Abstract
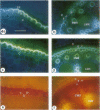
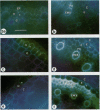
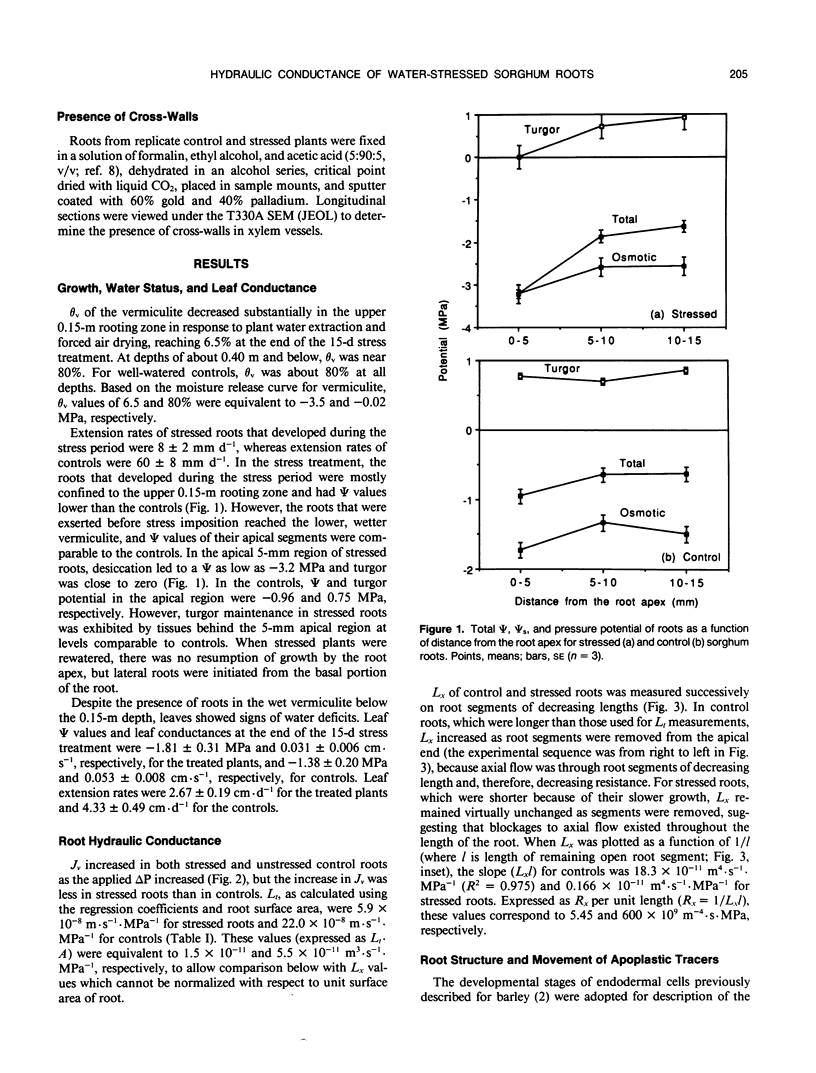
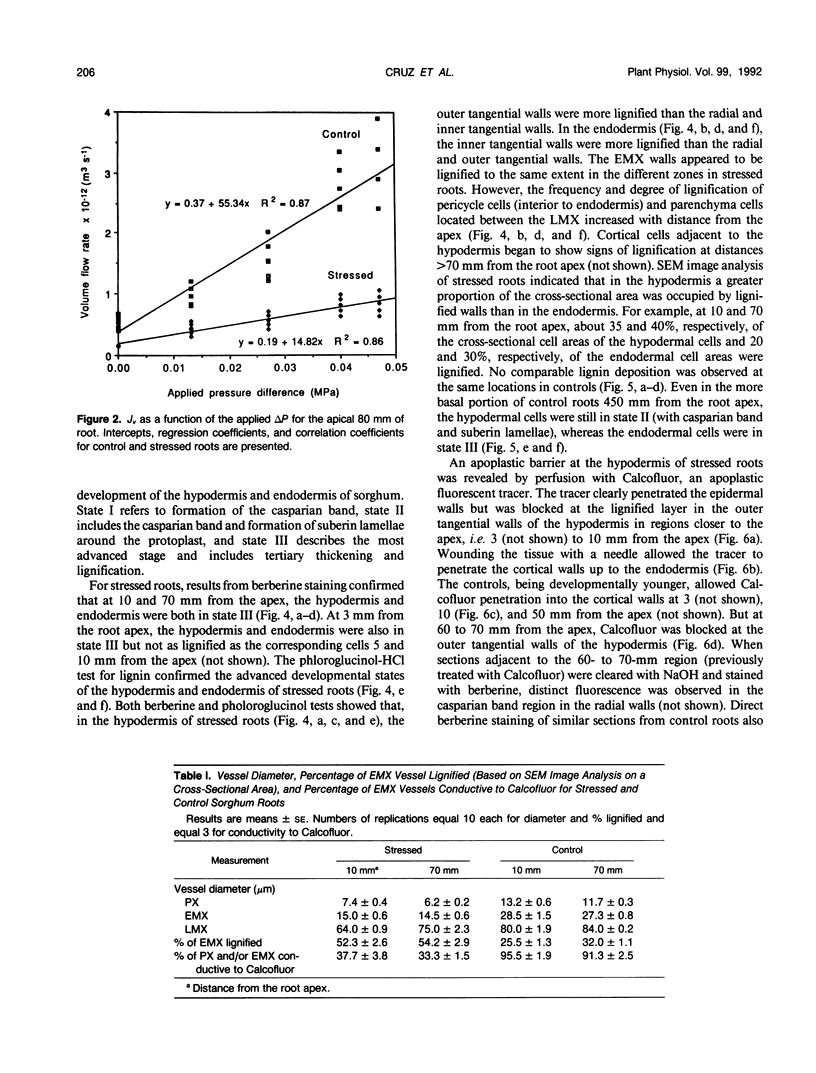
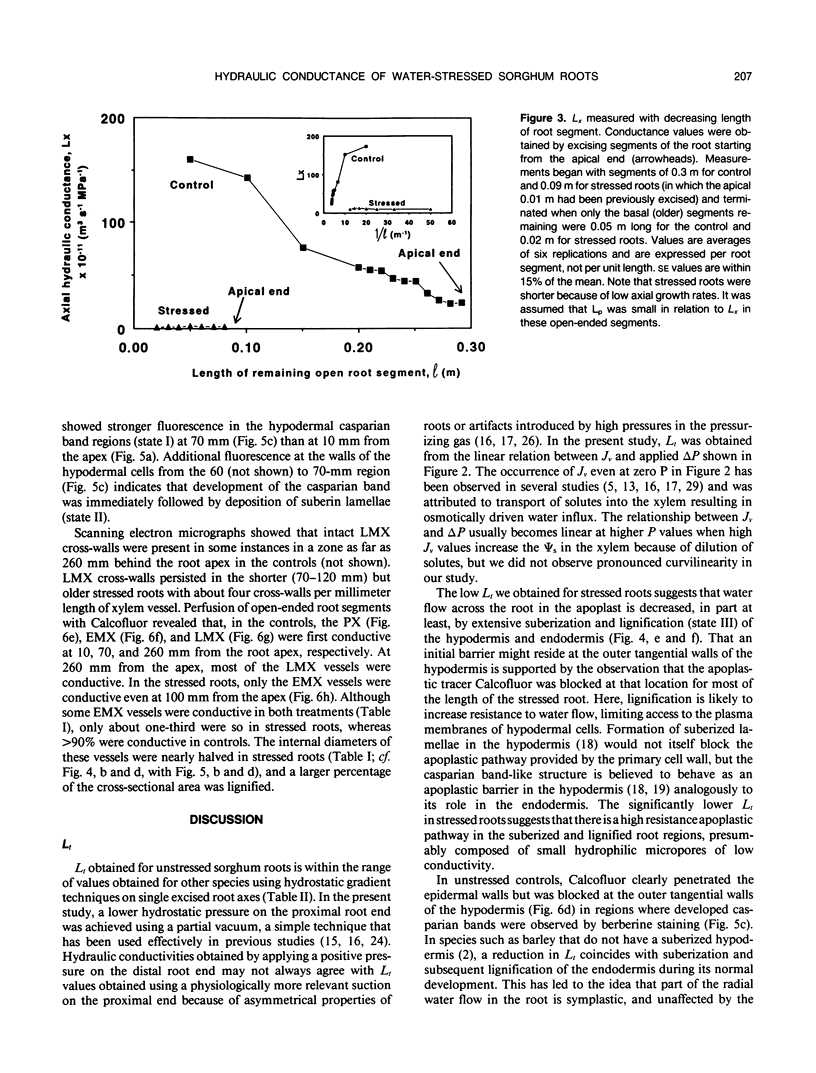
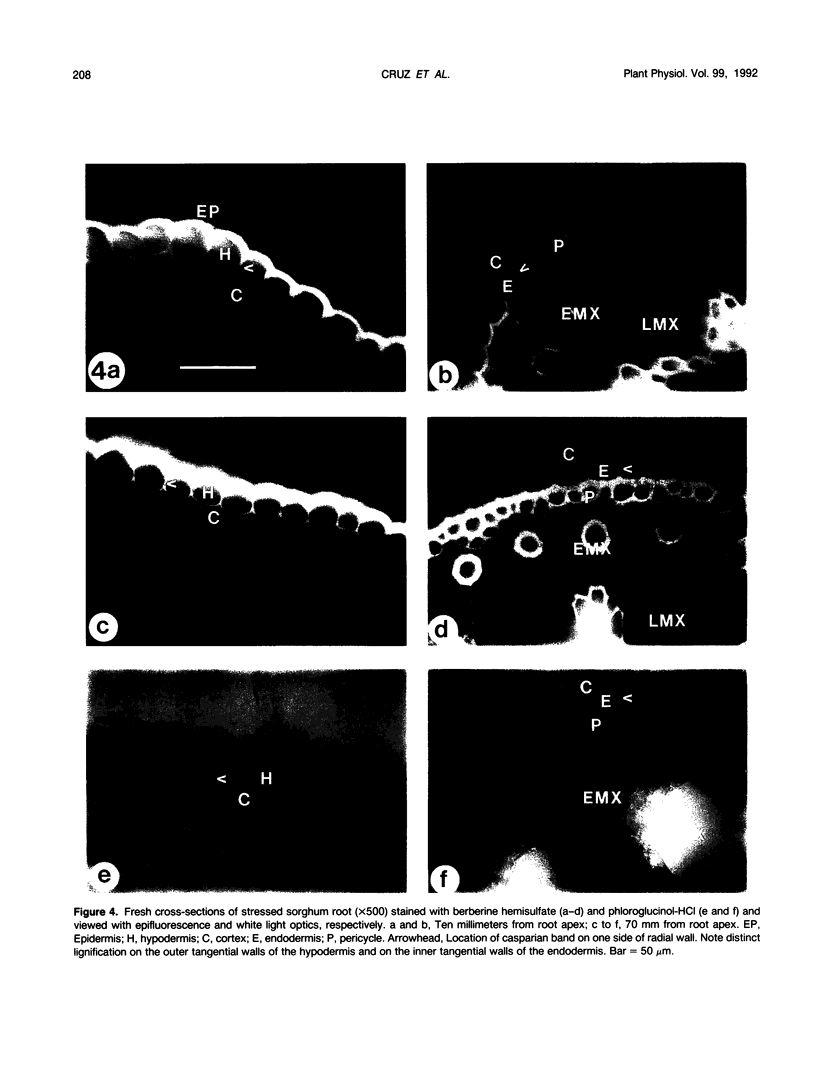
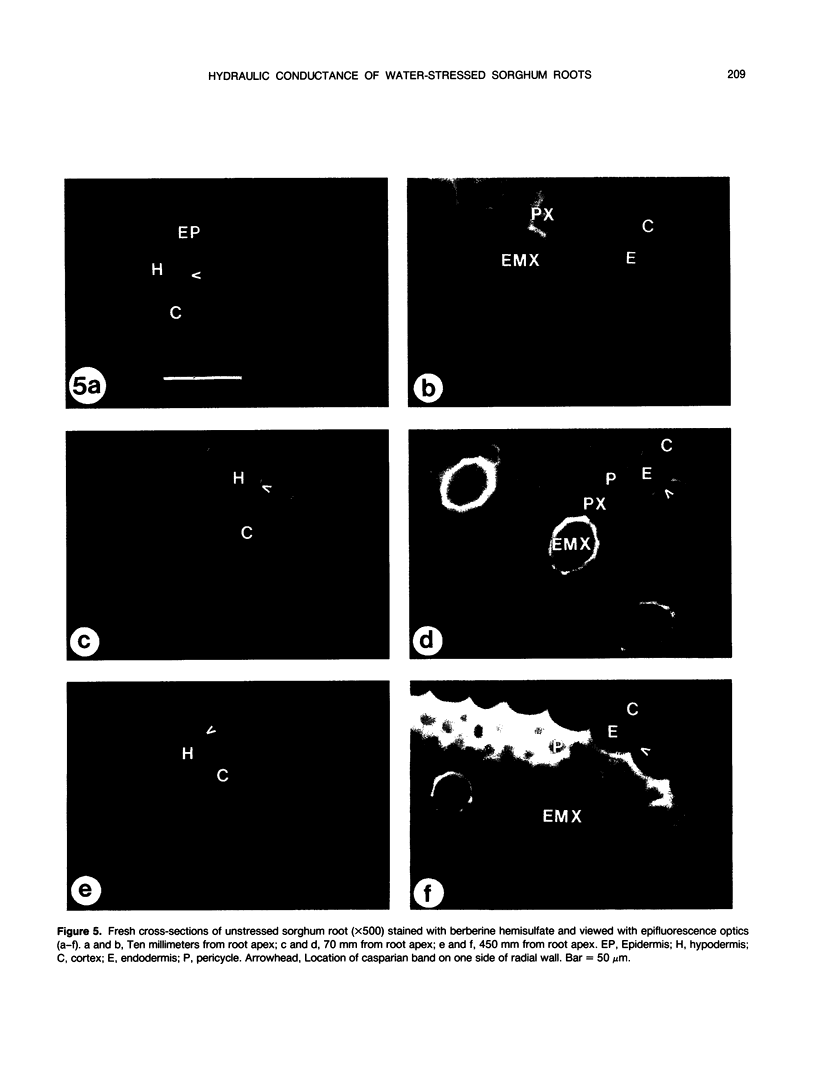
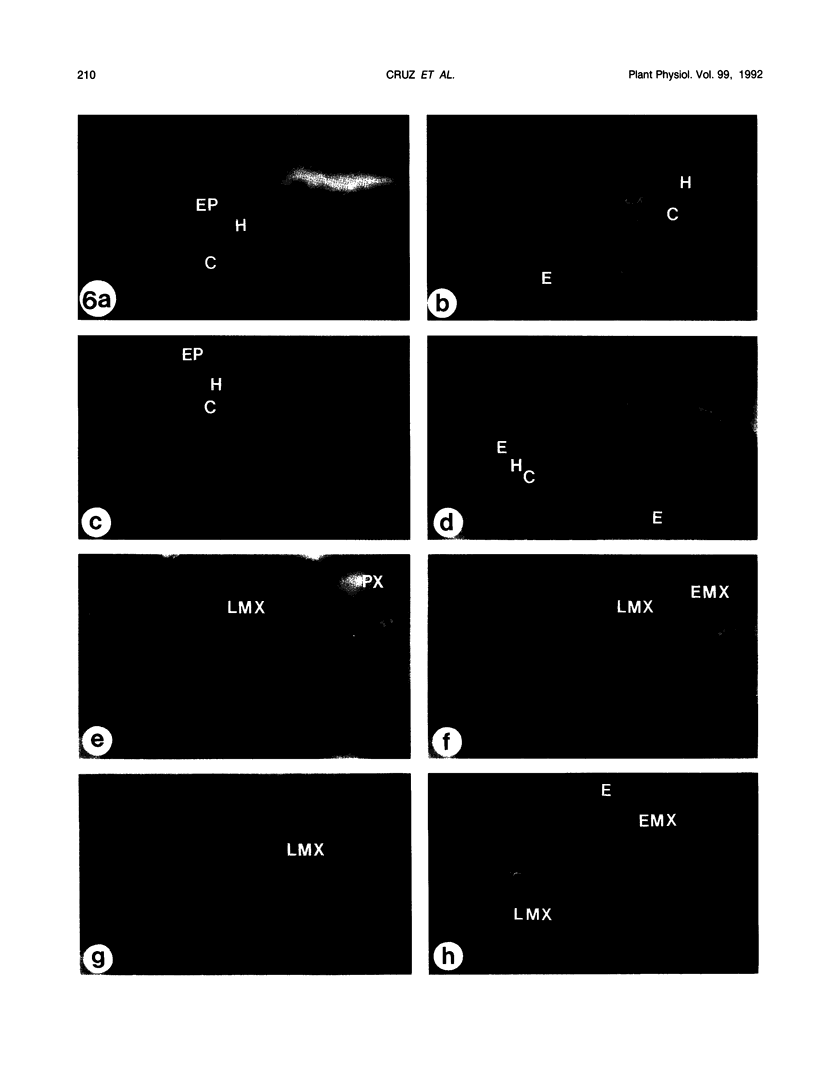
The effects of a severe water deficit on total root (Lt) and axial (Lx) hydraulic conductances and on the development of the hypodermis, endodermis, and xylem were studied in sorghum (Sorghum bicolor L.). Water deficit was imposed