Abstract
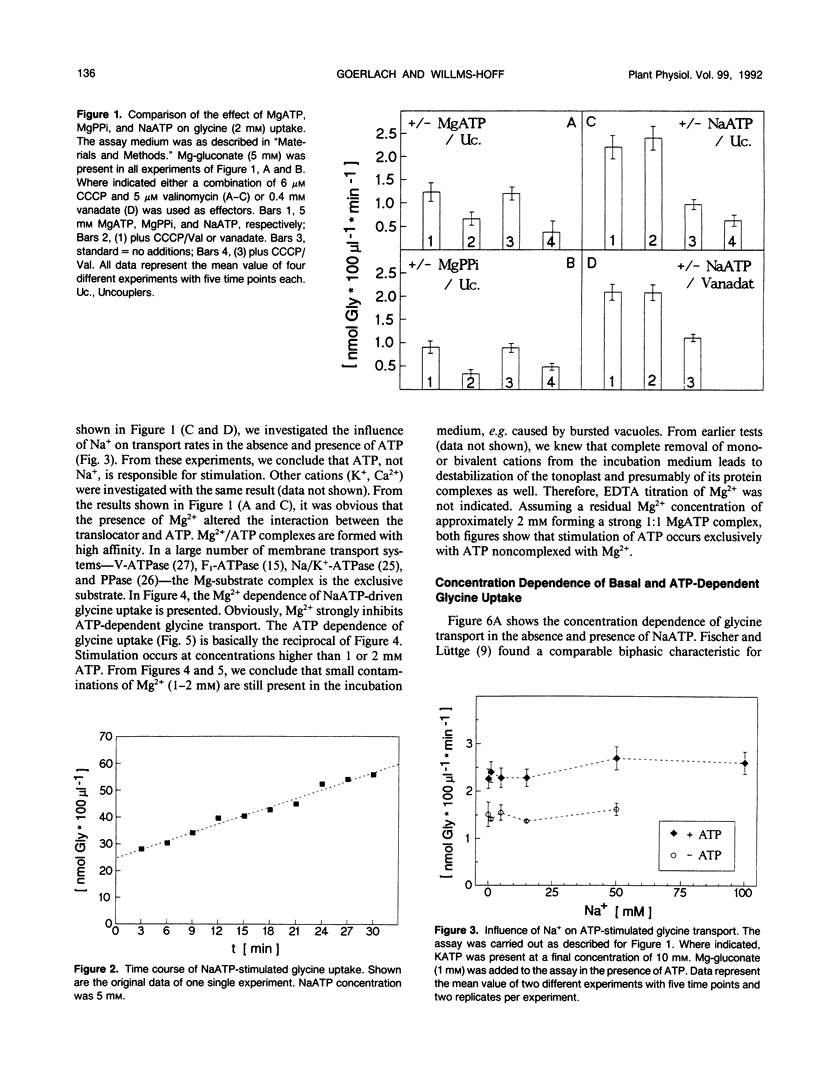
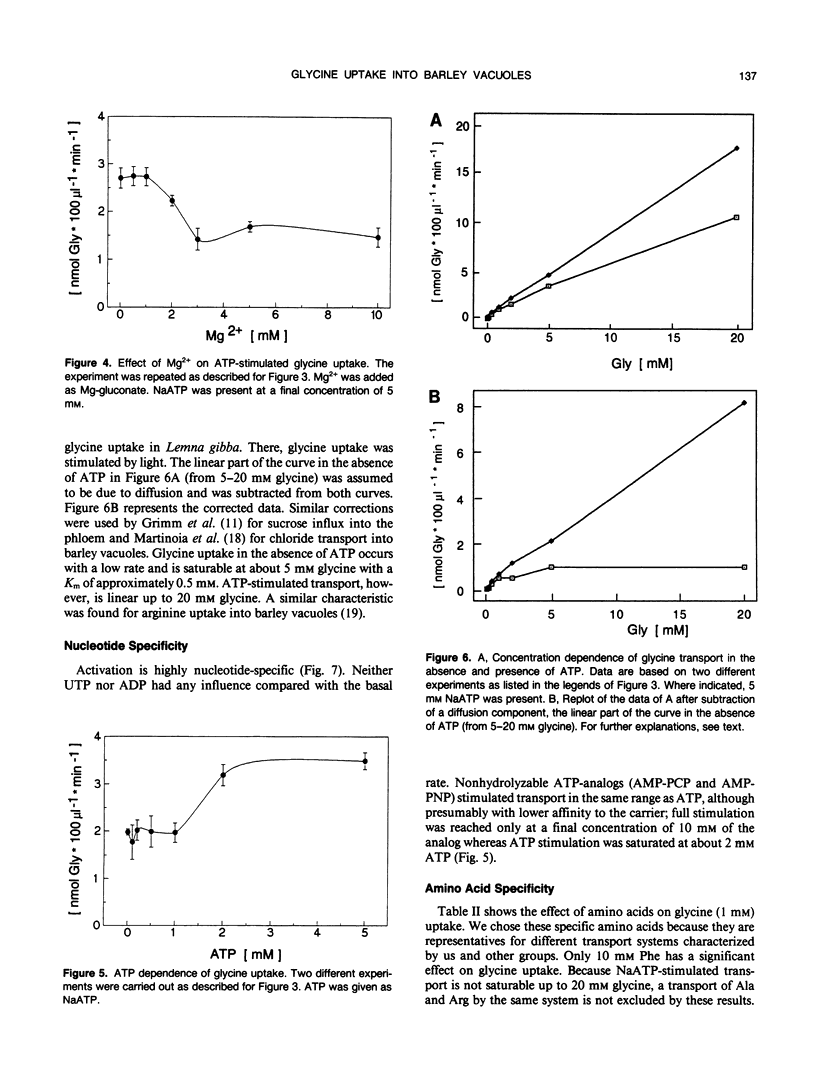
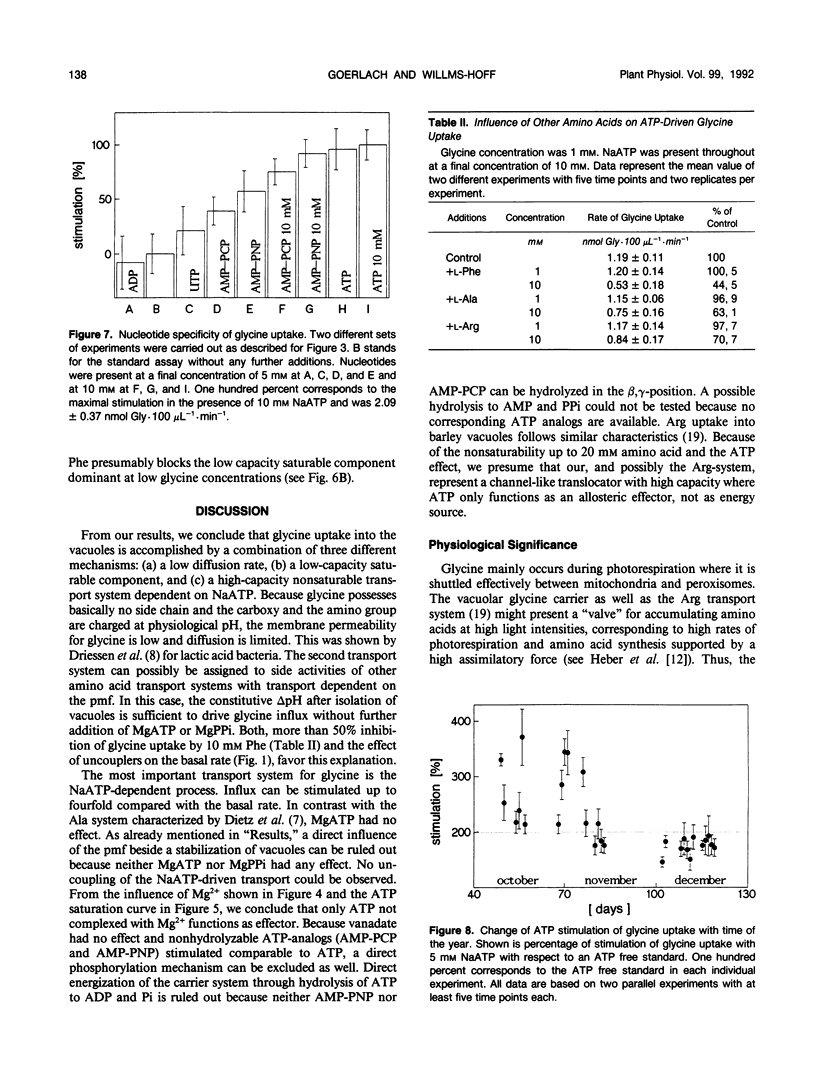
[U-14C]glycine uptake into barley (Hordeum vulgare cv Hasso) vacuoles was investigated. Glycine (2 millimolar) transport was stimulated two- to fourfold by NaATP. Stimulation was saturable with respect to ATP (1 millimolar) and linear up to 20 millimolar glycine. Stimulation by NaATP was suppressed by Mg2+ in equimolar amounts. Neither MgATP nor Mg-inorganic pyrophosphate had any effect on basal transport rate. Thus, the proton motive force can be excluded as the driving force. Uncouplers (valinomycine/carbonylcyanide-m-chlorophenylhydrazone) inhibited the basal rate up to 30% but had no influence on NaATP-stimulated uptake. Vanadate had no effect on either basal or NaATP-stimulated uptake. Nonhydrolyzable ATP analogs (adenylyl(β, γ-methylen)-diphosphate or adenylyl-imidodiphosphate) stimulated comparable to NaATP. Other nucleotides (UTP, ADP) had no effect. Some evidence exists that other amino acids (arginine, alanine, isoleucine, phenylalanine) are transported to a certain extent by a similar mechanism. The results indicate a high capacity channel-like translocator that is regulated but not energized by ATP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell R. D., Murray A. J., Lea P. J. Photorespiratory mutants of the mitochondrial conversion of glycine to serine. Plant Physiol. 1990 Nov;94(3):1316–1322. doi: 10.1104/pp.94.3.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K. J., Jäger R., Kaiser G., Martinoia E. Amino Acid Transport across the Tonoplast of Vacuoles Isolated from Barley Mesophyll Protoplasts : Uptake of Alanine, Leucine, and Glutamine. Plant Physiol. 