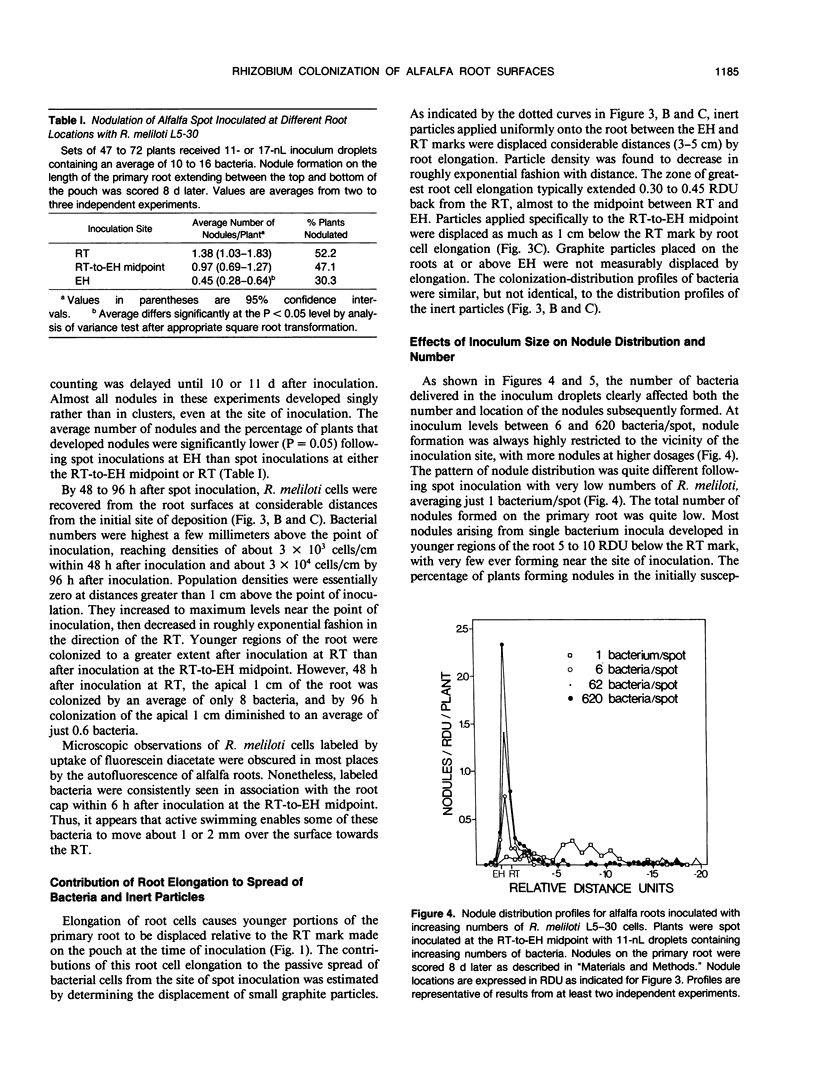
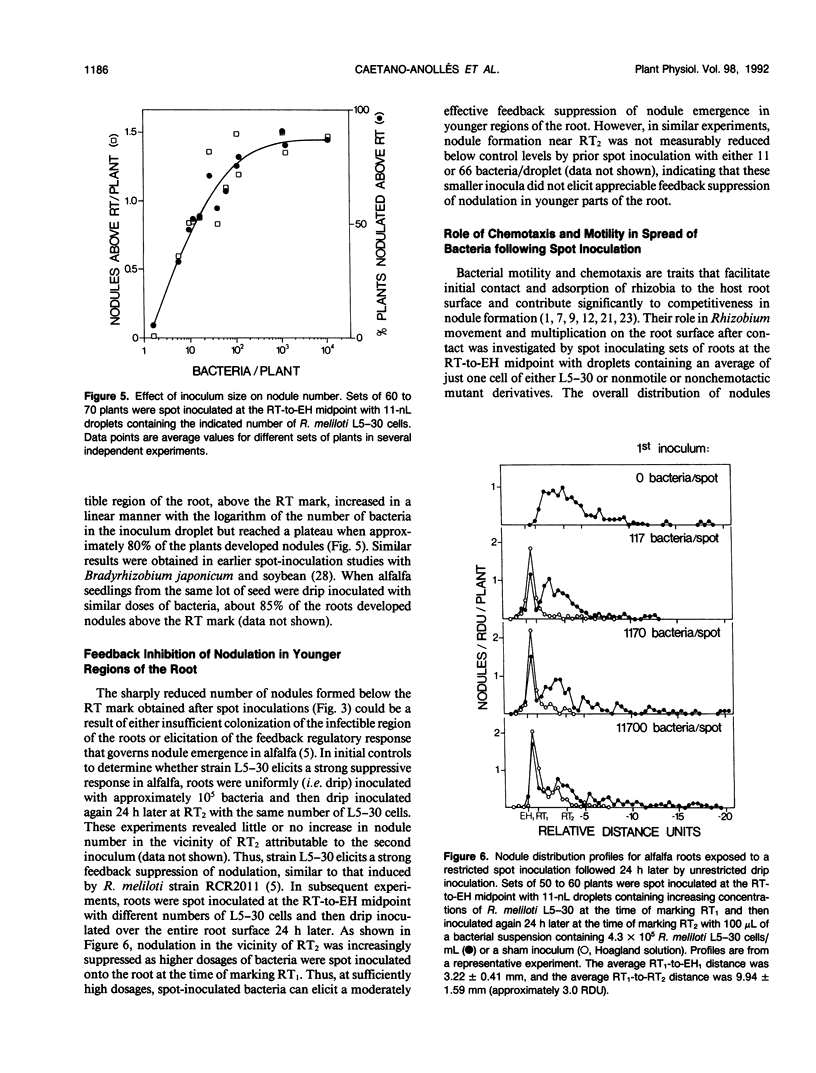
Inoculum droplets of approximately 10 nanoliter volume and containing about 10 Rhizobium meliloti cells were placed onto the root surface of alfalfa seedlings in plastic growth pouches at either the root tip, the position of the smallest emergent root hairs, or at a site midway between these points. The droplets were initially confined to an area of about 0.2 square millimeter at the point of application. By 48 and 96 hours after inoculation, the inoculum bacteria and their progeny were distributed over several centimeters of the root between the initial site of deposition and the growing root tip, reaching densities of 103 to 104 bacteria per centimeter near the site of initial deposition and decreasing exponentially from that point toward the root tip. Graphite particles deposited on the root surface close to the growing tip were similarly distributed along the root length by 48 and 96 hours, suggesting that passive displacement by root cell elongation was primarily responsible for the spread of bacteria. A nonmotile mutant of R. meliloti colonized alfalfa roots to the same extent as the wild type and was usually distributed in the same manner, indicating that bacterial motility contributed little under these conditions to long distance spread of the bacteria. However, when applied in low numbers, R. meliloti mutants defective in motility or chemotaxis were considerably less efficient in initiating nodules near the point of inoculation than the wild type. This implies that motility and/or chemotaxis contribute significantly to local exploration for suitable infection sites. Almost all nodules on the primary root formed within a few millimeters of the spot-inoculation site, indicating that, under our experimental conditions, movement and multiplication of R. meliloti on the root surface were not sufficient to maintain an adequate population in the infectible region of the root during root growth.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames P., Bergman K. Competitive advantage provided by bacterial motility in the formation of nodules by Rhizobium meliloti. J Bacteriol. 1981 Nov;148(2):728–p. doi: 10.1128/jb.148.2.728-729.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Bhagwat A. A., Bauer W. D. Transient susceptibility of root cells in four common legumes to nodulation by rhizobia. Plant Physiol. 1981 Nov;68(5):1144–1149. doi: 10.1104/pp.68.