Abstract
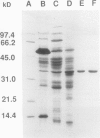
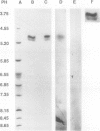
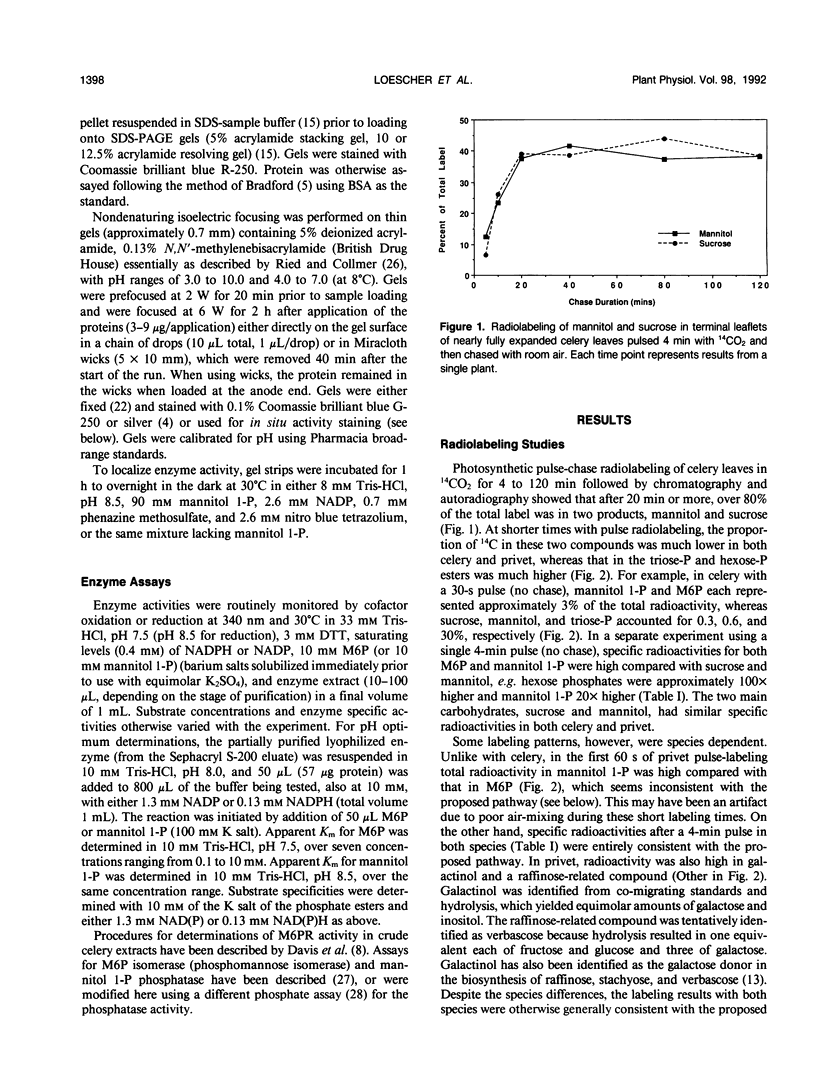
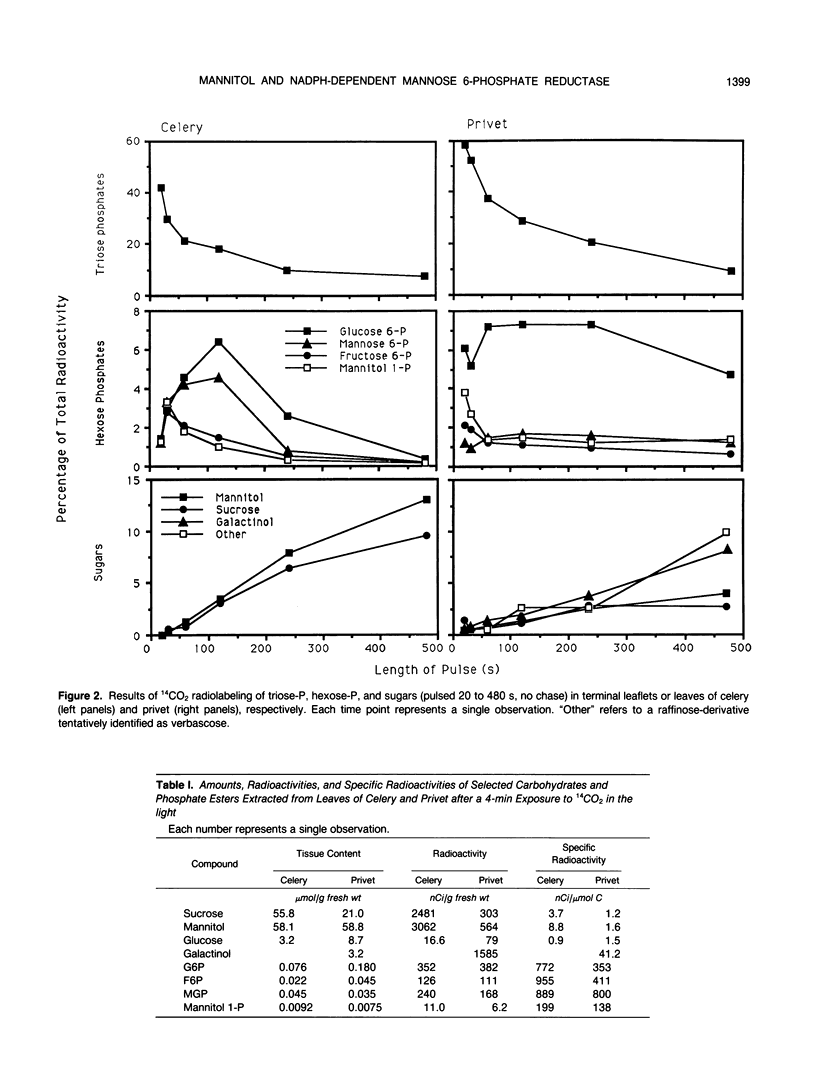
Mannitol is a major photosynthetic product in many algae and higher plants. Photosynthetic pulse and pulse-chase 14C-radiolabeling studies with the mannitol-synthesizing species, celery (Apium graveolens L.) and privet (Ligustrum vulgare L.), showed that mannose 6-phosphate (M6P) and mannitol 1-phosphate were among the early photosynthetic products. A NADPH-dependent M6P reductase was detected in these species (representing two different higher plant families), and the enzyme was purified to apparent homogeneity (68-fold with a 22% yield) and characterized from celery leaf extracts. The celery enzyme had a monomeric molecular mass, estimated from mobilities on sodium dodecyl sulfate-polyacrylamide gels, of 35 kilodaltons. The isoelectric point was pH 4.9; the apparent Km (M6P) was 15.8 millimolar, but the apparent Km (mannitol 1-phosphate) averaged threefold higher; pH optima were 7.5 with M6P/NADPH and 8.5 with mannitol 1-phosphate/NADP as substrates. Substrate and cofactor requirements were quite specific. NADH did not substitute for NADPH, and there was no detectable activity with fructose 6-phosphate, glucose 6-phosphate, fructose 1-phosphate, mannose 1-phosphate, mannose, or mannitol. NAD only partially substituted for NADP. Mg2+, Ca2+, Zn2+, and fructose-2,6-bisphosphate had no apparent effects on the purified enzyme's activity. In vivo radiolabeling results and the enzyme's kinetics, specificity, and distribution (in two-plant families) all suggest that NADPH-dependent M6P reductase plays an important role in mannitol biosynthesis in higher plants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIELESKI R. L. SEPARATION OF PHOSPHATE ESTERS BY THIN-LAYER CHROMATOGRAPHY AND ELECTROPHORESIS. Anal Biochem. 1965 Aug;12:230–234. doi: 10.1016/0003-2697(65)90086-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Fellman J. K., Loescher W. H. Biosynthesis of Sucrose and Mannitol as a Function of Leaf Age in Celery (Apium graveolens L.). Plant Physiol. 1988 Jan;86(1):129–133. doi: 10.1104/pp.86.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlert D. C. Ketose reductase activity in developing maize endosperm. Plant Physiol. 1987 Jul;84(3):830–834. doi: 10.1104/pp.84.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. C., Kennedy R. A., Loescher W. H. Developmental Changes in Photosynthetic Gas Exchange in the Polyol-Synthesizing Species, Apium graveolens L. (Celery). Plant Physiol. 