Abstract
Background
This study evaluated the non-inferiority of dexamethasone (DEX) on day 1, with sparing on days 2–4 in cisplatin-based chemotherapy.
Methods
Patients with malignant solid tumors who were treated with cisplatin (≥50 mg/m²) were randomly assigned (1:1) to receive either DEX on days 1–4 (Arm D4) or DEX on day 1 (Arm D1) plus palonosetron, NK-1 RA, and olanzapine (5 mg). The primary endpoint was complete response (CR) during the delayed (24–120 h) phase. The non-inferiority margin was set at −15%.
Results
A total of 281 patients were enrolled, 278 of whom were randomly assigned to Arm D4 (n = 139) or Arm D1 (n = 139). In 274 patients were included in the efficacy analysis, the rates of delayed CR in Arms D4 and D1 were 79.7% and 75.0%, respectively (risk difference −4.1%; 95% CI –14.1%–6.0%, P = 0.023). However, patients in Arm D1 had significantly lower total control rates during the delayed and overall phases, and more frequent nausea and appetite loss. There were no significant between-arm differences in the quality of life.
Conclusion
DEX-sparing is an alternative option for patients receiving cisplatin; however, this revised administration schedule should be applied on an individual basis after a comprehensive evaluation.
Clinical Trials Registry number
UMIN000032269
Subject terms: Chemotherapy, Oesophageal cancer, Head and neck cancer
Background
Chemotherapy-induced nausea and vomiting (CINV) are common adverse reactions associated with chemotherapy and considerably reduce patient quality of life (QOL). CINV has been classically assessed during the overall (0–120 h post-chemotherapy), acute (0–24 h), and delayed (24–120 h) treatment phases [1]. Intravenously administered cytotoxic agents are categorized into four emetic risk groups (highly, moderately, low, and minimal) [2]. Highly emetogenic chemotherapy (HEC)—including cisplatin-based chemotherapy and anthracycline plus cyclophosphamide (AC)—can increase the incidence of vomiting to >90% of patients without proper antiemetic prophylaxis [3]. CINV has been evaluated using composite endpoints, including nausea, vomiting, and rescue antiemetic use. Landmark CINV trials that have established the standard antiemetic regimens have adopted complete response (CR), defined as no vomiting and no rescue antiemetic use, as the primary endpoint [4].
A randomized, double-blinded, placebo-controlled phase III trial showed that combining 10 mg olanzapine with 5-hydroxytryptamine type 3 receptor antagonists (5-HT3-RA), neurokinin-1 receptor antagonists (NK1-RA), and dexamethasone (DEX) was more effective than placebo for preventing CINV in the acute and delayed phases in patients receiving HEC; however, 10 mg of olanzapine increased sedation [5]. Another pivotal phase3 trial (J-FORCE study) also found that adding 5 mg olanzapine to palonosetron, NK1-RA, and DEX resulted in a significantly higher CR rate than those without olanzapine during the delayed phase in patients receiving cisplatin-based chemotherapy without increased sedation [4]. Based on the results of these pivotal studies, current guidelines recommend the quadruple antiemetic therapy of 5-HT3-RA, NK1-RA, DEX, and 5–10 mg olanzapine as standard antiemetic therapy for preventing CINV in patients receiving HEC [6, 7]. Unnecessary sedation may be avoided by using 5, rather than 10 mg, olanzapine.
DEX is typically administered over multiple days from the start of chemotherapy to treat delayed CINV [8], whereas the frequent administration of corticosteroids is associated with numerous adverse effects—such as insomnia, reduced bone mineral density, and hyperglycemia [9–11]. A meta-analysis revealed that DEX-sparing strategies, which administer DEX only during the acute phase (day 1) and spare it during the delayed phase (day 2 and on), did not decrease the CR rate during the delayed phase for patients receiving AC regimen [12]. Our prior phase III study (DEX-1 study) sought to determine the non-inferiority of DEX sparing on days 2–3 for patients receiving HEC, including AC and cisplatin-based chemotherapy combined with palonosetron and NK1-RA [13]. The DEX-1 study met the primary endpoint of CR during the overall phase. However, in subgroup analyses, DEX sparing did not achieve non-inferiority in the delayed phase in patients receiving cisplatin-based chemotherapy; the CR rate was only 51%.
For patients receiving AC therapy, olanzapine significantly improved CINV during the delayed phase, even when DEX was spared [14]. The J-FORCE study suggested olanzapine greatly impacted antiemetic effects during the delayed phase in patients administered cisplatin-based regimens. However, J-FORCE adopted conventional DEX administration (days 1–4) [4]. Therefore, we hypothesized DEX sparing could be achieved without decreasing the CR rate during the delayed phase in patients receiving quadruple antiemetic therapy. The phase III SPARED study was designed to detect the non-inferiority of DEX sparing on days 2–4 when combined with palonosetron, NK1-RA, and 5 mg olanzapine in patients receiving cisplatin-based chemotherapy.
Methods
Study design and participants
This multicenter, placebo-controlled, double-blinded, randomized non-inferiority phase III study sought to clarify the non-inferiority of DEX on days 1–4 and DEX on days 1 in combination with palonosetron, NK1-RA, and 5 mg olanzapine in patients receiving cisplatin-based chemotherapy. The patients were recruited from 10 medical centers in Japan (including cancer centers, private hospitals, public hospitals, and university hospitals).
Inclusion criteria were patients with histologically or cytologically confirmed malignant solid tumors (excluding those with a hemopoietic malignancy), naïve to cisplatin, and scheduled to receive first-course cisplatin-based (≥50 mg/m2) chemotherapy; age 20–74 years; an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 or 1; no nausea and/or vomiting during the 24 h before registration; adequate organ function confirmed by laboratory tests within 2 weeks before registration (aspartate aminotransferase ≤100 IU/L, alanine transaminase ≤100 IU/L, total bilirubin ≤2.0 mg/dL, serum creatinine ≤1.5 mg/dL); life expectancy ≥3 months; and written informed consent from the patient before registration. We excluded patients with moderately emetogenic chemotherapy scheduled within 6 days before or after cisplatin initiation (minimal to low emetogenic agents were allowed) or radiation therapy to the abdomen or pelvis; symptomatic brain metastases; contraindications for corticosteroid use; and routine use of corticosteroids or any other agent with antiemetic potential. Those with a convulsive disorder requiring anticonvulsant therapy; diabetes requiring treatment with insulin, oral hypoglycemic agents, or both; and glycated hemoglobin (National Glycohemoglobin Standardization Program) ≥ 6.5% at the time of registration were also excluded. Details of the study protocol have been reported elsewhere [15].
