Abstract
Background
The median eating duration in the U.S. is 14.75 h, spread throughout the period of wakefulness and ending before sleep. Food intake at an inappropriate circadian time may lead to adverse metabolic outcomes. Emerging literature suggests that time restricted eating (TRE) may improve glucose tolerance and insulin sensitivity. The aim was to compare 24‐h glucose profiles and insulin sensitivity in participants after completing 12 weeks of a behavioral weight loss intervention based on early TRE plus daily caloric restriction (E‐TRE+DCR) or DCR alone.
Methods
Eighty‐one adults with overweight or obesity (age 18–50 years, BMI 25–45 kg/m2) were randomized to either E‐TRE+DCR or DCR alone. Each participant wore a continuous glucose monitor (CGM) for 7 days and insulin sensitivity was estimated using the homeostatic model assessment of insulin resistance (HOMA‐IR) at Baseline and Week 12. Changes in CGM‐derived measures and HOMA‐IR from Baseline to Week 12 were assessed within and between groups using random intercept mixed models.
Results
Forty‐four participants had valid CGM data at both time points, while 38 had valid glucose, insulin, HOMA‐IR, and hemoglobin A1c (A1c) data at both timepoints. There were no significant differences in sex, age, BMI, or the percentage of participants with prediabetes between the groups (28% female, age 39.2 ± 6.9 years, BMI 33.8 ± 5.7 kg/m2, 16% with prediabetes). After adjusting for weight, there were no between‐group differences in changes in overall average sensor glucose, standard deviation of glucose levels, the coefficient of variation of glucose levels, daytime or nighttime average sensor glucose, fasting glucose, insulin, HOMA‐IR, or A1c. However, mean amplitude of glycemic excursions changed differently over time between the two groups, with a greater reduction found in the DCR as compared to E‐TRE+DCR (p = 0.03).
Conclusion
There were no major differences between E‐TRE+DCR and DCR groups in continuous glucose profiles or insulin sensitivity 12 weeks after the intervention. Because the study sample included participants with normal baseline mean glucose profiles and insulin sensitivity, the ability to detect changes in these outcomes may have been limited.
Keywords: behavioral strategies, caloric restriction, continuous glucose monitoring, insulin sensitivity, time restricted eating, weight management
The aim was to compare 24‐h glucose profiles and insulin sensitivity in 44 adult participants with overweight or obesity completing a 12‐week behavioral intervention based on early time restricted eating plus daily caloric restriction (E‐TRE+DCR) or DCR alone. After adjusting for weight loss from the intervention, there were no between‐group differences in changes in overall average sensor glucose, standard deviation of glucose levels, the coefficient of variation of glucose levels, daytime or nighttime average sensor glucose, fasting glucose, insulin, homeostatic model of insulin resistance, or hemoglobin A1c although mean amplitude of glycemic excursions changed differently over time between the two groups, with a greater reduction found in the DCR as compared toE‐TRE+DCR (p = 0.03). Because the study sample included participants with normal baseline mean glucose profiles and insulin sensitivity, the ability to detect changes in these outcomes may have been limited.
1. INTRODUCTION
Dietary patterns have changed over recent decades, with a shift toward more frequent eating over a longer period throughout the day. 1 , 2 Recent data suggest that the median eating duration in US adults is 14.75 h, with many individuals exhibiting a pattern of eating episodes occurring throughout wakefulness and cessation of eating only during sleep. 2 Because metabolic processes are entrained to circadian rhythms, food intake at an inappropriate circadian time may lead to adverse metabolic outcomes, including the development of obesity, type 2 diabetes, and cardiovascular disease. 3 , 4 Indeed, data from studies of shift workers have consistently shown that night shift workers are at higher risk for overweight/obesity, metabolic syndrome, hypertension, type 2 diabetes, and cardiovascular disease. 5 Restricting feeding to an 8–12‐h window of time during the biological day has been shown to restore circadian rhythmicity in peripheral metabolic organs, improve glucose tolerance, and reduce hepatosteatosis in animal models of diet‐induced obesity. 6 , 7 Further, human studies of short‐term, eucaloric time restricted eating (TRE, <5 weeks with caloric intake matched to energy requirements) have demonstrated improvements in glucose homeostasis, insulin sensitivity, β‐cell responsiveness, increased fat oxidation, and reductions in blood pressure as well as appetite. 8 , 9 , 10 , 11 , 12 , 13 , 14 A number of studies have also evaluated the impact of TRE on glycemic parameters using a 24‐h continuous glucose monitor (CGM) and have found favorable effects of TRE on mean glucose, time‐in‐range, glycemic variability, and glucose excursions. 8 , 9 , 15 , 16 , 17 , 18 , 19 The TRE interventions in these studies utilized a variety of eating windows (timing and duration) and the interventions ranged from 4 days to 12 weeks in length. However, none of these studies provided recommendations on calorie restriction or implemented guidelines‐based behavioral support for weight loss. 20
In contrast, the authors recently completed a 39‐week trial comparing the effects of early TRE plus daily caloric restriction (E‐TRE+DCR) to DCR alone, with all participants receiving a group‐based behavioral weight loss intervention. They found that both groups lost significant weight at 12 weeks as compared to baseline (E‐TRE+DCR −6.3% and DCR −5.5%) with minimal weight regain at week 39 (E‐TRE+DCR −5.2% and DCR −4.7%), with no between‐group differences in weight loss. 21 Therefore, the primary aim of this exploratory secondary analysis was to compare 24‐h glucose profiles and insulin sensitivity in participants after completing 12 weeks of a behavioral weight loss intervention based on E‐TRE+DCR or DCR alone. The hypothesis was that glycemic outcomes would improve in both groups with weight loss, but that there would be greater improvements in the E‐TRE+DCR group. The authors additionally explored associations between measures of glucose and measures of both body composition and meal timing at baseline.
2. MATERIALS AND METHODS
2.1. Participants
Adults aged 18–50 years with a BMI of 27–45 kg/m2 and weight stable (≤5% change by self‐report over the previous 6 months) with a self‐reported typical eating duration >12 h per day were recruited for a behavioral weight loss trial from the University of Colorado Anschutz Medical Campus and surrounding community. The Colorado Multiple Institutional Review Board approved the study protocol and all participants provided written informed consent prior to participation (approval code: 18‐0487, approval date: 5/7/2018). This study was conducted in accordance with the principles expressed in the Declaration of Helsinki. Clinicaltrials.gov: NCT03571048.
2.2. Study design
The present study is a secondary analysis focusing on the first 12 weeks of a 39‐week randomized clinical trial that has been previously published. 21 Methods relevant to this secondary analysis are briefly described below.
2.2.1. Screening, baseline, and randomization
All participants completed screening labs and were evaluated by the study physician prior to the collection of baseline measures. Baseline assessments relevant to this secondary analysis included body composition, physical activity, 24‐h glucose profiles, and fasting insulin sensitivity (described below). Following baseline assessments, participants were randomized 1:1 to E‐TRE+DCR or DCR.
2.2.2. Group‐based behavioral weight loss intervention
The randomized groups met separately and were led by registered dietitians who met weekly with participants during the first 12 weeks of the behavioral weight loss program. The curriculum for the intervention was based on the PreventT2 curriculum and utilized a skills‐based approach and cognitive behavioral strategies for lifestyle modification with a dietary focus on daily caloric restriction. 22 Participants in both groups were given personalized calorie goals based on their measured resting energy expenditure (indirect calorimetry) reduced by 10% (∼35% caloric restriction). Participants in the DCR group were not given any specific instruction regarding timing of food intake, whereas participants in the E‐TRE+DCR group were instructed to eat only during a window of 10 h, starting within 3 h of waking. Volunteers in both groups were counseled on the importance of physical activity for weight loss and received a recommendation to perform 150 min/week of moderate intensity physical activity.
