Abstract
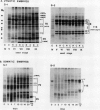
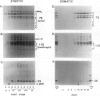
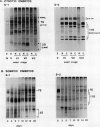
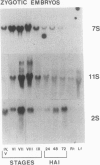
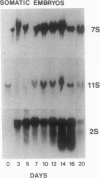
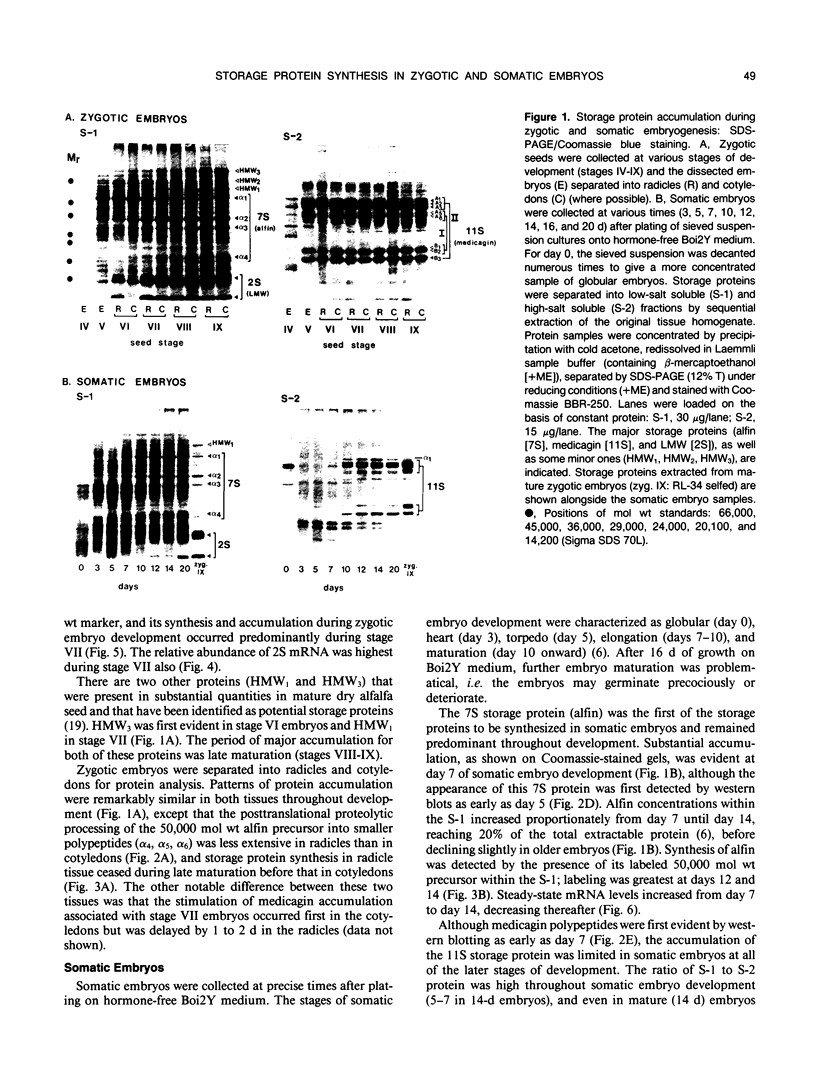
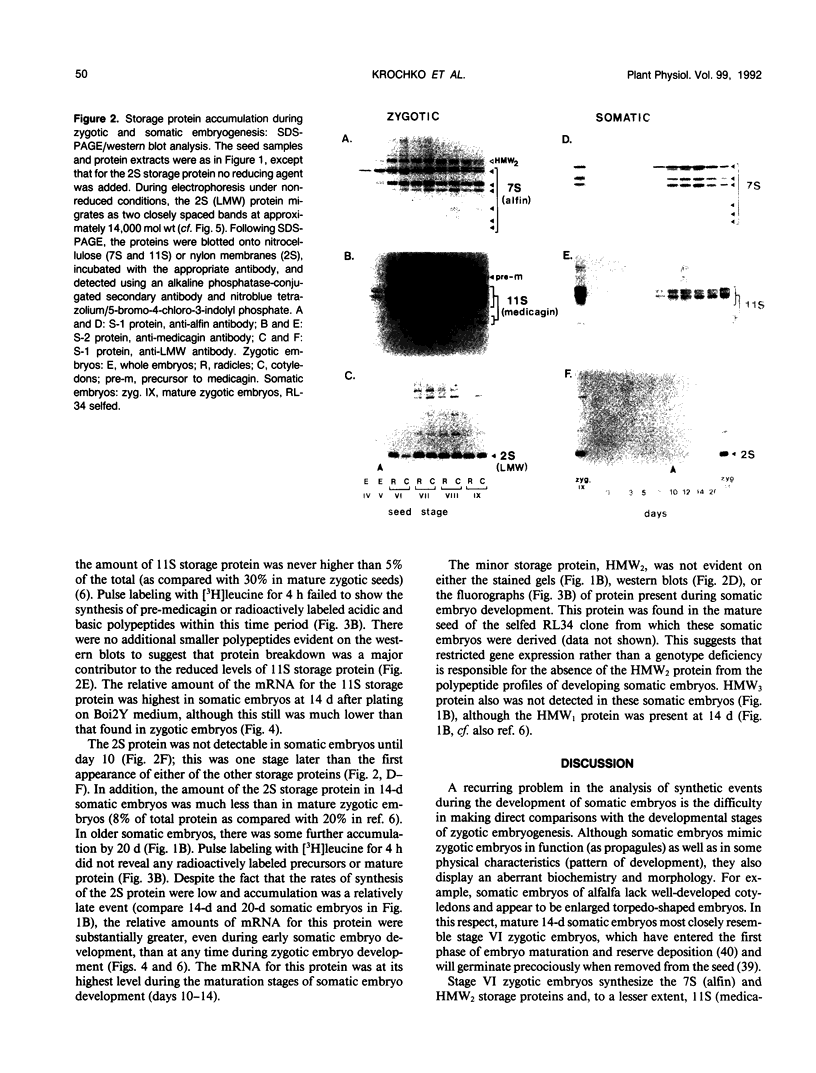
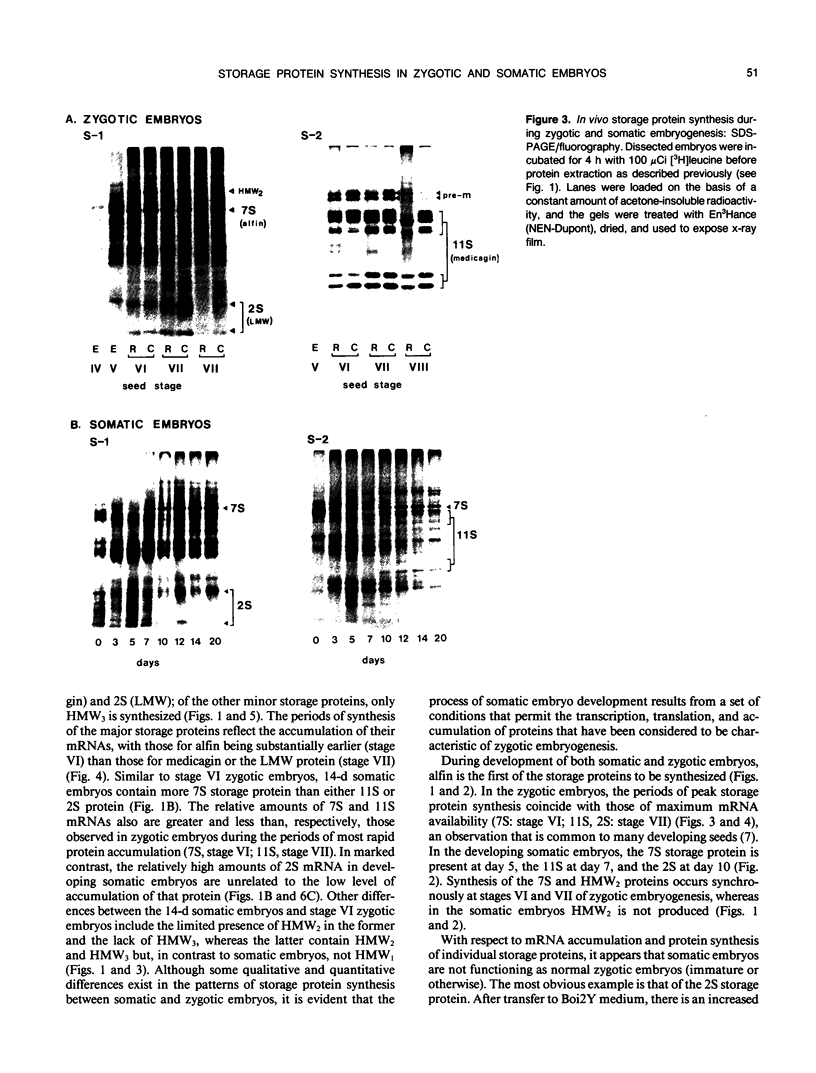
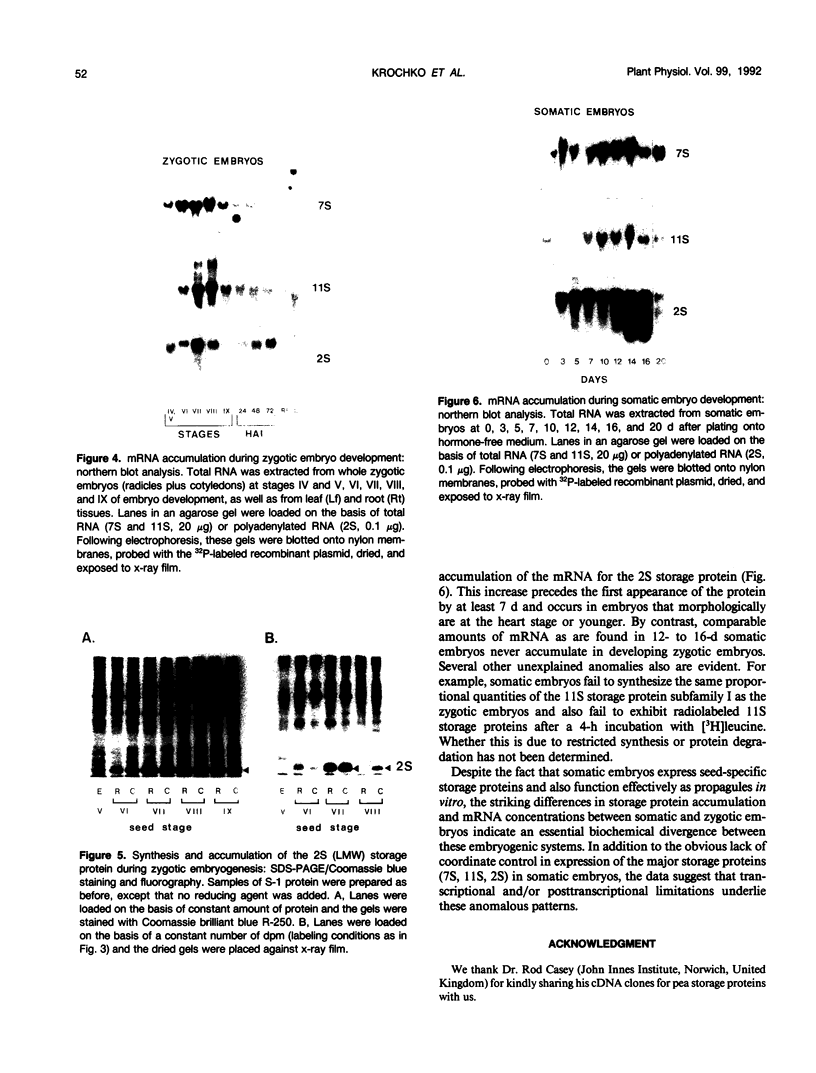
During development on hormone-free media, somatic embryos pass through distinct morphological stages that superficially resemble those of zygotic embryo development (globular, heart, torpedo, cotyledonary stages). Despite these similarities, they differ from zygotic embryos in the extent of cotyledonary development and the patterns of synthesis and quantitative expression of seed-specific storage proteins (7S, 11S, and 2S proteins). Alfin (7S) is the first storage protein synthesized in developing zygotic embryos (stage IV). The 11S (medicagin) and 2S (Low Molecular Weight, LMW) storage proteins are not detectable until the following stage of development (stage V), although all three are present before the completion of embryo enlargement. Likewise, the 7S storage protein is the first to be synthesized in developing somatic embryos (day 5). Medicagin is evident by day 7 and the LMW protein by day 10. In contrast to zygotic embryos, alfin remains the predominant storage protein in somatic embryos