Randomization and masking
Eligible patients were randomly assigned (1:1) to either DEX on days 1–4 (Arm D4) or day 1 with placebo on days 2–4 (Arm D1). The registration was done by a blinded pharmacist using a password-protected web entry system. Inquiries about patient registration and the web entry system were managed at the Department of Clinical Trial Data Management at the University of Tokyo and by the study’s secretariat. Randomization was centrally performed by random allocation modules of electronic data captures using the minimization method, with balancing prognostic factors for age (<60 vs. ≥60 years), sex, cisplatin dose level (≥70 vs. <70 mg/m2), and institution. The dispensing pharmacists independently maintained the allocation information to ensure double-blinding. Patients, medical staff, investigators, and individuals handling data were blinded to the treatment assignment. Placebo (plain saline) was visually indistinguishable from the intravenous DEX.
Procedures
Patients in both treatment arms were administered palonosetron (0.75 mg intravenous infusion on day 1, 30 min before the start of cisplatin), NK1-RA (125 mg of aprepitant administered orally on day 1; 80 mg on days 2 and 3; or 150 mg fosaprepitant administered intravenously on day 1, 1 h before the start of cisplatin), and olanzapine (5 mg administered orally on days 1–4 after dinner). Patients in both arms were administered a 9.9 mg intravenous infusion of DEX on day 1. On days 2–4, the patients in Arm D4 were intravenously administered 6.6 mg of DEX (when using fosaprepitant, 13.2 mg was administered on days 3 and 4 to diminish the interaction between DEX and NK-1 RA). Patients in Arm D1 were intravenously administered a placebo. Patients were allowed rescue medication throughout the study period for nausea or vomiting. The choice of recommended rescue medication was determined by each investigator from prochlorperazine, metoclopramide, domperidone, chlorpheniramine, alprazolam, lorazepam, and haloperidol. The patients were admitted to the hospital and observed for 120 h after initiation of cisplatin.
During the 120 h after initiation of cisplatin, the patients recorded the following items in their symptom diary every 24 h: the number of emetic episodes; nausea severity using an 11-point numerical rating scale (NRS); the number of rescue medications; and adverse events (AEs) according to Patient-Reported Outcomes (PRO) version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) version 1·0 questionnaires [16], with 19 treatment-associated symptoms. The AEs were also evaluated by the blinded investigators according to CTCAE version 4.0 during the overall period. QOL was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ C30) before the start of cisplatin and on day 8 after starting cisplatin administration. PRO and QOL data were collected electronically from the patients on electronic tablet devices using the EDC system Viedoc me (Viedoc Technologies, Sweden).
Outcomes
The primary endpoint was the CR during the delayed phase. Secondary endpoints were CR during the acute and overall phases; complete control (CC; defined as no vomiting, no use of rescue medications, and no significant nausea) during the overall, acute, and delayed phases; total control (TC; defined as no vomiting, no rescue use, and no nausea) during the overall, acute, and delayed phases; rates of no vomiting and no nausea during the overall, acute, and delayed phases; time to antiemetic treatment failure (i.e., time to the first vomiting or use of rescue medications, whichever occurred first); nausea severity during the overall phase; AEs; and QOL.
Statistical analyses
The sample size was calculated based on an analysis of the primary endpoint. In previous studies with olanzapine added to 5-HT3-RA, NK1-RA, and DEX for cisplatin [17–19], the CR rate during the delayed phase was 75%–85%. Therefore, we expected the delayed phase’s CR rate to be 75% in both treatment arms. The non-inferiority margin for the primary endpoint was set at −15.0%. We required 262 patients to achieve 80% power and confirm non-inferiority at a one-sided significance level of 2.5%. After considering the possibility of attrition, the sample size was set at 280. All statistical procedures were detailed in the statistical analysis plan before data evaluation. No interim analysis was planned, and we used a full analysis set comprising the registered participants who underwent at least part of the protocol therapy. Those deemed ineligible for the study after registration and those not administered cisplatin-based chemotherapy were excluded. Point estimates and confidence intervals (CIs) were calculated for the CR, TC, CC, no nausea, and no vomiting rates. Between-group comparisons of the primary endpoint were completed using the Cochran-Mantel-Haenszel test, with adjustment for allocation factors (age <60 or ≥60 years, sex, and cisplatin dose level ≥70 or <70 mg/m2). The CR excluding for primary endpoint, TC, CC, no nausea, and no vomiting rates were compared between groups using a chi-squared test. For the primary analysis, non-CR patients missed the primary endpoints. Subgroup analyses were also performed to explore possible relationships between baseline patient characteristics and CR, CC, TC, and nausea using a logistic regression model. For the time to antiemetic treatment failure, we calculated the estimates of the median and the 95% CI for each group using the Kaplan–Meier method and compared between groups using a log-rank test. Between-group comparisons of AEs were completed using the Mantel test. We compared QOL changes from baseline between groups using a two-sample t test. All statistical analyses were completed with SAS (version 9.4).
Results
Patient characteristics
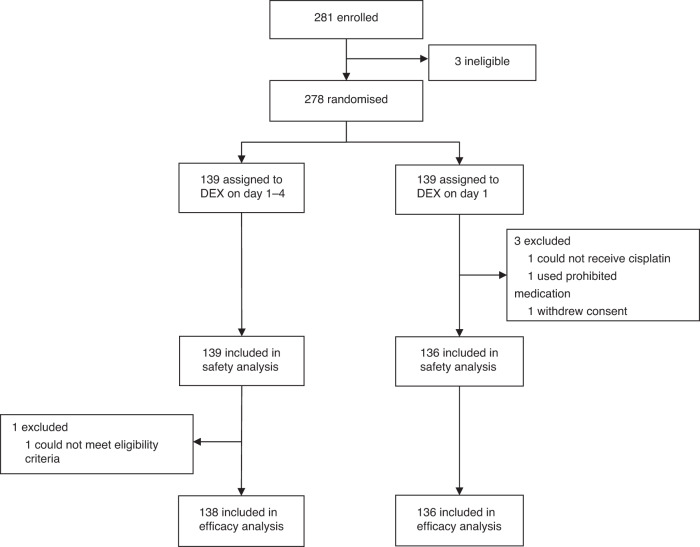
Between Oct 2018, and Mar 2021, 281 patients were enrolled, and three patients were excluded due to discontinuing chemotherapy before randomization. A total of 278 patients were randomly assigned to Arm D4 (n = 139) or D1 (n = 139). Three patients in Arm D1 did not receive chemotherapy; thus, 275 patients (139 in Arm D4 and 136 in Arm D1) were analyzed for safety. One patient in Arm D4 had a major protocol deviation for eligibility criteria. In total, the full analysis set included 274 patients (138 and 136 patients in Arms D4 and D1, respectively) (Fig. 1). The patients’ characteristics were well balanced between the two arms (Table 1). Most patients (70%) were male, and the major primary tumor sites were the esophagus (39%), head and neck (25%), and lung (19%).