1990 Jan;92(1):123–129. doi: 10.1104/pp.92.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Hellingwerf K. J., Konings W. N. Mechanism of energy coupling to entry and exit of neutral and branched chain amino acids in membrane vesicles of Streptococcus cremoris. J Biol Chem. 1987 Sep 15;262(26):12438–12443. [PubMed] [Google Scholar]
- Fischer E., Lüttge U. Membrane Potential Changes Related to Active Transport of Glycine in Lemna gibba G1. Plant Physiol. 1980 May;65(5):1004–1008. doi: 10.1104/pp.65.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarino J., Dupont F. M. Rapid induction of na/h exchange activity in barley root tonoplast. Plant Physiol. 1989 Jan;89(1):1–4. doi: 10.1104/pp.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homeyer U., Litek K., Huchzermeyer B., Schultz G. Uptake of Phenylalanine into Isolated Barley Vacuoles Is Driven by Both Tonoplast Adenosine Triphosphatase and Pyrophosphatase : Evidence for a Hydrophobic l-Amino Acid Carrier System. Plant Physiol. 1989 Apr;89(4):1388–1393. doi: 10.1104/pp.89.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchzermeyer B., Strotmann H. Acid/base-induced exchange of adenine nucleotides on chloroplast coupling factor (CF1). Z Naturforsch C. 1977 Sep-Oct;32(9-10):803–809. doi: 10.1515/znc-1977-9-1024. [DOI] [PubMed] [Google Scholar]