1986 Sep;82(1):307–311. doi: 10.1104/pp.82.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M. Purification and Characteristics of Sorbitol-6-phosphate Dehydrogenase from Loquat Leaves. Plant Physiol. 1981 Feb;67(2):221–224. doi: 10.1104/pp.67.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo T. M., Doehlert D. C., Crawford C. G. Sugar metabolism in germinating soybean seeds: evidence for the sorbitol pathway in soybean axes. Plant Physiol. 1990 Aug;93(4):1514–1520. doi: 10.1104/pp.93.4.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Negm F. B., Loescher W. H. Characterization and Partial Purification of Aldose-6-phosphate Reductase (Alditol-6-Phosphate:NADP 1-Oxidoreductase) from Apple Leaves. Plant Physiol. 1981 Jan;67(1):139–142. doi: 10.1104/pp.67.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negm F. B., Marlow G. C. Partial Purification and Characterization of d-Ribose-5-phosphate Reductase from Adonis vernalis L. Leaves. Plant Physiol. 1985 Aug;78(4):758–761. doi: 10.1104/pp.78.4.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negm F. B. Purification and Properties of an NADPH-Aldose Reductase (Aldehyde Reductase) from Euonymus japonica Leaves. Plant Physiol. 1986 Apr;80(4):972–977. doi: 10.1104/pp.80.4.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney C. L. A simple micro-assay for inorganic phosphate. Anal Biochem. 1976 Sep;75(1):201–210. doi: 10.1016/0003-2697(76)90071-3. [DOI] [PubMed] [Google Scholar]
- Redgwell R. J. Fractionation of plant extracts using ion-exchange Sephadex. Anal Biochem. 1980 Sep 1;107(1):44–50. doi: 10.1016/0003-2697(80)90489-3. [DOI] [PubMed] [Google Scholar]
- Ried J. L., Collmer A. Activity stain for rapid characterization of pectic enzymes in isoelectric focusing and sodium dodecyl sulfate-polyacrylamide gels. Appl Environ Microbiol. 1985 Sep;50(3):615–622. doi: 10.1128/aem.50.3.615-622.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpho M. E., Edwards G. E., Loescher W. H. A pathway for photosynthetic carbon flow to mannitol in celery leaves : activity and localization of key enzymes. Plant Physiol. 1983 Dec;73(4):869–873. doi: 10.1104/pp.73.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki S., Takeda A., Shimazu T. Assay of inorganic phosphate in the mild pH range, suitable for measurement of glycogen phosphorylase activity. Anal Biochem. 1985 Aug 1;148(2):277–281. doi: 10.1016/0003-2697(85)90229-5. [DOI] [PubMed] [Google Scholar]