Fig. 1. CONSORT diagram.
Of the 281 randomly assigned patients, 139 were confirmed eligible in each arm. Arm D4, dexamethasone days 1–4; Arm D1, dexamethasone day 1.
Table 1.
Patient characteristics.
| Characteristic | Arm D4 (n = 139) | Arm D1 (n = 139) |
|---|---|---|
| Median age | 63 (35–74) | 64 (25–74) |
| Gender, n (%) | ||
| Male | 95 (68.3) | 97 (69.8) |
| Female | 44 (31.7) | 42 (30.2) |
| ECOG PS, n (%) | ||
| 0 | 107 (77.0) | 106 (76.3) |
| 1 | 32 (23.0) | 33 (23.7) |
| Primary tumor, n (%) | ||
| Esophageal | 56 (40.3) | 53 (38.1) |
| Head and neck | 32 (23.0) | 37 (26.6) |
| Lung | 25 (18.0) | 28 (20.1) |
| Gastric | 10 (7.2) | 6 (4.3) |
| Others | 16 (11.5) | 15 (10.8) |
| Dose of CDDP, n (%) | ||
| ≥70 mg/m2 | 111 (79.9) | 113 (81.3) |
| <70 mg/m2 | 28 (20.1) | 26 (18.7) |
| Concurrent radiotherapy, n (%) | ||
| Yes | 51 (36.7) | 49 (35.3) |
| No | 88 (63.3) | 90 (64.7) |
| Drinking habits | ||
| Yes | 56 (40.3) | 60 (43.2) |
| No | 83 (59.7) | 77 (55.4) |
| N/A | 0 | 2 (1.4) |
| Motion sickness | ||
| Yes | 15 (10.8) | 18 (12.9) |
| No | 124 (89.2) | 119 (85.6) |
| N/A | 0 | 2 (1.4) |
| Morning sicknessa | ||
| Yes | 22 (50.0) | 23 (54.8) |
| No | 22 (50.0) | 18 (42.9) |
| N/A | 0 (0.0) | 1 (2.3) |
CDDP cisplatin, N/A not available, ECOG PS eastern cooperative oncology group performance status.
aThe percentage of patients with or without morning sickness was calculated using the number of female patients as the denominator.
Efficacy
The rates of CR, CC, TC, no nausea, and no vomiting are summarized in Table 2. The CR rates during the delayed phase were 79.7% in Arm D4 and 75.0% in Arm D1, with a difference of −4.1% (95% CI − 14.1% to 6.0%; P non-inferior = 0.023); the CR rate in the delayed phase, the primary endpoint, was met.
Table 2.
Rates of complete response, complete control, total control, no nausea, and no vomiting.
| Outcome | Arm D4 (n = 138) | Arm D1 (n = 136) | Risk Difference [95% CI] | P-value | |
|---|---|---|---|---|---|
| CR, n (%) | Acute | 133 (96.4) | 132 (97.1) | 0.7 [–3.5, 4.9] | 0.75 |
| Delayeda | 110 (79.7) | 102 (75.0) | –4.1 [–14.1, 6.0]b | 0.023 | |
| Overall | 109 (79.0) | 99 (72.8) | –6.2 [–16.3, 3.9] | 0.23 | |
| CC, n (%) | Acute | 130 (94.2) | 129 (94.9) | 0.7 [–4.7, 6.0] | 0.81 |
| Delayed | 98 (71.0) | 90 (66.2) | –4.8 [–15.8, 6.1] | 0.39 | |
| Overall | 96 (69.6) | 88 (64.7) | –4.9 [–16.0, 6.3] | 0.39 | |
| TC, n (%) | Acute | 124 (89.9) | 119 (87.5) | –2.4 [–9.9, 5.2] | 0.54 |
| Delayed | 83 (60.1) | 65 (47.8) | –12.4 [–24.1, –0.6] | 0.040 | |
| Overall | 81 (58.7) | 63 (46.3) | –12.4 [–24.1, –0.6] | 0.040 | |
| No nausea, n (%) | Acute | 124 (89.9) | 120 (88.2) | –1.6 [–9.0, 5.8] | 0.67 |
| Delayed | 89 (64.5) | 71 (52.2) | –12.3 [–23.9, –0.7] | 0.039 | |
| Overall | 86 (62.3) | 68 (50.0) | –12.3 [–24.0, –0.7] | 0.040 | |
| No vomiting, n (%) | Acute | 137 (99.3) | 135 (99.3) | –0.01 [–2.0, 2.0] | 0.99 |
| Delayed | 131 (94.9) | 129 (94.9) | –0.1 [–5.3, 5.1] | 0.98 | |
| Overall | 131 (94.9) | 128 (94.1) | –0.8 [–6.2, 4.6] | 0.77 | |
Arm D4 dexamethasone days 1–4, Arm D1 dexamethasone day 1, CC complete control (no emetic episodes, no use of rescue medication, and no more than mild nausea), CR complete response (no emetic episodes and no use of rescue medication), TC total control (no emetic episodes, no use of rescue medication, and no nausea).
aOne-sided P-value ≤ 0.025 was regarded as an indication of statistical significance with adjustment for allocation factors.
bRisk difference = –15% indicates noninferiority margin.
The CR rates in Arm D1 during the acute and overall phases were not different from those in Arm D4 (acute phase: 96.4% and 97.1% [95% CI of the difference, −3.5% to 4.9%; P = 0.75]; overall phase: 79.0% and 72.8% [95% CI of the difference, −16.3% to 3.9%; P = 0.23] in Arms D4 and D1, respectively). The CC rates for Arms D4 and D1 were 94.2% and 94.9% (95% CI − 4.7% to 6.0%, P = 0.81) in the acute phase, 71.0% and 66.2% (95% CI − 15.8% to −6.1%, P = 0.39) in the delayed phase, and 69.6% and 64.7% (95% CI − 16.0% to 6.3%, P = 0.39) in the overall phase, respectively. The TC rates for Arms D4 and D1 were 89.9% and 87.5% (95% CI − 9.9% to 5.2%, P = 0.54) in the acute phase, 60.1% and 47.8% (95% CI − 24.1% to −0.64%, P = 0.040) in the delayed phase, and 58.7% and 46.3% (95% CI − 24.1% to −0.64%, P = 0.040) in the overall phase, respectively.