- Martinoia E., Schramm M. J., Kaiser G., Kaiser W. M., Heber U. Transport of anions in isolated barley vacuoles : I. Permeability to anions and evidence for a cl-uptake system. Plant Physiol. 1986 Apr;80(4):895–901. doi: 10.1104/pp.80.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E., Thume M., Vogt E., Rentsch D., Dietz K. J. Transport of arginine and aspartic Acid into isolated barley mesophyll vacuoles. Plant Physiol. 1991 Oct;97(2):644–650. doi: 10.1104/pp.97.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. J., Neuburger M., Bourguignon J., Douce R. Interaction between the Component Enzymes of the Glycine Decarboxylase Multienzyme Complex. Plant Physiol. 1990 Oct;94(2):833–839. doi: 10.1104/pp.94.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. Solubilization and reconstitution of the oat root vacuolar h/ca exchanger. Plant Physiol. 1990 Feb;92(2):340–345. doi: 10.1104/pp.92.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev V. P. Integrating functions of biomembranes. Problems of lateral transport of energy, metabolites and electrons. Biochim Biophys Acta. 1980 Dec 31;604(3):297–310. doi: 10.1016/0005-2736(80)90576-3. [DOI] [PubMed] [Google Scholar]
- Villalobo A. Reconstitution of ion-motive transport ATPases in artificial lipid membranes. Biochim Biophys Acta. 1990 May 15;1017(1):1–48. doi: 10.1016/0005-2728(90)90176-5. [DOI] [PubMed] [Google Scholar]
- Wang Y., Leigh R. A., Kaestner K. H., Sze H. Electrogenic h-pumping pyrophosphatase in tonoplast vesicles of oat roots. Plant Physiol. 1986 Jun;81(2):497–502. doi: 10.1104/pp.81.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Sze H. Similarities and differences between the tonoplast-type and the mitochondrial H+-ATPases of oat roots. J Biol Chem. 1985 Sep 5;260(19):10434–10443. [PubMed] [Google Scholar]


