Abstract
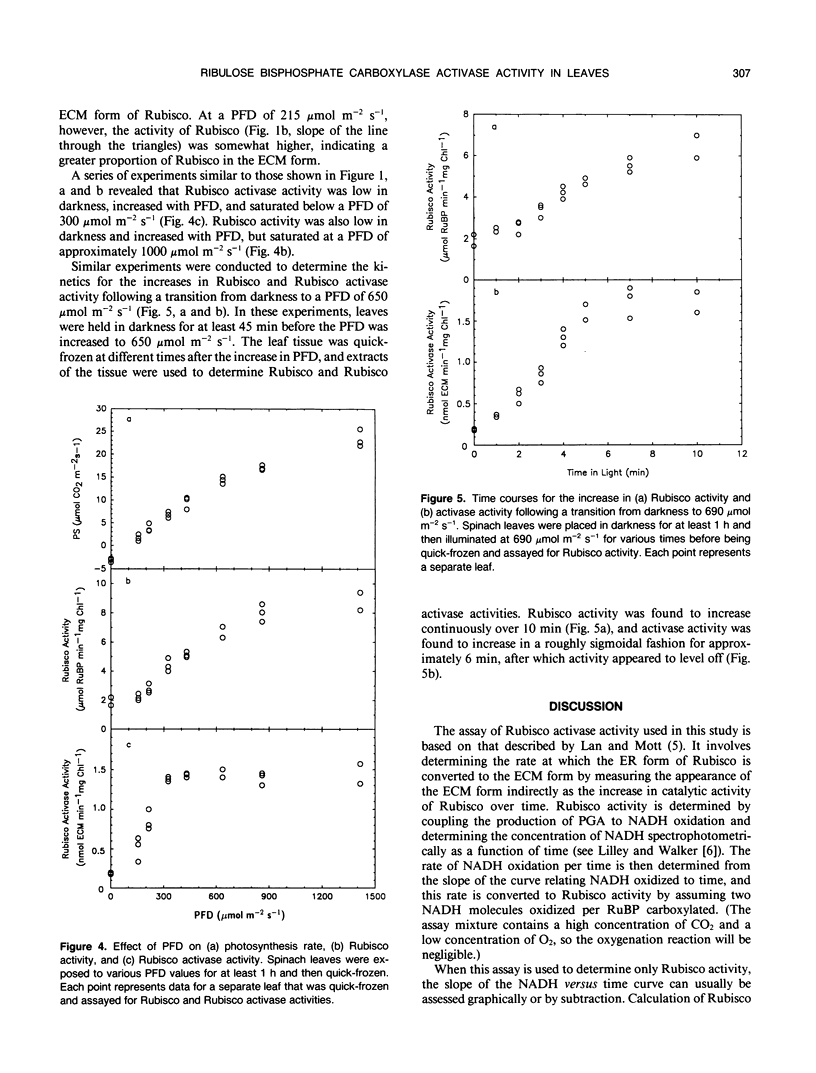
An assay for the activity of ribulose bisphosphate carboxylase (Rubisco) activase in crude leaf extracts was developed. The assay is based on a spectrophotometric assay of Rubisco, and activase activity (in nanomoles activated Rubisco per minute per milligram chlorophyll) was calculated from the rate of increase in Rubisco activity over time. Activase activity measurements were made using samples from spinach (Spinacia oleracea) leaves undergoing (a) steady-state photosynthesis at various photon flux density (PFD) values and (b) nonsteady-state photosynthesis following an increase from darkness to a high PFD. Analysis of these samples showed that steady-state Rubisco activase activity was relatively low in darkness, increased with PFD, and saturated below 300 micromoles per square meter per second. Rubisco activity (measured spectrophotometrically) was also found to be low in darkness and to increase with PFD, but it saturated at much higher PFD values (approximately 1000 micromoles per square meter per second) along with the rate of photosynthesis. Following an increase in PFD from darkness to 650 micromoles per square meter per second, activase activity increased more or less linearly over a period of 5 to 6 minutes, after which it was constant. Rubisco activity, however, increased more slowly. The light-dependence of Rubisco activase is consistent with previous gas-exchange data showing two interdependent processes in the activation of Rubisco following an increase in PFD.