2.2.3. COVID‐19 related intervention modification
Participants were recruited and enrolled in 3 cohorts between July 2018 and February 2020. Cohort 1 (n = 29) and Cohort 2 (n = 26) completed all study measures prior to the start of the 2020 COVID‐19 pandemic in the United States. Cohort 3 (n = 26) started the intervention in February 2020 and was at Week 6 of the intervention when a stay‐at‐home order was issued in Colorado. The behavioral weight loss intervention was moved to a secure virtual platform and Week 12 assessments of body composition, 24‐h glucose levels and insulin sensitivity were not performed due to restrictions on in‐person research. Therefore, only data from Cohorts 1 and 2 are included in this analysis.
2.3. Assessments
Changes in CGM profiles and insulin sensitivity were the primary goals of this secondary analysis and these measures were performed at baseline and week 12.
2.3.1. 24‐h glucose levels
Free‐living plasma glucose levels were measured using a continuous glucose monitoring system (FreeStyle LibrePro) for 7 days. The device consists of a sensor applied to the back of the patient's upper arm that measures glucose in interstitial fluid every 15 min. Participants were blinded to continuous plasma glucose levels.
2.3.2. Management of implausible continuous glucose monitor data
The researchers used previously published criteria by Shah et al. to flag and exclude implausible glucose data from our CGM output. 23 Implausible data were defined in the following ways 1 : any low CGM readings <50 mg/dL (2.8 mmol/L) during sleep time as defined by the CGM logs when the participant was likely to be lying on the CGM sensor, 2 any low CGM readings <50 mg/dL on the first day of sensor wear for each participant, 3 any strings (three or more consecutive readings) of CGM‐measured lows <50 mg/dL flanked by CGM readings ≥80 mg/dL within 10 min before and after the string, and 4 any strings during which readings of >200 mg/dL were interspersed with readings of <70 mg/dL. Implausible CGM outputs were handled as missing data.
2.3.3. Insulin sensitivity
Blood samples were obtained at Baseline and Week 12 and were analyzed at the Colorado Clinical and Translational Sciences Institute Core Lab. Glucose was measured using an ultraviolet hexokinase assay and insulin was measured via chemiluminescent immunoassay. 24 , 25 A Homeostasis Model Assessment of Insulin Resistance (HOMA‐IR) was calculated using the formula: fasting serum insulin (μU/ml) × fasting plasma glucose (mmol/L)/22.5. 26 Hemoglobin A1c (A1c) was additionally measured using potassium ferricyanide. 27
A secondary goal of the present analysis was to explore the associations among glycemic parameters, body composition, physical activity, and meal timing at Baseline. Methodology for the assessments relevant to this analysis conducted at Baseline and Week 12 are as follows:
2.3.4. Body composition
Participants underwent measurement of body composition by dual x‐ray absorptiometry (Hologic Inc.).
2.3.5. Physical activity
Activity levels and postural changes were measured over 7 days, 24 h/day using ActivPAL (PALTechnologies). The ActivPAL was placed on the participant's anterior thigh and accelerometer‐derived information was used to spent in different body positions. A time‐stamped “event” data file was used to determine the time spent sitting, standing, and stepping per day. The data file also estimated daily energy expenditure expressed in metabolic equivalents (METs) per hour.
2.3.6. Meal timing
Participants used personal smartphones to take pictures before and after consuming all calorie‐containing food and beverages during the 7‐day period and then texted these photographs directly to a secure account that was monitored in real time by study personnel. 28 The timestamps on the photographs were used to estimate the frequency and timing of energy intake across the daily eating window. For the purpose of quantifying the timing of energy intake, days were considered to start at 4 AM and include the following 24 h through 4 AM on the next day. Valid days were defined as those with at least two eating episodes at least 5 h apart. Invalid days were excluded from the analysis.
2.4. Sample size determination and power analysis
Assuming 80% power and a 5% significance level, the main trial aimed to randomize 80 participants in order to detect an effect size of 0.63 Cohen's d. 21 Given a standard deviation of 9.9 for average glucose, a corresponding detectable difference in a 6.24 mg/dL change in average glucose existed between the groups. However, due to COVID‐19 interruptions, only n = 44 children were included in this analysis. With this sample size, there was 80% power to detect an effect size of 0.87 Cohen's d, corresponding to a detectable difference in a 8.61 mg/dL change in average glucose.
2.5. Statistical analysis
Continuous glucose monitor variables were derived using the “cgm analysis” package for R (R Foundation for Statistical Computing). 29 Two‐sample t‐tests, Chi‐square tests, or Fisher's exact tests were used to assess differences in baseline characteristics by randomized group. Random intercept mixed models with unstructured covariance matrices were used to assess whether there were between group and within group differences in various measures of glucose. These models included time, randomized group, and time by randomized group interactions, as well as an adjustment for weight. Additionally, Spearman correlations were included for associations between measures of glucose and measures of body composition, physical activity, and meal timing at Baseline. Finally, rank sum tests were used to assess whether measures of glucose differed between those who lost ≥5% of their body weight and those who did not. Because of small sample sizes, additional analyses of changes in glycemic parameters and insulin sensitivity were not performed among participants with prediabetes at baseline (n = 7). Due to the exploratory nature of these analyses, p‐values were not adjusted for multiple testing.
3. RESULTS
3.1. Participants
Out of the 95 participants who were assessed for eligibility, N = 85 individuals were enrolled and N = 81 started the 12‐week intervention. Since volunteers in Cohort 3 were affected by COVID‐19‐related restrictions on in‐person research, they did not have 24‐h glucose levels and insulin sensitivity performed at Week 12. Therefore, only data from Cohorts 1 and 2 are included in the present analysis and include n = 44 participants who had complete CGM data and n = 38 participants who had complete glycemic parameter data (Figure 1). Baseline demographic characteristics of the participants from Cohorts 1 and 2 included in this study are shown in Table 1. There were no differences in age, sex, body composition, or prediabetes status at baseline, but there was a higher proportion of white participants in the E‐TRE+DCR group compared with DCR (p = 0.042). Differences in weight, body composition, eating behaviors, energy intake, physical activity and sedentary behavior, and sleep in all participants who started the intervention—including a separate secondary analysis on how these parameters were affected by COVID‐19—were previously reported. 21 , 30 Amongst all 3 cohorts, the E‐TRE+DCR group had an eating duration 1.6 (95% CI: −2.6, −1.0, p < 0.001) hours shorter than DCR group at Week 12, and the E‐TRE+DCR group ate their last meal 0.93 (95% CI: −1.59, −0.27) hours earlier, on average, than those in DCR (p = 0.007). 21
FIGURE 1.