The no nausea rate during the delayed and overall phases for Arm D1 was significantly lower than that for Arm D4 (64.5% vs. 52.2%, P = 0.039 and 62.3% vs. 50.0%, P = 0.040, respectively). However, the patients with severe nausea (NRS ≥ 3) during the delayed and overall phases for Arm D1 were similar to those for Arm D4 (Supplemental Table S1). Additionally, the severity of patient-reported nausea by NRS, vomiting, and the use of rescue medications was not different between the two arms (Supplemental Fig. S1 and Supplemental Tables S1, 2).
The subgroup analyses showed that morning sickness identified possible interaction in CR, CC, TC, and no nausea (Supplemental Fig. S2–5). For younger patients (<60 years), Arm D4 demonstrated better TC and no nausea. No other significant interactions were observed in the subgroup analysis. There was no significant between-arm difference in time to antiemetic treatment failure (Supplemental Fig. S6).
Safety
The patient-reported AEs are listed in Table 3. In PRO-CTCAE, appetite loss (severity P = 0.0023), nausea (frequency P = 0.0033), diarrhea (frequency P = 0.015), and headache (frequency P = 0.0021, severity P = 0.020) were observed more often in Arm D1. Nausea severity did not differ between the two arms. In CTCAE evaluated by each investigator (Supplemental Table S3), nausea (P = 0.031) and anorexia (P = 0.0067) were observed more often in Arm D1. There were no other significant between-arm differences for any other symptom.
Table 3.
Patient-reported adverse events.
| Symptom | Arm D4 (n = 139) | Arm D1 (n = 136) | P-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild, n (%) | Moderate, n (%) | Severe, n (%) | Very severe, n (%) | ≥Mild, n (%) | ≥Severe, n (%) | Mild, n (%) | Moderate, n (%) | Severe, n (%) | Very severe, n (%) | ≥Mild, n (%) | ≥Severe, n (%) | |||
| Dry mouth | Severity | 43 (30.9) | 32 (23.0) | 13 (9.4) | 1 (0.7) | 89 (64.0) | 14 (10.1) | 41 (30.1) | 28 (20.6) | 14 (10.3) | 1 (0.7) | 84 (61.8) | 15 (11.0) | 0.82 |
| Mouth/throat sores | Severity | 43 (30.9) | 24 (17.3) | 0 (0.0) | 0 (0.0) | 67 (48.2) | 0 (0.0) | 38 (27.9) | 13 (9.6) | 2 (1.5) | 1 (0.7) | 54 (39.7) | 3 (2.2) | 0.24 |
| Taste changes | Severity | 32 (23.0) | 9 (6.5) | 6 (4.3) | 1 (0.7) | 48 (34.5) | 7 (5.0) | 37 (27.2) | 13 (9.6) | 4 (2.9) | 1 (0.7) | 55 (40.4) | 5 (3.7) | 0.54 |
| Appetite loss | Severity | 32 (23.0) | 27 (19.4) | 17 (12.2) | 7 (5.0) | 83 (59.7) | 24 (17.3) | 34 (25.0) | 31 (22.8) | 32 (23.5) | 8 (5.9) | 105 (77.2) | 40 (29.4) | 0.0023 |
| Nausea | Frequency | 25 (18.0) | 24 (17.3) | 7 (5.0) | 0 (0.0) | 56 (40.3) | 7 (5.0) | 30 (22.1) | 41 (30.1) | 3 (2.2) | 5 (3.7) | 79 (58.1) | 8 (5.9) | 0.0033 |
| Severity | 32 (23.0) | 19 (13.7) | 2 (1.4) | 1 (0.7) | 54 (38.8) | 3 (2.2) | 52 (38.2) | 18 (13.2) | 4 (2.9) | 1 (0.7) | 75 (55.1) | 5 (3.7) | 0.063 | |
| Vomiting | Frequency | 9 (6.5) | 6 (4.3) | 0 (0.0) | 0 (0.0) | 15 (10.8) | 0 (0.0) | 11 (8.1) | 5 (3.7) | 0 (0.0) | 0 (0.0) | 16 (11.8) | 0 (0.0) | 0.95 |
| Severity | 11 (7.9) | 6 (4.3) | 0 (0.0) | 0 (0.0) | 17 (12.2) | 0 (0.0) | 16 (11.8) | 4 (2.9) | 0 (0.0) | 0 (0.0) | 20 (14.7) | 0 (0.0) | 0.84 | |
| Bloating | Frequency | 21 (15.1) | 47 (33.8) | 7 (5.0) | 7 (5.0) | 82 (59.0) | 14 (10.1) | 24 (17.6) | 46 (33.8) | 11 (8.1) | 3 (2.2) | 84 (61.8) | 14 (10.3) | 0.98 |
| Severity | 48 (34.5) | 21 (15.1) | 8 (5.8) | 1 (0.7) | 78 (56.1) | 9 (6.5) | 49 (36.0) | 21 (15.4) | 7 (5.1) | 1 (0.7) | 78 (57.4) | 8 (5.9) | 0.97 | |
| Hiccups | Frequency | 15 (10.8) | 49 (35.3) | 23 (16.5) | 4 (2.9) | 91 (65.5) | 27 (19.4) | 30 (22.1) | 51 (37.5) | 22 (16.2) | 2 (1.5) | 105 (77.2) | 24 (17.6) | 0.51 |