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Mills K. K., Crist D. K., Evans W. R., Bauer W. D. Effects of culture age on symbiotic infectivity of Rhizobium japonicum. J Bacteriol. 1983 Jan;153(1):443–451. doi: 10.1128/jb.153.1.443-451.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano-Anollés G., Wall L. G., De Micheli A. T., Macchi E. M., Bauer W. D., Favelukes G. Role of Motility and Chemotaxis in Efficiency of Nodulation by Rhizobium meliloti. Plant Physiol. 1988 Apr;86(4):1228–1235. doi: 10.1104/pp.86.4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weger L. A., van der Vlugt C. I., Wijfjes A. H., Bakker P. A., Schippers B., Lugtenberg B. Flagella of a plant-growth-stimulating Pseudomonas fluorescens strain are required for colonization of potato roots. J Bacteriol. 1987 Jun;169(6):2769–2773. doi: 10.1128/jb.169.6.2769-2773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dershaw D. D., Scher H. I. Serial transabdominal sonography of bladder cancer. AJR Am J Roentgenol. 1988 May;150(5):1055–1059. doi: 10.2214/ajr.150.5.1055. [DOI] [PubMed] [Google Scholar]
- Harris P. J., Northcote D. H. Patterns of polysaccharide biosynthesis in differentiating cells of maize root-tips. Biochem J. 1970 Dec;120(3):479–491. doi: 10.1042/bj1200479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. M., Alexander M. Bacterial Growth Rates and Competition Affect Nodulation and Root Colonization by Rhizobium meliloti. Appl Environ Microbiol. 1986 Oct;52(4):807–811. doi: 10.1128/aem.52.4.807-811.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Ruilong, Tran Van Mai, Schmidt E. L. Nodulating Competitiveness of a Nonmotile Tn7 Mutant of Bradyrhizobium japonicum in Nonsterile Soil. Appl Environ Microbiol. 1989 Aug;55(8):1895–1900. doi: 10.1128/aem.55.8.1895-1900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M., Bauer W. D. A rapid regulatory response governing nodulation in soybean. Plant Physiol. 1983 Oct;73(2):286–290. doi: 10.1104/pp.73.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent L., Huang S. Z., Rolfe B. G., Djordjevic M. A. Split-Root Assays Using Trifolium subterraneum Show that Rhizobium Infection Induces a Systemic Response That Can Inhibit Nodulation of Another Invasive Rhizobium Strain. Appl Environ Microbiol. 1987 Jul;53(7):1611–1619. doi: 10.1128/aem.53.7.1611-1619.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler R. J., Peirce C., Bergman K. Mapping and cloning of a fla-che region of the Rhizobium meliloti chromosome. J Bacteriol. 1986 Nov;168(2):785–790. doi: 10.1128/jb.168.2.785-790.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