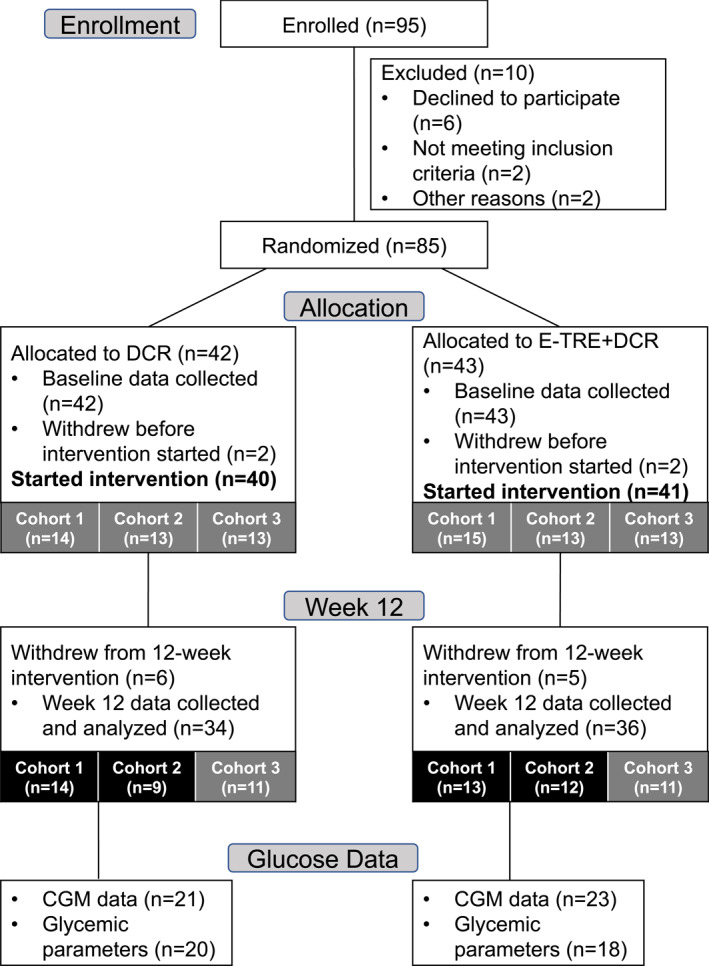
Flowchart of participants included in this analysis from the main study. Eighty‐five participants were randomized to the parent trial, but only 81 individuals began the intervention. Cohort 1 (n = 29) and Cohort 2 (n = 26) completed all study measures prior to the start of the 2020 COVID‐19 pandemic in the United States. Cohort 3 (n = 26) started the intervention in February 2020 and had to move their participation to a virtual platform starting week 6 given restrictions on in‐person research. 24‐h glucose levels and insulin sensitivity were not performed in Cohort 3. Therefore, only data from Cohorts 1 and 2 are included in the present analysis, which includes 44 individuals with valid CGM data and 38 individuals with valid glycemic parameter data. CGM, continuous glucose monitor.
TABLE 1.
Participant characteristics at baseline.
| DCR (n = 21) | E‐TRE+DCR (n = 23) | p‐value | |
|---|---|---|---|
| Age (years) | 38.3 (7.1) | 39.9 (6.7) | 0.440 |
| Height (m) | 1.6 (0.1) | 1.7 (0.1) | 0.755 |
| Weight (kg) | 88.4 (18.4) | 95.4 (19.5) | 0.233 |
| BMI (kg/m2) | 32.5 (4.7) | 34.8 (6.4) | 0.182 |
| Sex—n (%) | 0.348 | ||
| Male | 1 (4.8) | 4 (17.4) | |
| Female | 20 (95.2) | 19 (82.6) | |
| Race—n (%) | 0.042 | ||
| Other | 9 (42.9) | 3 (13.0) | |
| White | 12 (57.1) | 20 (87.0) | |
| Prediabetes—n (%) | 0.448 | ||
| No | 16 (76.2) | 20 (87.0) | |
| Yes | 5 (23.8) | 3 (13.0) |
Note: Mean (standard deviation) are presented. p‐values were calculated using two sample t‐tests, Chi‐Square tests or Fisher's exact tests when appropriate. Bold values indicates statistical significance p < 0.05.
3.2. Mealtiming and eating duration of participants in cohorts 1 and 2
Among those with valid meal timing data in this secondary analysis, Baseline median (interquartile range, IQR) first mealtimes were 08:42 (07:41–09:15) for E‐TRE+DCR and 09:00 (07:30–10:12) for DCR, and Baseline last mealtimes were 20:09 (19:20–20:31) for E‐TRE+DCR and 18:45 (17:43–20:07) for DCR. At Week 12, the first mealtimes were 09:58 (08:36–10:17) for E‐TRE+DCR and 09:24 (07:20–10:27) for DCR, while the last mealtimes were 18:53 (17:48–19:22) for E‐TRE+DCR and 18:53 (18:20–19:45) for DCR. The median (IQR) eating duration for E‐TRE+DCR was 11:53 h (10:25–12:29 h) at Baseline and 09:17 h (08:29–09:46 h) at Week 12, while the eating duration for the DCR group was 10:45 h (09:52–11:43 h) at Baseline and 10:28 h (08:54–11:19 h) at Week 12. 21
3.3. Correlations between glycemic parameters and body composition, physical activity, and meal timing at baseline
Correlations between baseline CGM parameters and glycemic measures with body composition, physical activity, and meal timing were assessed (Figure 2). No significant correlations existed between CGM profiles and body composition, physical activity, or meal timing. However, there were significant correlations between insulin sensitivity, physical activity, and meal timing. Specifically, there were significant negative correlations between the HOMA‐IR and step count (r = −0.36, p = 0.022), and HOMA‐IR and METs (r = −0.36, p = 0.023). There were also significant positive correlations between HOMA‐IR and weight (r = 0.41, p = 0.009), fat mass (r = 0.52, p < 0.001), and the time of the first meal (r = 0.41, p = 0.012). Similarly, there were significant negative correlations between insulin and step count (r = −0.39, p = 0.015) and METs (r = −0.39, p = 0.015), and there were significant positive correlations between insulin and weight (r = 0.43, p = 0.007), fat mass (r = 0.52, p < 0.001), and the first mealtime (r = 0.43, p = 0.009). Finally, there was a significant positive correlation between A1c and fat mass (r = 0.33, p = 0.027).
FIGURE 2.
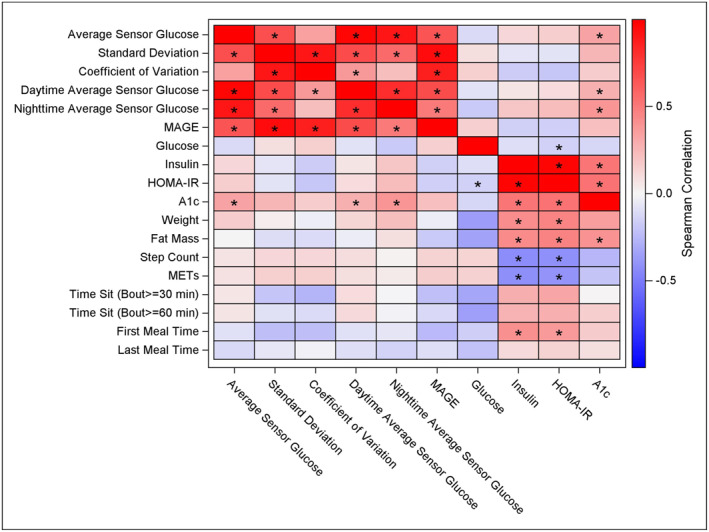
Spearman correlations of Baseline parameters. Spearman correlations of glucose and measures of body composition, physical activity, and meal timing at Baseline. Statistically significant correlations (p < 0.05) are marked with an asterisk. A1c, hemoglobin A1c; HOMA‐IR, homeostatic model assessment of insulin resistance; MAGE, mean amplitude of glycemic excursions; METs, metabolic equivalents.