throughout development. Not only are the relative amounts of medicagin and the LMW protein reduced in somatic embryos but the LMW protein is accumulated much later than the other proteins. Quantification of the storage protein mRNAs (7S, 11S, and 2S) by northern blot analysis confirms that there are substantial differences in the patterns of message accumulation in zygotic and somatic embryos of alfalfa (Medicago sativa). In zygotic embryos, the 7S, 11S, and 2S storage protein mRNAs are abundant during maturation and, in particular, during the stages of maximum protein synthesis (alfin, stages VI and VII; medicagin, stage VII; LMW, stage VII). In somatic embryos, the predominance of the 7S storage protein is correlated with increased accumulation of its mRNA, whereas the limited synthesis of the 11S storage protein is associated with much lower steady-state levels of its message. The mRNA for the LMW protein is present already by 3 days after transfer to hormone-free media, yet that protein is not evident on stained gels until day 10. Thus, both transcriptional and posttranscriptional events appear to be important in determining the protein complement of these seed tissues. On the basis of storage protein and mRNA accumulation, mature (14 days) somatic embryos most closely resemble stage VI zygotic embryos. The results of the developmental comparison also suggest that the patterns of synthesis of the individual storage proteins (7S, 11S, or 2S) are regulated independently of each other during embryogenesis in alfalfa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach L. R., Spencer D., Randall P. J., Higgins T. J. Transcriptional and post-transcriptional regulation of storage protein gene expression in sulfur-deficient pea seeds. Nucleic Acids Res. 1985 Feb 11;13(3):999–1013. doi: 10.1093/nar/13.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley J. D., Marcus A. Gene expression in seed development and germination. Prog Nucleic Acid Res Mol Biol. 1990;38:165–193. doi: 10.1016/s0079-6603(08)60711-4. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Gatehouse J. A., Lycett G. W., Croy R. R., Boulter D. The post-translational proteolysis of the subunits of vicilin from pea (Pisum sativum L.). Biochem J. 1982 Dec 1;207(3):629–632. doi: 10.1042/bj2070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada J. J., Barker S. J., Goldberg R. B. Soybean beta-conglycinin genes are clustered in several DNA regions and are regulated by transcriptional and posttranscriptional processes. Plant Cell. 1989 Apr;1(4):415–425. doi: 10.1105/tpc.1.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins T. J., Chandler P. M., Randall P. J., Spencer D., Beach L. R., Blagrove R. J., Kortt A. A., Inglis A. S. Gene structure, protein structure, and regulation of the synthesis of a sulfur-rich protein in pea seeds. J Biol Chem. 1986 Aug 25;261(24):11124–11130. [PubMed] [Google Scholar]