| Severity | 49 (35.3) | 29 (20.9) | 10 (7.2) | 1 (0.7) | 89 (64.0) | 11 (7.9) | 61 (44.9) | 20 (14.7) | 14 (10.3) | 0 (0.0) | 95 (69.9) | 14 (10.3) | 0.74 | |
| Diarrhea | Frequency | 13 (9.4) | 18 (12.9) | 9 (6.5) | 0 (0.0) | 40 (28.8) | 9 (6.5) | 23 (16.9) | 22 (16.2) | 10 (7.4) | 5 (3.7) | 60 (44.1) | 15 (11.0) | 0.015 |
| Constipation | Severity | 32 (23.0) | 45 (32.4) | 26 (18.7) | 3 (2.2) | 106 (76.3) | 29 (20.9) | 35 (25.7) | 44 (32.4) | 15 (11.0) | 6 (4.4) | 100 (73.5) | 21 (15.4) | 0.40 |
| Headache | Frequency | 15 (10.8) | 13 (9.4) | 2 (1.4) | 0 (0.0) | 30 (21.6) | 2 (1.4) | 29 (21.3) | 24 (17.6) | 3 (2.2) | 0 (0.0) | 56 (41.2) | 3 (2.2) | 0.0021 |
| Severity | 19 (13.7) | 6 (4.3) | 1 (0.7) | 0 (0.0) | 26 (18.7) | 1 (0.7) | 41 (30.1) | 6 (4.4) | 1 (0.7) | 0 (0.0) | 48 (35.3) | 1 (0.7) | 0.020 | |
| Insomnia | Severity | 20 (14.4) | 20 (14.4) | 7 (5.0) | 0 (0.0) | 47 (33.8) | 7 (5.0) | 21 (15.4) | 17 (12.5) | 3 (2.2) | 0 (0.0) | 41 (30.1) | 3 (2.2) | 0.28 |
| Fatigue | Severity | 37 (26.6) | 32 (23.0) | 11 (7.9) | 2 (1.4) | 82 (59.0) | 13 (9.4) | 45 (33.1) | 37 (27.2) | 7 (5.1) | 4 (2.9) | 93 (68.4) | 11 (8.1) | 0.31 |
| Anxious | Frequency | 8 (5.8) | 13 (9.4) | 2 (1.4) | 1 (0.7) | 24 (17.3) | 3 (2.2) | 16 (11.8) | 10 (7.4) | 2 (1.5) | 2 (1.5) | 30 (22.1) | 4 (2.9) | 0.59 |
| Severity | 14 (10.1) | 11 (7.9) | 2 (1.4) | 1 (0.7) | 28 (20.1) | 3 (2.2) | 15 (11.0) | 8 (5.9) | 4 (2.9) | 1 (0.7) | 28 (20.6) | 5 (3.7) | 0.87 | |
| Discouraged | Frequency | 18 (12.9) | 26 (18.7) | 1 (0.7) | 1 (0.7) | 46 (33.1) | 2 (1.4) | 23 (16.9) | 16 (11.8) | 6 (4.4) | 2 (1.5) | 47 (34.6) | 8 (5.9) | 0.71 |
| Severity | 30 (21.6) | 13 (9.4) | 1 (0.7) | 1 (0.7) | 45 (32.4) | 2 (1.4) | 29 (21.3) | 13 (9.6) | 3 (2.2) | 0 (0.0) | 45 (33.1) | 3 (2.2) | 0.85 | |
| Sad or unhappy feelings | Frequency | 8 (5.8) | 16 (11.5) | 1 (0.7) | 1 (0.7) | 26 (18.7) | 2 (1.4) | 14 (10.3) | 7 (5.1) | 0 (0.0) | 0 (0.0) | 21 (15.4) | 0 (0.0) | 0.096 |
| Severity | 8 (5.8) | 11 (7.9) | 3 (2.2) | 0 (0.0) | 22 (15.8) | 3 (2.2) | 10 (7.4) | 4 (2.9) | 2 (1.5) | 1 (0.7) | 17 (12.5) | 3 (2.2) | 0.35 | |
| Hot flashes | Frequency | 27 (19.4) | 30 (21.6) | 2 (1.4) | 0 (0.0) | 59 (42.4) | 2 (1.4) | 30 (22.1) | 32 (23.5) | 2 (1.5) | 0 (0.0) | 64 (47.1) | 2 (1.5) | 0.53 |
| Severity | 39 (28.1) | 11 (7.9) | 2 (1.4) | 0 (0.0) | 52 (37.4) | 2 (1.4) | 44 (32.4) | 13 (9.6) | 1 (0.7) | 0 (0.0) | 58 (42.6) | 1 (0.7) | 0.52 | |
| Somnolence | Severity | 43 (30.9) | 35 (25.2) | 6 (4.3) | 1 (0.7) | 85 (61.2) | 7 (5.0) | 38 (27.9) | 41 (30.1) | 10 (7.4) | 1 (0.7) | 90 (66.2) | 11 (8.1) | 0.17 |
| Dyssomnia | Severity | 30 (21.6) | 7 (5.0) | 4 (2.9) | 1 (0.7) | 42 (30.2) | 5 (3.6) | 26 (19.1) | 9 (6.6) | 5 (3.7) | 2 (1.5) | 42 (30.9) | 7 (5.1) | 0.54 |
Arm D4 dexamethasone days 1–4, Arm D1 dexamethasone day 1.
Quality of Life
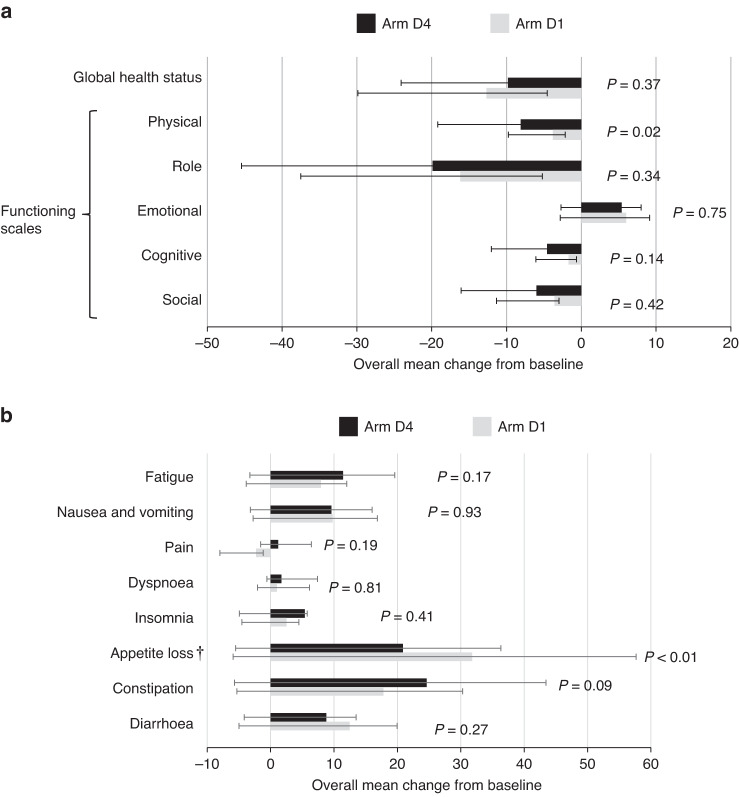
QOL questionnaires were collected from all patients at baseline and 97.8% at the end of the overall period. For Arm D4, the mean global health status scores were 59.1 (95% CI 55.0–63.1) before the start of cisplatin and 49.3 (95% CI 45.0–53.7) at the end of the overall period; for Arm D1, the scores were 63.4 (95% CI 59.1–67.6) and 51.4 (95% CI, 47.5–55.3), respectively (Supplemental Table S4). The change in the global health status score was not different between the two arms (Fig. 2a). Arm D1 demonstrated significantly poorer scores than Arm D4 for appetite loss (Fig. 2b). In contrast, Arm D1 displayed better physical functioning scores (Fig. 2a). There were no other significant between-arm differences for any other items.