3.4. Continuous glucose profile
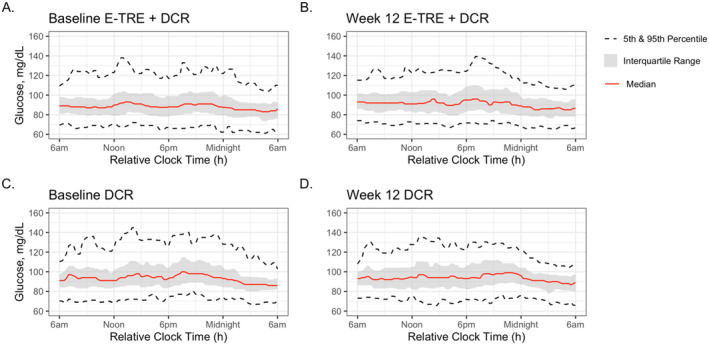
Continuous glucose profiles of the E‐TRE+DCR and DCR groups pre‐ and post‐intervention are shown in Figure 3. At Baseline, the 24‐h average sensor glucose, glucose standard deviation, nighttime average sensor glucose, mean amplitude of glycemic excursions of glucose, and A1c were all higher in DCR as compared to E‐TRE+DCR (p < 0.05 for all parameters, Table 2). Among those in DCR, there was a significant decrease from Baseline to Week 12 in glucose standard deviation, coefficient of variation, and mean amplitude of glycemic excursions (p < 0.05 for all parameters, Figure 4B). Among those in E‐TRE+DCR, there were no significant changes across the 12‐week intervention (Table 3). There was a significant difference in the change in mean amplitude of glycemic excursions across the 12‐week period between the groups (p = 0.03), with a greater reduction in mean amplitude of glycemic excursions in the DCR as compared to the E‐TRE+DCR. Average sensor glucose, glucose standard deviation, nighttime average sensor glucose, and A1c did not change within or between the groups over the 12‐week duration of this study.
FIGURE 3.
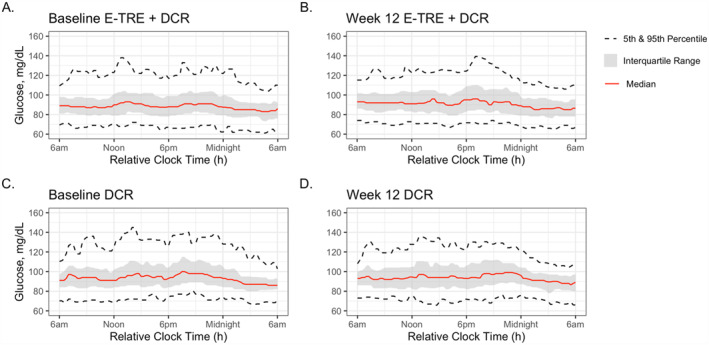
Continuous glucose tracings. Continuous glucose tracings of the E‐TRE+DCR and DCR groups at Baseline and after the 12‐week intervention.
TABLE 2.
Baseline continuous glucose and insulin sensitivity parameters.
| DCR | E‐TRE+DCR | p‐value | |
|---|---|---|---|
| Average sensor glucose (mg/dL) | 95.8 (9.0) | 90.5 (10.2) | 0.042 |
| Standard deviation (mg/dL) | 15.8 (3.6) | 13.6 (3.9) | 0.034 |
| Coefficient of variation | 0.16 (0.03) | 0.15 (0.03) | 0.122 |
| Daytime average sensor glucose (mg/dL) | 97.0 (8.6) | 92.0 (10.3) | 0.067 |
| Nighttime average sensor glucose (mg/dL) | 92.8 (11.9) | 87.3 (10.9) | 0.032 |
| MAGE (mg/dL) | 35.2 (8.6) | 29.1 (9.6) | 0.018 |
| Glucose (mg/dL) | 77.2 (16.9) | 81.2 (7.0) | 0.146 |
| Insulin (µIU/mL) | 7.8 (4.9) | 8.3 (4.8) | 0.989 |
| HOMA‐IR | 1.6 (1.1) | 1.7 (1.1) | 0.965 |
| A1c | 5.4 (0.3) | 5.2 (0.3) | 0.042 |
Note: p‐value calculated from weight‐adjusted mixed model. Mean (standard deviation) are presented. Bold values indicates statistical significance p < 0.05.
Abbreviations: A1c, hemoglobin A1c; HOMA‐IR, homeostatic model assessment of insulin resistance; MAGE, mean amplitude of glycemic excursions.
FIGURE 4.
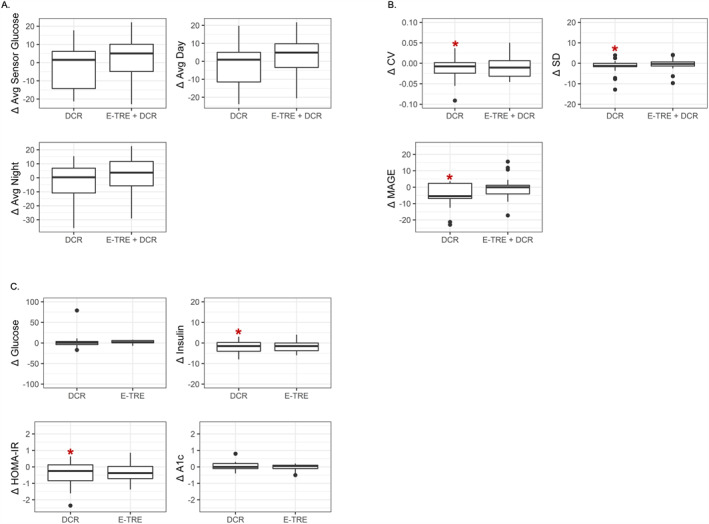
Change in glucose and insulin variables from Baseline to Week 12. Changes are shown for average changes in (A) average overall glucose, average glucose during the day, average glucose during the night, and day/night ratio; (B) coefficient of variation, standard deviation, and mean amplitude of glycemic excursions; and (C) glucose, insulin, homeostatic model assessment of insulin resistance (HOMA‐IR), and A1c. Boxplots show the median, 25th and 75th percentiles (IQR). Whiskers are 1.5× IQR. *Statistically significant change from baseline (p < 0.05 extracted from mixed models adjusting for weight).
TABLE 3.
Measures of continuous glucose and insulin sensitivity parameters for both randomized groups.
| Variable | DCR baseline | DCR 12 weeks | Change | p‐value a | E‐TRE baseline | E‐TRE 12 weeks | Change | p‐value a | p‐value b |
|---|---|---|---|---|---|---|---|---|---|
| Average sensor glucose | 95.8 (9.0) | 93.2 (9.2) | −2.5 (12.0) | 0.430 | 90.5 (10.2) | 92.7 (11.1) | 2.1 (11.9) | 0.278 | 0.187 |
| Standard deviation | 15.8 (3.6) | 14.0 (2.7) | −1.8 (3.7) | 0.022 | 13.6 (3.9) | 13.0 (3.0) | −0.6 (3.0) | 0.474 | 0.228 |
| Coefficient of variation | 0.16 (0.03) | 0.15 (0.03) | −0.01 (0.03) | 0.041 | 0.15 (0.03) | 0.14 (0.03) | −0.01 (0.03) | 0.177 | 0.566 |
| Daytime average sensor glucose | 97.0 (8.6) | 94.4 (10.8) | −2.6 (11.9) | 0.396 | 92.0 (10.3) | 94.6 (11.5) | 2.7 (11.2) | 0.195 | 0.131 |
| Nighttime average sensor glucose | 92.8 (11.9) | 90.5 (7.3) | −2.3 (13.4) | 0.564 | 87.3 (10.9) | 88.3 (10.7) | 1.0 (13.8) | 0.533 | 0.396 |
| MAGE | 35.2 (8.6) | 29.8 (6.5) | −5.4 (7.3) | 0.002 | 29.1 (9.6) | 28.6 (8.3) | −0.6 (7.0) | 0.801 | 0.030 |
| Glucose | 77.2 (16.9) | 80.4 (6.8) | 3.2 (19.3) | 0.386 | 81.2 (7.0) | 83.7 (5.5) | 2.2 (4.3) | 0.532 | 0.879 |
| Insulin | 7.8 (4.9) | 5.9 (3.7) | −1.9 (3.2) | 0.022 | 8.3 (4.8) | 6.8 (3.3) | −1.8 (2.8) | 0.057 | 0.817 |
| HOMA‐IR | 1.6 (1.1) | 1.2 (0.7) | −0.4 (0.7) | 0.025 | 1.7 (1.1) | 1.4 (0.7) | −0.3 (0.6) | 0.111 | 0.666 |
| A1c | 5.4 (0.3) | 5.4 (0.4) | 0.0 (0.3) | 0.234 | 5.2 (0.3) | 5.2 (0.4) | 0.0 (0.2) | 0.601 | 0.618 |
Note: Mean (standard deviation) are presented. Bold values indicates statistical significance p < 0.05.
