Abstract
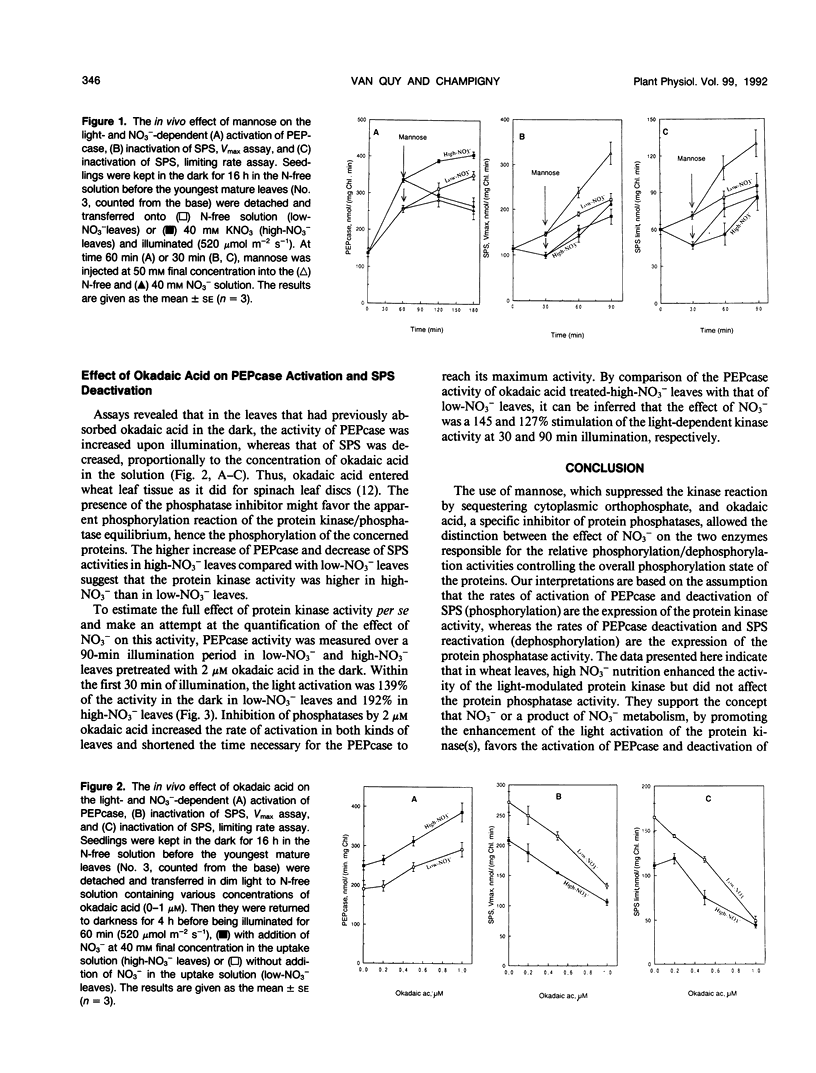
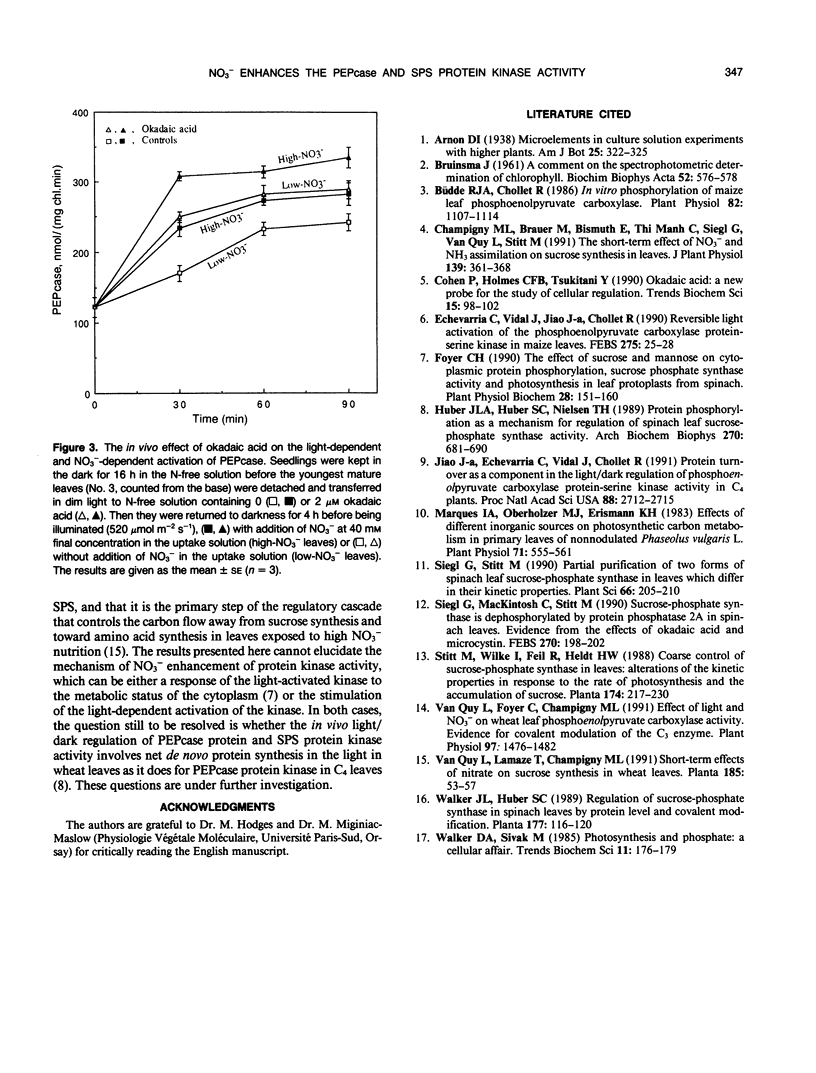
The aim of this work was to determine which of the two reactions (i.e. phosphorylation or dephosphorylation) involved in the establishment of the phosphorylated status of the wheat leaf phosphoenolpyruvate carboxylase and sucrose phosphate synthase protein responds in vivo to NO3− uptake and assimilation. Detached mature leaves of wheat (Triticum aestivum L. cv Fidel) were fed with N-free (low-NO3− leaves) or 40 mm NO3− solution (high-NO3− leaves). The specific inhibition of the enzyme-protein kinase or phosphatase activities was obtained in vivo by addition of mannose or okadaic acid, respectively, in the uptake solution. Mannose at 50 mm, by blocking the kinase reaction, inhibited the processes of NO3−-dependent phosphoenolpyruvate carboxylase activation and sucrose phosphate synthase deactivation. Following the addition of mannose, the deactivation of phosphoenolpyruvate carboxylase and the activation of sucrose phosphate synthase, both due to the enzyme-protein dephosphorylation, were at the same rate in low-NO3− and high-NO3− leaves, indicating that NO3− had no effect per se on the enzyme-protein phosphatase activity. Upon treatment with okadaic acid, the higher increase of phosphoenolpyruvate carboxylase and decrease of sucrose phosphate synthase activities observed in high NO3− compared with low NO3− leaves showed evidence that NO3− enhanced the protein kinase activity. These results support the concept that NO3−, or a product of its metabolism, favors the activation of phosphoenolpyruvate carboxylase and deactivation of sucrose phosphate synthase in wheat leaves by promoting the light activation of the enzyme-protein kinase(s) without affecting the phosphatase(s).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUINSMA J. A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta. 1961 Sep 30;52:576–578. doi: 10.1016/0006-3002(61)90418-8. [DOI] [PubMed] [Google Scholar]