in the upper rooting zone while the lower zones were kept moist. Lt and Lx were based on water flow rates obtained by applying suction to proximal xylem ends of excised roots. The development of the hypodermis, endodermis, and other tissues were examined by staining with fluorescent berberine hemisulfate and phloroglucinol-HCl. The Lt value (x 10−8 meters per second per megapascal) for unstressed control roots was 22.0 and only 5.9 for stressed roots. The low Lt in stressed roots was attributed, in part, to accelerated deposition of lignin and suberin in the hypodermis and endodermis. Calcofluor, an apoplastic tracer that binds to cellulose, was blocked in stressed roots at the lignified and suberized outer tangential walls of the hypodermis but readily penetrated the cortical walls of similar root regions in controls where the casparian band was not developed. Lx per unit root length was about 100 times lower in stressed roots than in controls because of the persistence of late metaxylem cross-walls and the smaller diameter and lower number of conductive protoxylem and early metaxylem vessels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Drew M. C., He C. J., Morgan P. W. Decreased Ethylene Biosynthesis, and Induction of Aerenchyma, by Nitrogen- or Phosphate-Starvation in Adventitious Roots of Zea mays L. Plant Physiol. 1989 Sep;91(1):266–271. doi: 10.1104/pp.91.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiscus E. L. The Interaction between Osmotic- and Pressure-induced Water Flow in Plant Roots. Plant Physiol. 1975 May;55(5):917–922. doi: 10.1104/pp.55.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frensch J., Steudle E. Axial and Radial Hydraulic Resistance to Roots of Maize (Zea mays L.). Plant Physiol. 1989 Oct;91(2):719–726. doi: 10.1104/pp.91.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner J. E., Lin L. S. Plant cell wall architecture. Cell. 1989 Jan 27;56(2):231–239. doi: 10.1016/0092-8674(89)90896-9. [DOI] [PubMed] [Google Scholar]
- Wenzel C. L., McCully M. E., Canny M. J. Development of water conducting capacity in the root systems of young plants of corn and some other c4 grasses. Plant Physiol. 1989 Apr;89(4):1094–1101. doi: 10.1104/pp.89.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]