Abbreviations: A1c, hemoglobin A1c; HOMA‐IR, homeostatic model assessment of insulin resistance; MAGE, mean amplitude of glycemic excursions; METs, metabolic equivalents.
p‐value calculated from weight‐adjusted mixed model tests whether baseline differs from Week 12 within each randomized group.
p‐value calculated from randomized group × time interaction of weight‐adjusted mixed model tests whether the changes in each outcome from baseline to week 12 differed by randomized group.
3.5. Insulin sensitivity
There were no differences between DCR and E‐TRE+DCR in fasting glucose levels, insulin levels, or insulin sensitivity by HOMA‐IR at Baseline. There was a significant decrease in HOMA‐IR in the DCR group between Baseline and Week 12, which was driven by a significant decrease in insulin (p < 0.05, Figure 4C). These glycemic parameters did not change significantly in the E‐TRE+DCR group. The changes in fasting glucose, insulin levels, and HOMA‐IR did not statistically differ between the groups between Baseline and Week 12 (Table 3).
3.6. Changes in glycemic parameters stratified by weight loss
In a post‐hoc analysis, there were no differences in the changes in glucose variables, insulin, or HOMA‐IR from Baseline to Week 12 in participants who lost 5% or more body weight versus those who did not (Table 4).
TABLE 4.
Changes in continuous glucose and insulin sensitivity measures stratified by weight loss over 12 weeks.
| Lost <5% or gained weight (n = 25) | Lost ≥5% (n = 19) | p‐value | |
|---|---|---|---|
| Change in average sensor glucose | 2.4 (−4.8, 7.4) | 1.5 (−14.2, 9.9) | 0.742 |
| Change in SD | −0.5 (−1.4, 0.5) | −1.2 (−1.6, 0.6) | 0.452 |
| Change in CV | −0.0 (−0.0, 0.0) | −0.0 (−0.0, 0.0) | 0.573 |
| Change in daytime average sensor glucose | 2.1 (−5.4, 8.0) | 0.9 (−11.5, 9.4) | 0.869 |
| Change in nighttime average sensor glucose | 2.7 (−4.3, 9.1) | 1.6 (−17.2, 12.4) | 0.605 |
| Change in MAGE | −3.1 (−6.7, 2.7) | −2.9 (−6.9, 0.8) | 0.605 |
| Change in glucose | 1.5 (−4.0, 7.0) | 1.5 (−2.0, 3.0) | 0.860 |
| Change in insulin | −2.0 (−4.0, 0.0) | −0.5 (−5.0, 0.5) | 0.712 |
| Change in HOMA‐IR | −0.4 (−0.7, 0.1) | −0.1 (−1.0, 0.1) | 0.826 |
| Change in A1c | 0.1 (0.0, 0.2) | −0.1 (−0.1, 0.1) | 0.137 |
Note: Median (IQR) presented. Differences in change variables between those who lost 5% or more weight versus those who did not were assessed using Wilcoxon rank sum tests.
Abbreviations: A1c, hemoglobin A1c; HOMA‐IR, homeostatic model assessment of insulin resistance; MAGE, mean amplitude of glycemic excursions.
4. DISCUSSION
The primary goal of this secondary analysis was to compare 24‐h glucose profiles and insulin sensitivity in participants completing 12 weeks of a behavioral weight loss intervention based on E‐TRE+DCR or DCR alone. In contrast to prior studies that showed improvements in glycemic outcomes with short‐term TRE, there were no clinically significant differences between E‐TRE+DCR and DCR in glucose profiles or insulin sensitivity after 12 weeks of the dietary interventions. Due to COVID‐19‐related interruptions in clinical research, the current analysis includes only a subset of participants in whom there were unexpected baseline between‐group differences. Specifically, there were fewer white participants and higher 24‐h average sensor glucose, standard deviation, nighttime average sensor glucose, mean amplitude of glycemic excursions, and A1c in DCR as compared to E‐TRE+DCR. Glucose parameters are expected to improve with weight loss in individuals with abnormal glucose levels or insulin resistance at baseline. Indeed, among the DCR, there were reductions in HOMA‐IR, mean amplitude of glycemic excursions, glucose standard deviation, and coefficient of variation, whereas there were no significant changes in the E‐TRE+DCR. The only statistically significant difference between groups was a greater reduction in mean amplitude of glycemic excursions in the DCR as compared to the E‐TRE+DCR, which is of uncertain clinical significance and was likely driven by the higher mean amplitude of glycemic excursions in the DCR at baseline. The generalizability of the present findings may also be limited due to the small number of males included in the analyses (Table 1). Additionally, the ability to detect significant differences between the groups was likely due to the reduction in the sample size from the original study.
Time restricted eating is a relatively new dietary strategy that has been gaining popularity for the treatment of obesity and related cardiometabolic complications. The literature to date has shown an overall beneficial trend of TRE on glycemic parameters, 8 , 9 , 15 , 17 , 18 , 19 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 although these findings have not been universal. 11 , 16 , 32 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 The improvements in fasting glucose, insulin resistance, and insulin sensitivity that have been observed in studies have often been attributed to calorie restriction and weight loss, 8 , 31 , 46 , 52 , 53 , 54 , 55 though there are a handful of TRE trials that demonstrated a positive effect of TRE on glucose metabolism in the absence of significant changes in energy intake or weight. 9 , 10 , 32 , 33 , 34 , 35 , 36 , 37 The conflicting results of these studies may be related to variable timing and duration of eating windows, short duration of intervention, and small sample sizes in many of the studies.
Previous studies have shown improvements in glycemic outcomes with weight loss from TRE only in those with impairments at baseline. For example, in a recent study by Manoogian et al. 13 A1c, fasting glucose, fasting insulin, and HOMA‐IR did not change over 12 weeks in 137 firefighters working 24‐h shifts who followed TRE. However, the subset of TRE participants who had impaired fasting glucose or HOMA‐IR at baseline had significant improvements in these parameters in comparison to the control group participants who had similar metabolic abnormalities at study start. Similarly, in a 2020 meta‐analysis by Moon et al. that included 10 studies of TRE, 56 the five studies that included participants with a metabolic abnormality all showed a significant change in fasting glucose, whereas the five studies with healthy participants did not show any significant change in this parameter. In contrast, in Xie et al's 2022 study examining TRE in healthy volunteers without obesity, participants randomized to E‐TRE showed significant improvements in both fasting plasma glucose and HOMA‐IR compared to controls over 5 weeks. 57 The discrepancy in the results between these three studies may have been due to the large variability in time windows used in the TRE arms.