- Jensenius J. C., Andersen I., Hau J., Crone M., Koch C. Eggs: conveniently packaged antibodies. Methods for purification of yolk IgG. J Immunol Methods. 1981;46(1):63–68. doi: 10.1016/0022-1759(81)90333-1. [DOI] [PubMed] [Google Scholar]
- Krochko J. E., Bewley J. D. Use of electrophoretic techniques in determining the composition of seed storage proteins in alfalfa. Electrophoresis. 1988 Nov;9(11):751–763. doi: 10.1002/elps.1150091111. [DOI] [PubMed] [Google Scholar]
- Ladin B. F., Tierney M. L., Meinke D. W., Hosángadi P., Veith M., Beachy R. N. Developmental Regulation of beta-Conglycinin in Soybean Axes and Cotyledons. Plant Physiol. 1987 May;84(1):35–41. doi: 10.1104/pp.84.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- STEWARD F. C., MAPES M. O., KENT A. E., HOLSTEN R. D. GROWTH AND DEVELOPMENT OF CULTURED PLANT CELLS. Science. 1964 Jan 3;143(3601):20–27. doi: 10.1126/science.143.3601.20. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling L., Drews G. N., Goldberg R. B. Transcriptional and post-transcriptional regulation of soybean seed protein mRNA levels. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2123–2127. doi: 10.1073/pnas.83.7.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]