Fig. 2. EORTC QLQ-C 30 scores.
Overall mean change from baseline in the EORTC QLQ-C 30 Global Health Status and Functioning subscale scores (a) and symptom scores (b). The overall mean change from baseline was estimated using repeated-measurement models; changes > 0 indicate improvement; bars indicate 95% confidence intervals (CIs) for Global Health Status and Functioning subscale scores; changes < 0 indicate improvement from baseline; bars indicate 95% CIs for symptom scores. QOL questionnaires were available for 137 patients in arm D4 and 131 patients in arm D1.
Discussion
To the best of our knowledge, this is the first phase III study to demonstrate the non-inferiority of DEX sparing on days 2–4 in terms of CR rates during the delayed phase when combined with palonosetron, NK1-RA, and 5 mg olanzapine in patients receiving cisplatin-based chemotherapy (79.7% vs. 75.0%, P non-inferior = 0.023). Additionally, we found that patients’ global health status scores and the incidence of severe nausea were not worsened by DEX sparing.
However, DEX sparing induced a significant increase in mild to moderate nausea and appetite loss, leading to a reduction in the TC rate during the overall and delayed phases. Therefore, we did not confirm our study hypothesis that 5 mg olanzapine could substitute for DEX on days 2–4 to prevent CINV in the delayed phase in patients receiving cisplatin-based chemotherapy. We should determine the clinical implication of a test regimen in CINV more comprehensively evaluating secondary endpoints including patient-reported outcomes and QOL, patient’s preference, cost-effectiveness.
For the reasons stated above, patients receiving cisplatin-based chemotherapy with DEX sparing should be carefully selected. We found younger age to be a significant risk factor for nausea in the subgroup analysis, consistent with the results of the previous study [20]. This means that DEX sparing should be avoided in younger patients. Although a history of morning sickness was a favorable factor in all the subgroup analyses, this finding was inconsistent with the previous reports [20]. This might have been caused by the small number of patients who reported a history of morning sickness. Even after the subgroup analyses, we could not determine good candidates for DEX sparing.
Systemic corticosteroids are immunosuppressants that reduce lymphocytes essential for the immune response to viruses [21]; consequently, steroid use may increase the incidence of viral and infectious respiratory complications with increasing doses [22, 23]. Although no studies have identified an association between corticosteroids doses as antiemetic therapy and the risk or severity of coronavirus disease 2019 (COVID-19), a previous report showed lymphopenia to be associated with more severe disease in COVID-19 [24]. These findings demonstrated the importance of minimizing corticosteroid exposure in patients with cancer during the COVID-19 pandemic. The European Society for Medical Oncology guidance for supportive care recommends a reduced dose of DEX on day 1 without additional use in the following days should be considered for patients receiving HEC during the COVID-19 pandemic [25]. Conversely, the antiemetic guidelines established by Cancer Care Ontario in Canada during the COVID-19 pandemic showed that single-day DEX dosing is recommended over multiple-day dosing for patients receiving HEC regimens, excluding high-dose cisplatin [26]. Our data support the administration of reduced DEX doses as antiemetic therapy in patients treated with cisplatin-based chemotherapy during any viral pandemic such as COVID-19.
Aside from its immunosuppressive effects, we believed DEX sparing could reduce the incidence of hot flashes, mood disorders, insomnia, and anxiety—commonly reported as DEX-related AEs. However, the two study arms demonstrated no significant differences. Olanzapine has been used as an antipsychotic drug and increases somnolence [27]. Nikbaksh et al. reported that olanzapine reduced depression in patients with gastric cancer receiving chemotherapy [28]. Therefore, no significant differences in depression, insomnia, or anxiety were observed. The DEX-1 study demonstrated that DEX sparing significantly reduced hot flashes on days 4 and 5 in patients administered HEC [13]. The patient backgrounds of this current study were different from those of the DEX-1 study, especially the proportion of females in the DEX-1 study (80.8%). Therefore, it is possible that this current study did not find any difference in hot flashes regardless of DEX sparing.
This study has certain limitations. First, females represented approximately 30% of the study population; however, this figure is consistent with recent evidence regarding patients who receive cisplatin-based chemotherapy [4, 29]. The primary tumors were esophageal, head, neck, and lung—all of which were well balanced between the two arms. However, our results might not apply to other primary tumors. The current study did not evaluate toxicities from long-term administration of DEX—such as decreased bone mineral density, impaired glucose tolerance, and infections. Lastly, although fosnetupitant, a novel NK 1-RA, may improve CR in the delayed phase better than fosaprepitant [30], but was not evaluated in this current study and needs to be investigated further.
In conclusion, DEX sparing on days 2–4 could prevent CINV and maintain the global health status in patients receiving cisplatin-based chemotherapy when combined with palonosetron, NK1-RA, and 5 mg olanzapine. Since DEX sparing worsens mild to moderate nausea and appetite loss, it should be carefully applied on a patient-by-patient basis after a comprehensive evaluation.
Supplementary information
Acknowledgements
The authors thank all the patients and their families, investigators, and institutions involved in this study. We are also grateful to Tomoe Mashiko for data management, Shunsuke Oyamada for data analysis, and Yoshiki Horie for helpful discussions. This study is supported by Japan Agency for Medical Research and Development (AMED) grant number 21ck0106501h0003. AMED had and will have no involvement in the design and conduct of the study, collection, management, analysis, and interpretation of the data, and preparation, review, or approval of the manuscript. The authors would like to thank Enago (www.enago.jp) for the English language review.
Author contributions
HM, NI and TEN contributed to the trial conception and are the principal investigators. HM, NI, TM, TK, TY, and TEN participated in the design of the study. TY did the statistical analysis. TM and TK contributed to data management. HM, NI, statistician (TY), and TEN had full access to the data, conducted data analysis and interpretation, and take responsibility for the integrity of the data and adherence to the study protocol. HM and NI contributed to the initial draft of the manuscript and are responsible for the decision to submit the manuscript for publication. All authors contributed to data collection and interpretation, and revision of the manuscript for important content.