Full text
PDF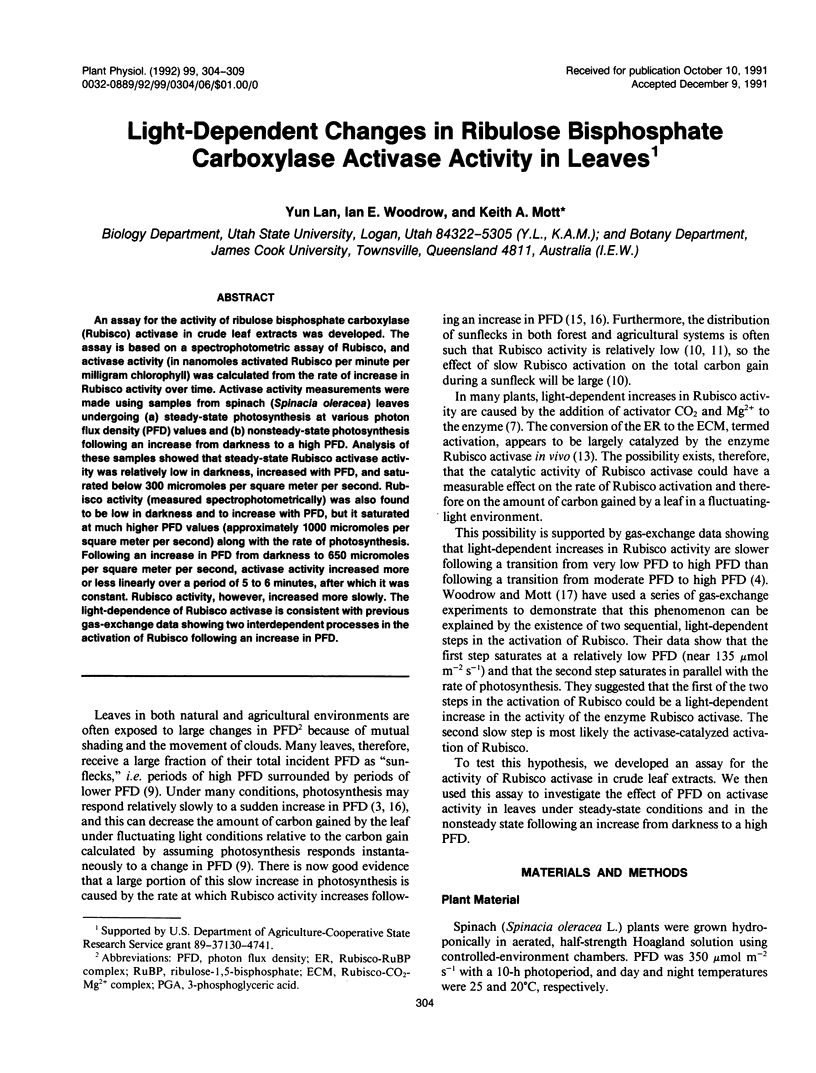




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell W. J., Ogren W. L. A novel role for light in the activation of ribulosebisphosphate carboxylase/oxygenase. Plant Physiol. 1990 Jan;92(1):110–115. doi: 10.1104/pp.92.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon Z. G., Mott K. A. Evidence that Ribulose 1,5-Bisphosphate (RuBP) Binds to Inactive Sites of RuBP Carboxylase in Vivo and an Estimate of the Rate Constant for Dissociation. Plant Physiol. 1989 Apr;89(4):1253–1257. doi: 10.1104/pp.89.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. B., Woodrow I. E., Mott K. A. Nonsteady-State Photosynthesis following an Increase in Photon Flux Density (PFD) : Effects of Magnitude and Duration of Initial PFD. Plant Physiol. 1991 Feb;95(2):498–503. doi: 10.1104/pp.95.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y., Mott K. A. Determination of Apparent K(m) Values for Ribulose 1,5-Bisphosphate Carboxylase/Oxygenase (Rubisco) Activase Using the Spectrophotometric Assay of Rubisco Activity. Plant Physiol. 1991 Feb;95(2):604–609. doi: 10.1104/pp.95.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. An improved spectrophotometric assay for ribulosebisphosphate carboxylase. Biochim Biophys Acta. 1974 Jul 17;358(1):226–229. doi: 10.1016/0005-2744(74)90274-5. [DOI] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Mott K. A. Do Stomata Respond to CO(2) Concentrations Other than Intercellular? Plant Physiol. 1988 Jan;86(1):200–203. doi: 10.1104/pp.86.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcy R. W., Seemann J. R. Photosynthetic induction state of leaves in a soybean canopy in relation to light regulation of ribulose-1-5-bisphosphate carboxylase and stomatal conductance. Plant Physiol. 1990 Oct;94(2):628–633. doi: 10.1104/pp.94.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R., Jr Rubisco activase. Biochim Biophys Acta. 1990 Jan 4;1015(1):15–28. doi: 10.1016/0005-2728(90)90211-l. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Streusand V. J., Chatfield J. M., Portis A. R. Purification and assay of rubisco activase from leaves. Plant Physiol. 1988 Dec;88(4):1008–1014. doi: 10.1104/pp.88.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J. R., Kirschbaum M. U., Sharkey T. D., Pearcy R. W. Regulation of Ribulose-1,5-Bisphosphate Carboxylase Activity in Alocasia macrorrhiza in Response to Step Changes in Irradiance. Plant Physiol. 1988 Sep;88(1):148–152. doi: 10.1104/pp.88.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrow I. E., Mott K. A. Biphasic Activation of Ribulose Bisphosphate Carboxylase in Spinach Leaves as Determined from Nonsteady-State CO(2) Exchange. Plant Physiol. 1992 May;99(1):298–303. doi: 10.1104/pp.99.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]