This trend toward improvement in glycemic parameters has also been demonstrated in at least 8 studies using continuous glucose monitoring. 8 , 9 , 15 , 16 , 17 , 18 , 19 , 31 For example, the randomized cross‐over studies by Jamshed et. al 9 and Andriessen et al. 17 both showed a reduction in the 24‐h mean glucose when participants followed TRE. This trend toward a lower 24‐h mean glucose was also notable in studies by Wilkinson et al. 16 and Zhao et al. 18 At least 6 of these studies commented on lowered fasting glucose trends in the TRE group. 8 , 9 , 16 , 17 , 18 , 31 However, it is important to note that the eating windows were of various lengths and had different starting or stopping times. Interestingly, studies by Jones et al. 15 and Haganes et al. 19 showed decreases in nocturnal glucose in the TRE groups although there were no differences in overall mean glucose in these interventional groups. 15 Other continuous glucose parameters that were positively impacted by TRE included reduced glycemic excursions, 9 glycemic variability, 15 and time‐in‐range. 8 , 17 Positive changes in continuous glucose parameters were again more notable in participants with glucose aberrations at baseline (i.e., prediabetes or diabetes) in these studies, 16 , 17 although a recent 4‐week study of TRE versus low‐carbohydrate diet to improve mean glucose in patients with type 2 diabetes showed that while TRE was associated with an improvement in the glucose management indicator, TRE participants also spent less time in the range and more time hyperglycemic than low‐carbohydrate diet. 58
The authors also assessed correlations between measures of glucose and body composition, physical activity, and meal timing at baseline. Unsurprisingly, there were negative correlations between HOMA‐IR and step count and METs, as well as between insulin levels and both step count and METs. Together, these correlations are in line with previous findings that suggest lower levels of physical activity are associated with greater insulin resistance. 59 HOMA‐IR and insulin also had significant positive correlations with weight, fat mass, and average first mealtime at Baseline. Similarly, there was a significant positive correlation between A1c and fat mass. It is well established that insulin resistance is more prevalent in those of higher weight and with increased adiposity. 60 However, the positive correlation between the HOMA‐IR and first mealtime indicates that individuals who start eating later in the day may be at higher risk for insulin resistance. This finding is supported by studies of breakfast skipping, which have shown that skipping breakfast is associated with type 2 diabetes 61 , 62 and insulin resistance. 63 This trend has also been demonstrated in previous TRE studies and supports the hypothesis that early TRE may play a role in improving circadian rhythmicity to improve insulin sensitivity. 10 , 11 , 12 , 13
This study has several limitations, including the reduction in sample size due to COVID‐19‐related restrictions on in‐person research, which resulted in baseline differences between groups and may have limited the ability to detect between group differences. Although the main study's inclusion criteria included a self‐reported eating duration of greater than 12 h, both groups in this subset of participants had baseline median eating durations that were less than 12 h, and notably shorter than the national median eating duration of 14.75 h. 2 It is certainly possible that not all eating episodes were recorded with the photographic food records. The E‐TRE+DCR group decreased their median duration of eating from 11.9 to 9.3 h over the 12 weeks, while the DCR group decreased their eating duration from 10.8 to 10.5 h over the 12 weeks of the intervention. When assessing the changes in eating duration across all 3 cohorts, 21 the E‐TRE+DCR group had an eating duration 1.6 (95% CI: −2.6, −1.0, p < 0.001) hours shorter than DCR group at Week 12, and the E‐TRE+DCR group ate their last meal 0.93 (95% CI: −1.59, −0.27) hours earlier, on average, than those in DCR (p = 0.007). 21 Given the smaller sample size in this secondary analysis, statistically significant differences in meal timing or meal duration were not assessed. Since the subset of participants included in this study in both groups had relatively short eating durations at baseline and reduced their eating duration to some degree during the study, any potential glycemic effects of the TRE intervention may have been blunted. Finally, the generalizability of this study's findings may also be limited due to the small number of males included in this analysis (Figure 1).
There are fortunately several upcoming studies that will further assess the role of TRE in glucose metabolism with the use of CGM. 64 , 65 , 66 This is particularly important in populations with dysglycemia at baseline as most studies to date that have found favorable changes in continuous glucose profiles or glycemic parameters have been in individuals with baseline‐impaired glucose. Another area of interest is assessing how the timing of the eating window may affect glucose outcomes as there is both pre‐clinical and clinical evidence that the 24‐h circadian rhythm, which is synchronized with behavioral and environmental patterns, impacts nutrient utilization and storage. 32 Upcoming trials will further examine changes in continuous glucose and glycemic parameters in early versus late TRE interventions. 66 It is of particular importance to determine whether late TRE results in cardiometabolic improvements, given that many individuals attempting to follow TRE prefer a delayed eating window to better align with social eating occasions. 67 While longer‐term studies are needed to understand the impact of TRE and the timing of eating windows (i.e., early vs. late) on continuous glucose and insulin sensitivity in the general population, future studies on TRE in individuals with prediabetes and diabetes are particularly necessary.
5. CONCLUSION
In summary, there were no significant differences between E‐TRE+DCR and DCR in glucose profiles or insulin sensitivity after 12 weeks of the dietary interventions, except for a significant difference in the decrease in the mean amplitude of glycemic variation. It is possible that this secondary analysis did not demonstrate favorable changes between E‐TRE and glycemic parameters due to between‐group differences at baseline. As only 16% of the study's cohort had prediabetes, the ability to detect changes in glucose profiles and insulin sensitivity may have been further limited. More studies utilizing CGM are needed to understand the impact of TRE and the timing of eating windows (i.e., early vs. late) on continuous glucose and insulin sensitivity, especially in individuals with prediabetes and diabetes. To further understand the potential glycemic effects of a TRE intervention, future studies could also prescribe shorter eating durations to achieve a greater separation in eating windows between groups.
AUTHOR CONTRIBUTIONS
Elizabeth A. Thomas, Victoria A. Catenacci, Marc‐Andre Cornier and Corey A. Rynders conceived the experiments. Adnin Zaman, Elizabeth A. Thomas, Corey A. Rynders, and Sheila Steinke carried out the experiments. Adnin Zaman, Elizabeth A. Thomas, Corey A. Rynders, Rebecca Jeffers, and Laura Grau analyzed and interpreted the data and wrote the first draft of the manuscript. All authors were involved in writing the paper and had final approval of the submitted and published versions.
CONFLICT OF INTEREST STATEMENT
No conflicts of interest to declare.
INSTITUTIONAL CONSENT
Informed consent was obtained from all individualss involved in the study.
ACKNOWLEDGMENTS
We would like to thank our study participants and the Colorado Clinical and Translational Research Center staff who assisted with data collection. This research was supported by NIH/National Center for Research Resources Colorado Clinical and Translational Sciences Institute Grant (UL1 RR025780); NIH/National Institute of Diabetes and Digestive and Kidney Diseases R21 DK117499 (EAT, MAC, VAC, and CAR), KL2 TR002534 (EAT), K01 DK113063 (CAR), and F32 DK123878 (AZ); and Doris Duke Charitable Foundation Grant 2015212 (EAT).