Funding
This study was funded by the Japan Agency for Medical Research and Development (grant number 21ck0106501h0003).
Data availability
Data were generated by the authors and are available on request.
Competing interests
HA reports personal fees from Ono, Bristol-Myers Squibb, Daiichi-Sankyo, Takeda, Chugai, Eisai and Delta-Fly Pharma. HI reports grants from Daiichi Sankyo Co., Ltd., Nippon Kayaku Co., Ltd., Chugai,,, Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Asahi Kasei Pharma Co., Ltd., Mochida Pharmaceutical Co., Ltd.; and reports personal fees from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., Astellas Pharma Co., Ltd., Eli Lilly and Company, Daiichi Sankyo Co., Ltd., AstraZeneca plc, Nippon Kayaku Co., Ltd., Ono, Pharmaceutical Co., Ltd. and Nippon Boehringer Ingelheim Co., Ltd. TM reports grants from AC Medical, A2 Healthcare, CAC Croit Corporation, Japan Tobacco Inc, Japan Media Corporation, Medidata Solutions, Inc, Ono Pharmaceutical, FMD K&L Japan, Intellim, Welby, Nipro Corporation, New Age Trading, NOBORI Ltd. And Medrio; and reports personal fees from AYUMI, Impute, Pfizer, Welby, EISAI, Merc and Takeda. KH reports grants from Pfizer. JE reports grants from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Nippon, Boehringer Ingelheim Co., Ltd., Kyorin Holdings, Inc. and Nippon Kayaku Co., Ltd.; and reports personal fees from AstraZeneca plc, Chugai Pharmaceutical Co., Ltd., Bristol-Myers Squibb Co., Ltd., Takeda Pharmaceutical Co., Ltd., Novartis Pharma K.K. and Daiichi Sankyo Co., Ltd. NF reports personal fees from Eli Lilly Japan, AstraZeneca, Boehringer Ingelheim Japan, Chugai, Bristol Myers Squibb, Taiho, Pfizer Japan and Novartis. TY reports grants from AC MEDICAL INC., A2 Healthcare Corporation, EP Croit Co., Ltd., ClinChoice., Japan Tobacco Inc., Japan Media Corporation, Medidata Solutions, Inc., ONO PHARMACEUTICAL CO., LTD., Kyowa Kirin Co., Ltd., TSUMURA & CO., DAIICHI, SANKYO COMPANY, LIMITED., Otsuka Pharmaceutical Co., Ltd. Eisai Co., Ltd., ASAHI, INTECC CO., LTD., 3H Clinical Trial Inc., Medrio, Inc., NIPRO CORPORATION, Intellim Corporation, Welby Inc., 3H Medi Solution Inc., NIPRO CORPORATION, Baseconnect Inc., Nobori Ltd., Puravida Technologies LLC. and Hemp Kitchen Inc.; and reports personal fees from EPS Corporation, Japan Tobacco Inc., Medidata Solutions, Inc., ONO PHARMACEUTICAL CO., LTD., Kowa Company, Ltd., CHUGAI PHARMACEUTICAL CO. LTD., TSUMURA & CO., DAIICHI SANKYO COMPANY, LIMITED., Eisai Co., Ltd., ASAHI INTECC CO., LTD., ASAHI KASEI PHARMA CORPORATION, 3H Clinical Trial Inc. Intellim Corporation, Takeda, AstraZeneca, SONIRE Therapeutics Inc., SEIKAGAKU CORPORATION, Merck & Co., Inc. and NIPRO CORPORATION. TEN reports grants from Sumitomo Dainippon Pharma Co., Ono Pharmaceutical Co., Taiho Pharmaceutical Co., Takeda Pharmaceutical Co., Chugai Pharmaceutical Co., Sanofi K.K., Nippon Kayaku Co., MSD K.K. and Eli Lilly Japan K.K.; and reports personal fees from Thyas Co. Ltd., Rebirthel Co., Ltd., Japan Clinical Research Operations, Sumitomo Dainippon Pharma Co., Boehringer Ingelheim, Bristol-Myers Squibb, Ono Pharmaceutical Co., Taiho Pharmaceutical Co., Amgen, Takeda Pharmaceutical Co., Chugai Pharmaceutical Co., Sanofi K.K., Novartis Japan, Nippon Kayaku Co., MSD K.K., Eli Lilly Japan K.K., Bayer Yakuhin, Pfizer Japan Inc., Daiichi, Sankyo Co., Yakult Honsha Co., Nipro Co, Merck Serono Co., AstraZeneca, IQVIA and GlaxoSmithKline. All other authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board at each site. The study was registered with the University Hospital Medical Information Network, Clinical Trials Registry number UMIN000032269. All patients included in this study provided written, informed consent.
Consent for publication
All patients provided written, informed consent.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hiroko Minatogawa, Naoki Izawa.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02493-7.