- Budde R. J., Chollet R. In vitro phosphorylation of maize leaf phosphoenolpyruvate carboxylase. Plant Physiol. 1986 Dec;82(4):1107–1114. doi: 10.1104/pp.82.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Echevarría C., Vidal J., Jiao J. A., Chollet R. Reversible light activation of the phosphoenolpyruvate carboxylase protein-serine kinase in maize leaves. FEBS Lett. 1990 Nov 26;275(1-2):25–28. doi: 10.1016/0014-5793(90)81430-v. [DOI] [PubMed] [Google Scholar]
- Huber J. L., Huber S. C., Nielsen T. H. Protein phosphorylation as a mechanism for regulation of spinach leaf sucrose-phosphate synthase activity. Arch Biochem Biophys. 1989 May 1;270(2):681–690. doi: 10.1016/0003-9861(89)90551-1. [DOI] [PubMed] [Google Scholar]
- Jiao J., Echevarría C., Vidal J., Chollet R. Protein turnover as a component in the light/dark regulation of phosphoenolpyruvate carboxylase protein-serine kinase activity in C4 plants. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2712–2715. doi: 10.1073/pnas.88.7.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van Quy, Foyer C., Champigny M. L. Effect of Light and NO(3) on Wheat Leaf Phosphoenolpyruvate Carboxylase Activity: Evidence for Covalent Modulation of the C(3) Enzyme. Plant Physiol. 1991 Dec;97(4):1476–1482. doi: 10.1104/pp.97.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques I. A., Oberholzer M. J., Erismann K. H. Effects of Different Inorganic Nitrogen Sources on Photosynthetic Carbon Metabolism in Primary Leaves of Non-nodulated Phaseolus vulgaris L. Plant Physiol. 1983 Mar;71(3):555–561. doi: 10.1104/pp.71.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., MacKintosh C., Stitt M. Sucrose-phosphate synthase is dephosphorylated by protein phosphatase 2A in spinach leaves. Evidence from the effects of okadaic acid and microcystin. FEBS Lett. 1990 Sep 17;270(1-2):198–202. doi: 10.1016/0014-5793(90)81267-r. [DOI] [PubMed] [Google Scholar]