Zaman A., Grau L., Jeffers R., et al. The effects of early time restricted eating plus daily caloric restriction compared to daily caloric restriction alone on continuous glucose levels. Obes Sci Pract. 2024;e702. 10.1002/osp4.702
REFERENCES
- 1. Kant AK, Graubard BI. 40‐year trends in meal and snack eating behaviors of American adults. J Acad Nutr Diet. 2015;115(1):50‐63. 10.1016/j.jand.2014.06.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22(5):789‐798. 10.1016/j.cmet.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bass J. Circadian topology of metabolism. Nature. 2012;491(7424):348‐356. 10.1038/nature11704 [DOI] [PubMed] [Google Scholar]
- 4. Oosterman JE, Kalsbeek A, la Fleur SE, Belsham DD. Impact of nutrients on circadian rhythmicity. Am J Physiol Regul Integr Comp Physiol. 2015;308(5):R337‐R350. 10.1152/ajpregu.00322.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hemmer A, Mareschal J, Dibner C, et al. The effects of shift work on cardio‐metabolic diseases and eating patterns. Nutrients. 2021;13(11):4178. 10.3390/nu13114178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaix A, Zarrinpar A, Miu P, Panda S. Time‐restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20(6):991‐1005. 10.1016/j.cmet.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hatori M, Vollmers C, Zarrinpar A, et al. Time‐restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high‐fat diet. Cell Metab. 2012;15(6):848‐860. 10.1016/j.cmet.2012.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chow LS, Manoogian ENC, Alvear A, et al. Time‐restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity. 2020;28(5):860‐869. 10.1002/oby.22756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time‐restricted feeding improves 24‐hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11(6):1234. 10.3390/nu11061234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time‐restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212‐1221.e1213. 10.1016/j.cmet.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li C, Xing C, Zhang J, Zhao H, Shi W, He B. Eight‐hour time‐restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J Transl Med. 2021;19(1):148. 10.1186/s12967-021-02817-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allison KC, Hopkins CM, Ruggieri M, et al. Prolonged, controlled daytime versus delayed eating impacts weight and metabolism. Curr Biol. 2021;31(4):650‐657.e653. 10.1016/j.cub.2021.01.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manoogian ENC, Zadourian A, Lo HC, et al. Feasibility of time‐restricted eating and impacts on cardiometabolic health in 24‐h shift workers: the Healthy Heroes randomized control trial. Cell Metab. 2022;34(10):1442‐1456.e1447. 10.1016/j.cmet.2022.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early time‐restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity. 2019;27(8):1244‐1254. 10.1002/oby.22518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones R, Pabla P, Mallinson J, et al. Two weeks of early time‐restricted feeding (eTRF) improves skeletal muscle insulin and anabolic sensitivity in healthy men. Am J Clin Nutr. 2020;112(4):1015‐1028. 10.1093/ajcn/nqaa192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilkinson MJ, Manoogian ENC, Zadourian A, et al. Ten‐hour time‐restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31(1):92‐104.e105. 10.1016/j.cmet.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andriessen C, Fealy CE, Veelen A, et al. Three weeks of time‐restricted eating improves glucose homeostasis in adults with type 2 diabetes but does not improve insulin sensitivity: a randomised crossover trial. Diabetologia. 2022;65(10):1710‐1720. 10.1007/s00125-022-05752-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao L, Hutchison AT, Liu B, et al. Time‐restricted eating improves glycemic control and dampens energy‐consuming pathways in human adipose tissue. Nutrition. 2022;96:111583. 10.1016/j.nut.2021.111583 [DOI] [PubMed] [Google Scholar]
- 19. Haganes KL, Silva CP, Eyjolfsdottir SK, et al. Time‐restricted eating and exercise training improve HbA1c and body composition in women with overweight/obesity: a randomized controlled trial. Cell Metab. 2022;34(10):1457‐1471.e1454. 10.1016/j.cmet.2022.09.003 [DOI] [PubMed] [Google Scholar]
- 20. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63(25):2985‐3023. 10.1016/j.jacc.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 21. Thomas EA, Zaman A, Sloggett KJ, et al. Early time‐restricted eating compared with daily caloric restriction: a randomized trial in adults with obesity. Obesity. 2022;30(5):1027‐1038. 10.1002/oby.23420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Diabetes Prevention Program Curricula and Handouts . 2018. Accessed January 9, 2021. https://www.cdc.gov/diabetes/prevention/resources/curriculum.html
- 23. Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356‐4364. 10.1210/jc.2018-02763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen Y, Prinyawiwatkul W, Xu Z. Insulin: a review of analytical methods. Analyst. 2019;144(14):4139‐4148. 10.1039/c9an00112c [DOI] [PubMed] [Google Scholar]
- 25. Kubihal S, Goyal A, Gupta Y, Khadgawat R. Glucose measurement in body fluids: a ready reckoner for clinicians. Diabetes Metabol Syndr. 2021;15(1):45‐53. 10.1016/j.dsx.2020.11.021 [DOI] [PubMed] [Google Scholar]
- 26. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412‐419. 10.1007/bf00280883 [DOI] [PubMed] [Google Scholar]
- 27. Sakata M, Yoshida A, Haga M. Methemoglobin in blood as determined by double‐wavelength spectrophotometry. Clin Chem. 1982;28(3):508‐511. 10.1093/clinchem/28.3.508 [DOI] [PubMed] [Google Scholar]
- 28. Martin CK, Correa JB, Han H, et al. Validity of the Remote Food Photography Method (RFPM) for estimating energy and nutrient intake in near real‐time. Obesity. 2012;20(4):891‐899. 10.1038/oby.2011.344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vigers T, Chan CL, Snell‐Bergeon J, et al. cgmanalysis: an R package for descriptive analysis of continuous glucose monitor data. PLoS One. 2019;14(10):e0216851. 10.1371/journal.pone.0216851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zaman A, Sloggett KJ, Caldwell AE, et al. The effects of the COVID‐19 pandemic on weight loss in participants in a behavioral weight‐loss intervention. Obesity. 2022;30(5):1015‐1026. 10.1002/oby.23399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hutchison AT, Regmi P, Manoogian ENC, et al. Time‐restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity. 2019;27:724‐732. 10.1002/oby.22449 [DOI] [PubMed] [Google Scholar]
- 32. Gabel K, Varady KA. Current research: effect of time restricted eating on weight and cardiometabolic health. J Physiol. 2022;600(6):1313‐1326. 10.1113/jp280542 [DOI] [PubMed] [Google Scholar]
- 33. Schuppelius B, Peters B, Ottawa A, Pivovarova‐Ramich O. Time restricted eating: a dietary strategy to prevent and treat metabolic disturbances. Front Endocrinol. 2021;12:683140. 10.3389/fendo.2021.683140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Antoni R, Robertson TM, RObertson MD, Johnson JD. A pilot feasibility study exploring the effects of a moderate time‐restricted feeding intervention on energy intake, adiposity and metabolic physiology in free‐living human subjects. J Nutr Sci. 2018;7:e22. 10.1017/jns.2018.13 [DOI] [Google Scholar]