References
- 1.Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Eng J Med. 2016;374:1356–67. doi: 10.1056/NEJMra1515442. [DOI] [PubMed] [Google Scholar]
- 2.Hesketh PJ, Kris MG, Grunberg SM, Beck T, Hainsworth JD, Harker G, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15:103–9. doi: 10.1200/JCO.1997.15.1.103. [DOI] [PubMed] [Google Scholar]
- 3.Aogi K, Takeuchi H, Saeki T, Aiba K, Tamura K, Iino K, et al. Optimizing antiemetic treatment for chemotherapy-induced nausea and vomiting in Japan: update summary of the 2015 Japan Society of Clinical Oncology Clinical Practice Guidelines for Antiemesis. Int J Clin Oncol. 2021;26:1–17. doi: 10.1007/s10147-020-01818-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashimoto H, Abe M, Tokuyama O, Mizutani H, Uchitomi Y, Yamaguchi T, et al. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:242–9. doi: 10.1016/S1470-2045(19)30678-3. [DOI] [PubMed] [Google Scholar]
- 5.Navari RM, Qin R, Ruddy KJ, Liu H, Powell SF, Bajaj M, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Eng J Med. 2016;375:134–42. doi: 10.1056/NEJMoa1515725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, et al. Antiemetics: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35:3240–61. doi: 10.1200/JCO.2017.74.4789. [DOI] [PubMed] [Google Scholar]
- 7.Razvi Y, Chan S, McFarlane T, McKenzie E, Zaki P, DeAngelis C, et al. ASCO, NCCN, MASCC/ESMO: a comparison of antiemetic guidelines for the treatment of chemotherapy-induced nausea and vomiting in adult patients. Support Care Cancer. 2019;27:87–95. doi: 10.1007/s00520-018-4464-y. [DOI] [PubMed] [Google Scholar]
- 8.Grunberg S. Antiemetic activity of corticosteroids in patients receiving cancer chemotherapy: dosing, efficacy, and tolerability analysis. Ann Oncol. 2007;18:233–40. doi: 10.1093/annonc/mdl347. [DOI] [PubMed] [Google Scholar]
- 9.Vardy J, Chiew K, Galica J, Pond G, Tannock I. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer. 2006;94:1011–5. doi: 10.1038/sj.bjc.6603048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong Y, Han HS, Lee HD, Yang J, Jeong J, Choi MK, et al. A pilot study evaluating steroid-induced diabetes after antiemetic dexamethasone therapy in chemotherapy-treated cancer patients. Cancer Res Ttreat. 2016;48:1429–37. doi: 10.4143/crt.2015.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura M, Ishiguro A, Muranaka T, Fukushima H, Yuki S, Ono K, et al. A prospective observational study on effect of short‐term periodic steroid premedication on bone metabolism in gastrointestinal cancer (ESPRESSO‐01) Oncologist. 2017;22:592–600. doi: 10.1634/theoncologist.2016-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celio L, Bonizzoni E, Zattarin E, Codega P, De Braud F, Aapro M. Impact of dexamethasone-sparing regimens on delayed nausea caused by moderately or highly emetogenic chemotherapy: a meta-analysis of randomised evidence. BMC Cancer. 2019;19:1–13. doi: 10.1186/s12885-019-6454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito Y, Tsuda T, Minatogawa H, Kano S, Sakamaki K, Ando M, et al. Placebo-controlled, double-blinded phase III study comparing dexamethasone on day 1 with dexamethasone on days 1 to 3 with combined neurokinin-1 receptor antagonist and palonosetron in high-emetogenic chemotherapy. J Clin Oncol. 2018;36:1000–6. doi: 10.1200/JCO.2017.74.4375. [DOI] [PubMed] [Google Scholar]
- 14.Yeo W, Lau TK, Li L, Lai KT, Pang E, Cheung M, et al. A randomized study of olanzapine-containing versus standard antiemetic regimens for the prevention of chemotherapy-induced nausea and vomiting in Chinese breast cancer patients. Breast. 2020;50:30–38. doi: 10.1016/j.breast.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minatogawa H, Izawa N, Kawaguchi T, Miyaji T, Shimomura K, Kazunori H, et al. Study protocol for SPARED trial: randomised non-inferiority phase III trial comparing dexamethasone on day 1 with dexamethasone on days 1–4, combined with neurokinin-1 receptor antagonist, palonosetron and olanzapine (5 mg) in patients receiving cisplatin-based chemotherapy. BMJ Open. 2020;10:e041737. doi: 10.1136/bmjopen-2020-041737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyaji T, Iioka Y, Kuroda Y, Yamamoto D, Iwase S, Goto Y, et al. Japanese translation and linguistic validation of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) J Patient Rep Outcomes. 2017;1:1–10. doi: 10.1186/s41687-017-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011;9:188–95. doi: 10.1016/j.suponc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Yanai T, Iwasa S, Hashimoto H, Ohyanagi F, Takiguchi T, Takeda K, et al. A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol. 2018;23:382–8. doi: 10.1007/s10147-017-1200-4. [DOI] [PubMed] [Google Scholar]
- 19.Nakashima K, Murakami H, Yokoyama K, Omori S, Wakuda K, Ono A, et al. A phase II study of palonosetron, aprepitant, dexamethasone and olanzapine for the prevention of cisplatin-based chemotherapy-induced nausea and vomiting in patients with thoracic malignancy. Jpn J Clin Oncol. 2017;47:840–3. doi: 10.1093/jjco/hyx084. [DOI] [PubMed] [Google Scholar]
- 20.Dranitsaris G, Molassiotis A, Clemons M, Roeland E, Schwartzberg L, Dielenseger P, et al. The development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomiting. Ann Oncol. 2017;28:1260–7. doi: 10.1093/annonc/mdx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olnes MJ, Kotliarov Y, Biancotto A, Cheung F, Chen J, Shi R, et al. Effects of systemically administered hydrocortisone on the human immunome. Sci Rep. 2016;6:1–15. doi: 10.1038/srep23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11:954–63. doi: 10.1093/clinids/11.6.954. [DOI] [PubMed] [Google Scholar]
- 23.Fardet L, Petersen I, Nazareth I. Common infections in patients prescribed systemic glucocorticoids in primary care: a population-based cohort study. PLoS Med. 2016;13:e1002024. doi: 10.1371/journal.pmed.1002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–8. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Europiean Sociaty for Medical Oncology: Supportive Care Strategies During the COVID-19 Pandemic. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/supportive-care-in-the-covid-19-era
- 26.Grant RC, Rotstein C, Liu G, Forbes L, Vu K, Lee R, et al. Reducing dexamethasone antiemetic prophylaxis during the COVID-19 pandemic: recommendations from Ontario, Canada. Support Care Cancer. 2020;28:5031–6. doi: 10.1007/s00520-020-05588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schotte A, Janssen P, Gommeren W, Luyten W, Van Gompel P, Lesage A, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology. 1996;124:57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- 28.Nikbakhsh N, Sadeghi MV, Ramzani E, Moudi S, Bijani A, Yousefi R, et al. Efficacy of olanzapine in symptom relief and quality of life in gastric cancer patients receiving chemotherapy. J Res Med Sci. 2016;21:88. doi: 10.4103/1735-1995.192504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celio L, Cortinovis D, Cogoni AA, Cavanna L, Martelli O, Carnio S, et al. Dexamethasone-sparing regimens with oral netupitant and palonosetron for the prevention of emesis caused by high-dose cisplatin: a randomized noninferiority study. Oncologist. 2021;26:e1854–e61. doi: 10.1002/onco.13851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hata A, Okamoto I, Inui N, Okada M, Morise M, Akiyoshi K, et al. Randomized, double-blind, phase III study of fosnetupitant versus fosaprepitant for prevention of highly emetogenic chemotherapy-induced nausea and vomiting: CONSOLE. J Clin Oncol. 2022;40:180–8. doi: 10.1200/JCO.21.01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data were generated by the authors and are available on request.