Abstract
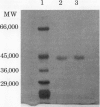
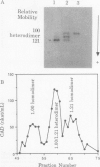
Cinnamyl alcohol dehydrogenase (CAD, EC 1.1.1. 195) has been purified to homogeneity from differentiating xylem tissue and developing seeds of loblolly pine (Pinus taeda L.). The enzyme is a dimer with a native molecular weight of 82,000 and a subunit molecular weight of 44,000, and is the only form of CAD involved in lignification in differentiating xylem. High levels of loblolly pine CAD enzyme were found in nonlignifying seed tissue. Characterization of the enzyme from both seeds and xylem demonstrated that the enzyme is the same in both tissues. The enzyme has a high affinity for coniferaldehyde (Km = 1.7 micromolar) compared with sinapaldehyde (Km in excess of 100 micromolar). Kinetic data strongly suggest that coniferin is a noncompetitive inhibitor of CAD enzyme activity. Protein sequences were obtained for the N-terminus (28 amino acids) and for two other peptides. Degenerate oligonucleotide primers based on the protein sequences were used to amplify by polymerase chain reaction a 1050 base pair DNA fragment from xylem cDNA. Nucleotide sequence from the cloned DNA fragment coded for the N-terminal protein sequence and an internal peptide of CAD. The N-terminal protein sequence has little similarity with the λCAD4 clone isolated from bean (MH Walter, J Grima-Pettenati, C Grand, AM Boudet, CJ Lamb [1988] Proc Natl Acad Sci USA 86:5546-5550), which has homology with malic enzyme.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dennis E. S., Gerlach W. L., Pryor A. J., Bennetzen J. L., Inglis A., Llewellyn D., Sachs M. M., Ferl R. J., Peacock W. J. Molecular analysis of the alcohol dehydrogenase (Adh1) gene of maize. Nucleic Acids Res. 1984 May 11;12(9):3983–4000. doi: 10.1093/nar/12.9.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind Y., Edwards R., Mavandad M., Hedrick S. A., Ribak O., Dixon R. A., Lamb C. J. Abnormal plant development and down-regulation of phenylpropanoid biosynthesis in transgenic tobacco containing a heterologous phenylalanine ammonia-lyase gene. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9057–9061. doi: 10.1073/pnas.87.22.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lüderitz T., Grisebach H. Enzymic synthesis of lignin precursors. Comparison of cinnamoyl-CoA reductase and cinnamyl alcohol:NADP+ dehydrogenase from spruce (Picea abies L.) and soybean (Glycine max L.). Eur J Biochem. 1981 Sep;119(1):115–124. doi: 10.1111/j.1432-1033.1981.tb05584.x. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Pey N. K., Herrnstadt C., Marcil R. A., Smith L. M. A universal method for the direct cloning of PCR amplified nucleic acid. Biotechnology (N Y) 1991 Jul;9(7):657–663. doi: 10.1038/nbt0791-657. [DOI] [PubMed] [Google Scholar]
- Napoli C., Lemieux C., Jorgensen R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in trans. Plant Cell. 1990 Apr;2(4):279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarni F., Grand C., Boudet A. M. Purification and properties of cinnamoyl-CoA reductase and cinnamyl alcohol dehydrogenase from poplar stems (Populus X euramericana). Eur J Biochem. 1984 Mar 1;139(2):259–265. doi: 10.1111/j.1432-1033.1984.tb08002.x. [DOI] [PubMed] [Google Scholar]
- Umezawa T., Davin L. B., Lewis N. G. Formation of lignans (-)-secoisolariciresinol and (-)-matairesinol with Forsythia intermedia cell-free extracts. J Biol Chem. 1991 Jun 5;266(16):10210–10217. [PubMed] [Google Scholar]
- Van Doorsselaere J., Villarroel R., Van Montagu M., Inzé D. Nucleotide sequence of a cDNA encoding malic enzyme from poplar. Plant Physiol. 1991 Aug;96(4):1385–1386. doi: 10.1104/pp.96.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M. H., Grima-Pettenati J., Grand C., Boudet A. M., Lamb C. J. Cinnamyl-alcohol dehydrogenase, a molecular marker specific for lignin synthesis: cDNA cloning and mRNA induction by fungal elicitor. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5546–5550. doi: 10.1073/pnas.85.15.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M. H., Grima-Pettenati J., Grand C., Boudet A. M., Lamb C. J. Extensive sequence similarity of the bean CAD4 (cinnamyl-alcohol dehydrogenase) to a maize malic enzyme. Plant Mol Biol. 1990 Sep;15(3):525–526. doi: 10.1007/BF00019173. [DOI] [PubMed] [Google Scholar]
- Wyrambik D., Grisebach H. Enzymic synthesis of lignin precursors. Further studies on cinnamyl-alcohol dehydrogenase from soybean-cell-suspension cultures. Eur J Biochem. 1979 Jul;97(2):503–509. doi: 10.1111/j.1432-1033.1979.tb13138.x. [DOI] [PubMed] [Google Scholar]
- Wyrambik D., Grisebach H. Purification and properties of isoenzymes of cinnamyl-alcohol dehydrogenase from soybean-cell-suspension cultures. Eur J Biochem. 1975 Nov 1;59(1):9–15. doi: 10.1111/j.1432-1033.1975.tb02418.x. [DOI] [PubMed] [Google Scholar]