Abstract
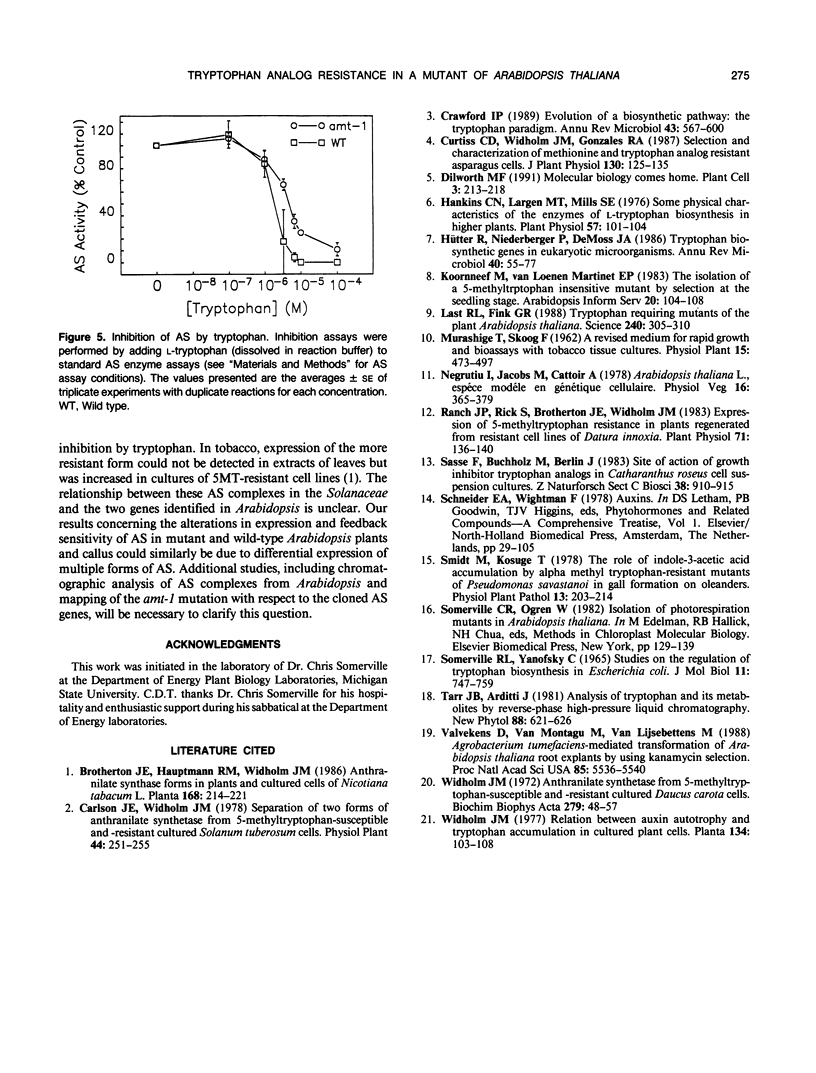
Mutants of Arabidopsis thaliana have been selected for resistance to growth inhibition at the seedling stage by α-methyltryptophan (aMT). One mutant, amt-1 has been characterized in detail. The appearance and growth rate of the mutant in the absence of the inhibitor are similar to wild type, both as plants and callus. However, mutant plant growth is unaffected by 25 micromolar aMT and mutant callus growth by 50 micromolar aMT, concentrations that completely inhibit the growth of wild-type plants and callus, respectively. Tryptophan levels in mutant and wild-type plants are 24.3 ± 2.7 and 4.7 ± 1.2 micrograms per gram fresh weight, respectively, and in the corresponding callus 64.0 ± 2.6 and 31.8 ± 8.4 micrograms per gram fresh weight, respectively. Anthranilate synthase (AS) activity levels in crude extracts from whole plants are 3.09 ± 0.54 nanomoles per milligram protein per hour in amt-1 and 1.32 ± 0.21 nanomoles per milligram protein per hour in wild-type plants. In crude extracts from callus, anthranilate synthase levels are 11.54 ± 2.05 nanomoles per milligram protein per hour and 7.74 ± 1.58 in amt-1 and wild type, respectively. Enzyme extracts are inhibited by l-tryptophan; the concentrations required for 50% inhibition (I50) are 3.9 and 1.9 micromolar for amt-1 and for wild type, respectively. The mutation segregates as a single nuclear allele and shows incomplete dominance. The concomitant increases in both AS activity and its I50 for tryptophan suggest that the mutation amt-1 either resides in one of the AS structural genes or causes increased expression of an AS isoform with an I50 greater than the average for the entire extract.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crawford I. P. Evolution of a biosynthetic pathway: the tryptophan paradigm. Annu Rev Microbiol. 1989;43:567–600. doi: 10.1146/annurev.mi.43.100189.003031. [DOI] [PubMed] [Google Scholar]
- Dilworth M. F. Molecular biology comes home. Plant Cell. 1991 Mar;3(3):213–218. doi: 10.1105/tpc.3.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins C. N., Largen M. T., Mills S. E. Some Physical Characteristics of the Enzymes of l-Tryptophan Biosynthesis in Higher Plants. Plant Physiol. 1976 Jan;57(1):101–104. doi: 10.1104/pp.57.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütter R., Niederberger P., DeMoss J. A. Tryptophan biosynthetic genes in eukaryotic microorganisms. Annu Rev Microbiol. 1986;40:55–77. doi: 10.1146/annurev.mi.40.100186.000415. [DOI] [PubMed] [Google Scholar]
- Last R. L., Fink G. R. Tryptophan-Requiring Mutants of the Plant Arabidopsis thaliana. Science. 1988 Apr 15;240(4850):305–310. doi: 10.1126/science.240.4850.305. [DOI] [PubMed] [Google Scholar]
- Ranch J. P., Rick S., Brotherton J. E., Widholm J. M. Expression of 5-Methyltryptophan Resistance in Plants Regenerated from Resistant Cell Lines of Datura innoxia. Plant Physiol. 1983 Jan;71(1):136–140. doi: 10.1104/pp.71.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMERVILLE R. L., YANOFSKY C. STUDIES ON THE REGULATION OF TRYPTOPHAN BIOSYNTHESIS IN ESCHERICHIA COLI. J Mol Biol. 1965 Apr;11:747–759. doi: 10.1016/s0022-2836(65)80032-8. [DOI] [PubMed] [Google Scholar]
- Valvekens D., Van Montagu M., Van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm J. M. Anthranilate synthetase from 5-methyltryptophan-susceptible and -resistant cultured Daucus carota cells. Biochim Biophys Acta. 1972 Aug 18;279(1):48–57. doi: 10.1016/0304-4165(72)90240-1. [DOI] [PubMed] [Google Scholar]