- 35. Martens CR, Rossman MJ, Mazzo MR, et al. Short‐term time‐restricted feeding is safe and feasible in non‐obese healthy midlife and older adults. Geroscience. 2020;42(2):667‐686. 10.1007/s11357-020-00156-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moro T, Tinsley G, Bianco A, et al. Effects of eight weeks of time‐restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance‐trained males. J Transl Med. 2016;14(1):290. 10.1186/s12967-016-1044-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parr EB, Devlin BL, Radford BE, Hawley JA. A delayed morning and earlier evening time‐restricted feeding protocol for improving glycemic control and dietary adherence in men with overweight/obesity: a randomized controlled trial. Nutrients. 2020;12(2):505. 10.3390/nu12020505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peeke PM, Greenway FL, Billes SK, Zhang D, Fujioka K. Effect of time restricted eating on body weight and fasting glucose in participants with obesity: results of a randomized, controlled, virtual clinical trial. Nutr Diabetes. 2021;11(1):6. 10.1038/s41387-021-00149-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Swiatkiewicz I, Wozniak A, Taub PR. Time‐restricted eating and metabolic syndrome: current status and future perspectives. Nutrients. 2021;13(1):221. 10.3390/nu13010221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anton SD, Lee SA, Donahoo WT, et al. The effects of time restricted feeding on overweight, older adults: a pilot study. Nutrients. 2019;11(7):1500. 10.3390/nu11071500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carlson O, Martin B, Stote KS, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal‐weight middle‐aged men and women. Metabolism. 2007;56(12):1729‐1734. 10.1016/j.metabol.2007.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stote KS, Baer DJ, Spears K, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal‐weight, middle‐aged adults. Am J Clin Nutr. 2007;85(4):981‐988. 10.1093/ajcn/85.4.981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tinsley GM, Moore ML, Graybeal AJ, et al. Time‐restricted feeding plus resistance training in active females: a randomized trial. Am J Clin Nutr. 2019;110(3):628‐640. 10.1093/ajcn/nqz126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parr EB, Devlin BL, Lim KHC, et al. Time‐restricted eating as a nutrition strategy for individuals with type 2 diabetes: a feasibility study. Nutrients. 2020;12(11):3228. 10.3390/nu12113228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cai H, Qin YL, Shi ZY, et al. Effects of alternate‐day fasting on body weight and dyslipidaemia in patients with non‐alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol. 2019;19(1):219. 10.1186/s12876-019-1132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cienfuegos S, Gabel K, Kalam F, et al. Effects of 4‐ and 6‐h time‐restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 2020;32(3):366‐378.e363. 10.1016/j.cmet.2020.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gabel K, Hoddy KK, Haggerty N, et al. Effects of 8‐hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging. 2018;4:345‐353. 10.3233/nha-170036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Karras SN, Koufakis T, Adamidou L, et al. Effects of orthodox religious fasting versus combined energy and time restricted eating on body weight, lipid concentrations and glycaemic profile. Int J Food Sci Nutr. 2021;72(1):82‐92. 10.1080/09637486.2020.1760218 [DOI] [PubMed] [Google Scholar]
- 49. Lowe DA, Wu N, Rohdin‐Bibby L, et al. Effects of time‐restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180(11):1491‐1499. 10.1001/jamainternmed.2020.4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McAllister MJ, Pigg BL, Renteria LI, Waldman HS. Time‐restricted feeding improves markers of cardiometabolic health in physically active college‐age men: a 4‐week randomized pre‐post pilot study. Nutr Res. 2020;75:32‐43. 10.1016/j.nutres.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 51. Phillips NE, Mareschal J, Schwab N, et al. The effects of time‐restricted eating versus standard dietary advice on weight, metabolic health and the consumption of processed food: a pragmatic randomised controlled trial in community‐based adults. Nutrients. 2021;13(3):1042. 10.3390/nu13031042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kahleova H, Belinova L, Malinska H, et al. Eating two larger meals a day (breakfast and lunch) is more effective than six smaller meals in a reduced‐energy regimen for patients with type 2 diabetes: a randomised crossover study. Diabetologia. 2014;57(8):1552‐1560. 10.1007/s00125-014-3253-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arnason TG, Bowen MW, Mansell KD. Effects of intermittent fasting on health markers in those with type 2 diabetes: a pilot study. World J Diabetes. 2017;8(4):154‐164. 10.4239/wjd.v8.i4.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Karras SN, Koufakis T, Adamidou L, et al. Similar late effects of a 7‐week orthodox religious fasting and a time restricted eating pattern on anthropometric and metabolic profiles of overweight adults. Int J Food Sci Nutr. 2021;72(2):248‐258. 10.1080/09637486.2020.1787959 [DOI] [PubMed] [Google Scholar]
- 55. Kesztyus D, Cermak P, Gulich M, Kesztyus T. Adherence to time‐restricted feeding and impact on abdominal obesity in primary care patients: results of a pilot study in a pre‐post design. Nutrients. 2019;11(12):2854. 10.3390/nu11122854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moon S, Kang J, Kim SH, et al. Beneficial effects of time‐restricted eating on metabolic diseases: a systemic review and meta‐analysis. Nutrients. 2020;12(5):1267. 10.3390/nu12051267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xie Z, Sun Y, Ye Y, et al. Randomized controlled trial for time‐restricted eating in healthy volunteers without obesity. Nat Commun. 2022;13(1):1003. 10.1038/s41467-022-28662-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Carbonel A, Velis E, Calinawan TL, et al. 28‐OR: effects of time‐restricted eating vs. Low‐carbohydrate diet on mean glucose in type 2 diabetes patients. Diabetes. 2022;71(Suppl_1):71. 10.2337/db22-28-or [DOI] [Google Scholar]
- 59. Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med. 2016;2(1):e000143. 10.1136/bmjsem-2016-000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang M, Hu T, Zhang S, Zhou L. Associations of different adipose tissue depots with insulin resistance: a systematic review and meta‐analysis of observational studies. Sci Rep. 2015;5(1):18495. 10.1038/srep18495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mekary RA, Giovannucci E, Willett WC, van Dam RM, Hu FB. Eating patterns and type 2 diabetes risk in men: breakfast omission, eating frequency, and snacking. Am J Clin Nutr. 2012;95(5):1182‐1189. 10.3945/ajcn.111.028209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Odegaard AO, Jacobs DR, Jr. , Steffen LM, Van Horn L, Ludwig DS, Pereira MA. Breakfast frequency and development of metabolic risk. Diabetes Care. 2013;36(10):3100‐3106. 10.2337/dc13-0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Monzani A, Ricotti R, Caputo M, et al. A systematic review of the association of skipping breakfast with weight and cardiometabolic risk factors in children and adolescents. What should we better investigate in the future? Nutrients. 2019;11(2):387. 10.3390/nu11020387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Santos‐Baez LS, Garbarini A, Shaw D, et al. Time‐restricted eating to improve cardiometabolic health: the New York time‐restricted EATing randomized clinical trial ‐ protocol overview. Contemp Clin Trials. 2022;120:106872. 10.1016/j.cct.2022.106872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Suthutvoravut U, Anothaisintawee T, Boonmanunt S, et al. Efficacy of time‐restricted eating and behavioural economic interventions in reducing fasting plasma glucose, HbA1c and cardiometabolic risk factors compared with time‐restricted eating alone or usual care in patients with impaired fasting glucose: protocol for an open‐label randomised controlled trial. BMJ Open. 2022;12(9):e058954. 10.1136/bmjopen-2021-058954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Peters B, Koppold‐Liebscher DA, Schuppelius B, et al. Effects of early vs. late time‐restricted eating on cardiometabolic health, inflammation, and sleep in overweight and obese women: a study protocol for the ChronoFast trial. Front Nutr. 2021;8:765543. 10.3389/fnut.2021.765543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Heilbronn LK, Regmi P. Will delaying breakfast mitigate the metabolic health benefits of time‐restricted eating? Obesity. 2020;28(Suppl 1):S6‐S7. 10.1002/oby.22776 [DOI] [PubMed] [Google Scholar]


