Abstract
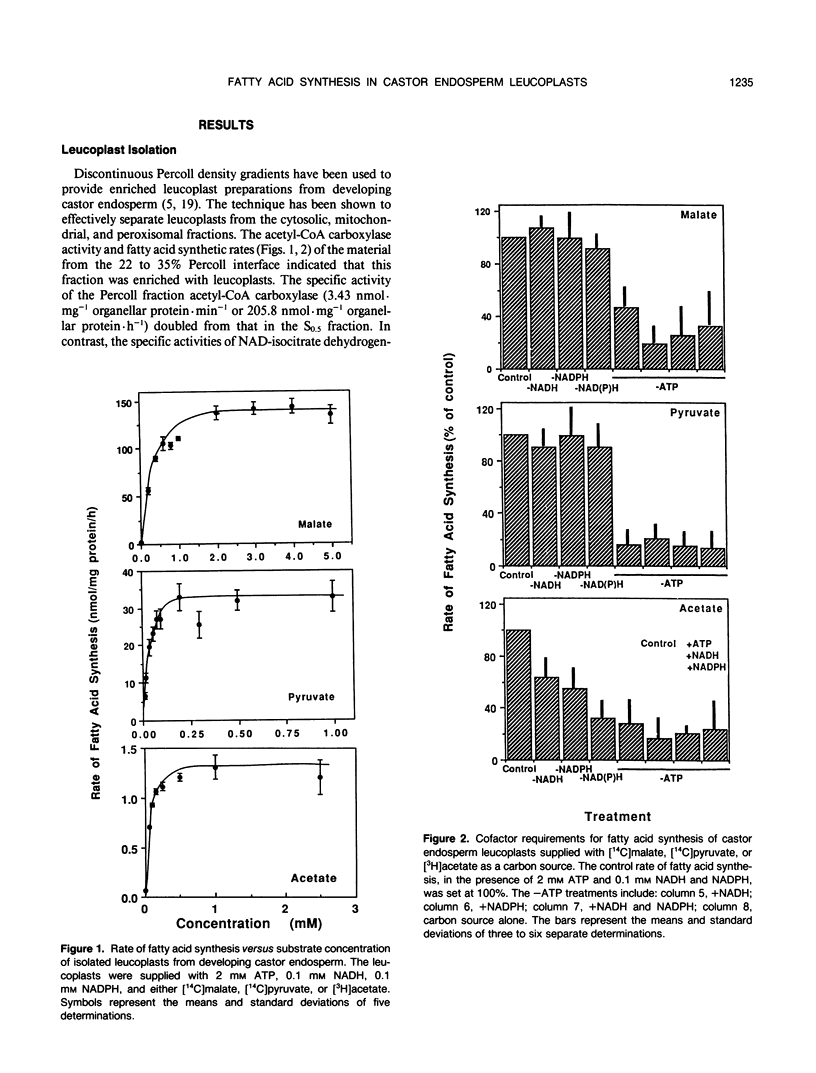
Leucoplasts were isolated from the endosperm of developing castor (Ricinis communis) endosperm using a discontinuous Percoll gradient. The rate of fatty acid synthesis was highest when malate was the precursor, at 155 nanomoles acetyl-CoA equivalents per milligram protein per hour. Pyruvate and acetate also were precursors of fatty acid synthesis, but the rates were approximately 4.5 and 120 times less, respectively, than when malate was the precursor. When acetate was supplied to leucoplasts, exogenous ATP, NADH, and NADPH were required to obtain maximal rates of fatty acid synthesis. In contrast, the incorporation of malate and pyruvate into fatty acids did not require a supply of exogenous reductant. Further, the incorporation of radiolabel into fatty acids by leucoplasts supplied with radiolabeled malate, pyruvate, or acetate was reduced upon coincubation with cold pyruvate or malate. The data suggest that malate and pyruvate may be good in vivo sources of carbon for fatty acid synthesis and that, in these preparations, leucoplast fatty acid synthesis may be limited by activity at or downstream of the acetyl-CoA carboxylase reaction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedict C. R., Beevers H. Formation of sucrose from malate in germinating castor beans. I. Conversion of malate to phosphoenol-pyruvate. Plant Physiol. 1961 Sep;36(5):540–544. doi: 10.1104/pp.36.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle S. A., Hemmingsen S. M., Dennis D. T. Energy Requirement for the Import of Protein into Plastids from Developing Endosperm of Ricinus communis L. Plant Physiol. 1990 Jan;92(1):151–154. doi: 10.1104/pp.92.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle S. A., Hemmingsen S. M., Dennis D. T. Uptake and processing of the precursor to the small subunit of ribulose 1,5-bisphosphate carboxylase by leucoplasts from the endosperm of developing castor oil seeds. Plant Physiol. 1986 Jul;81(3):817–822. doi: 10.1104/pp.81.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dennis D. T., Green T. R. Soluble and particulate glycolysis in developing castor bean endosperm. Biochem Biophys Res Commun. 1975 Jan 2;64(3):970–975. doi: 10.1016/0006-291x(75)90142-4. [DOI] [PubMed] [Google Scholar]
- Drennan C. H., Canvin D. T. Oleic acid synthesis by a particulate preparation from developing castor oil seeds. Biochim Biophys Acta. 1969;187(2):193–200. doi: 10.1016/0005-2760(69)90027-7. [DOI] [PubMed] [Google Scholar]
- Miernyk J. A., Dennis D. T. Isozymes of the glycolytic enzymes in endosperm from developing castor oil seeds. Plant Physiol. 1982 Apr;69(4):825–828. doi: 10.1104/pp.69.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid E. E., Lyttle C. R., Canvin D. T., Dennis D. T. Pyruvate dehydrogenase complex activity in proplastids and mitochondria of developing castor bean endosperm. Biochem Biophys Res Commun. 1975 Jan 6;62(1):42–47. doi: 10.1016/s0006-291x(75)80402-5. [DOI] [PubMed] [Google Scholar]
- Simcox P. D., Reid E. E., Canvin D. T., Dennis D. T. Enzymes of the Glycolytic and Pentose Phosphate Pathways in Proplastids from the Developing Endosperm of Ricinus communis L. Plant Physiol. 1977 Jun;59(6):1128–1132. doi: 10.1104/pp.59.6.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz W., Stitt M., Heldt H. W. Enzymic determination of metabolites in the subcellular compartments of spinach protoplasts. Plant Physiol. 1980 Jul;66(1):187–193. doi: 10.1104/pp.66.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilkey B., Canvin D. T. Subcellular localization of oleic acid biosynthesis enzymes in the developing castor bean endosperm. Biochem Biophys Res Commun. 1969 Mar 10;34(5):646–653. doi: 10.1016/0006-291x(69)90787-6. [DOI] [PubMed] [Google Scholar]


