Abstract
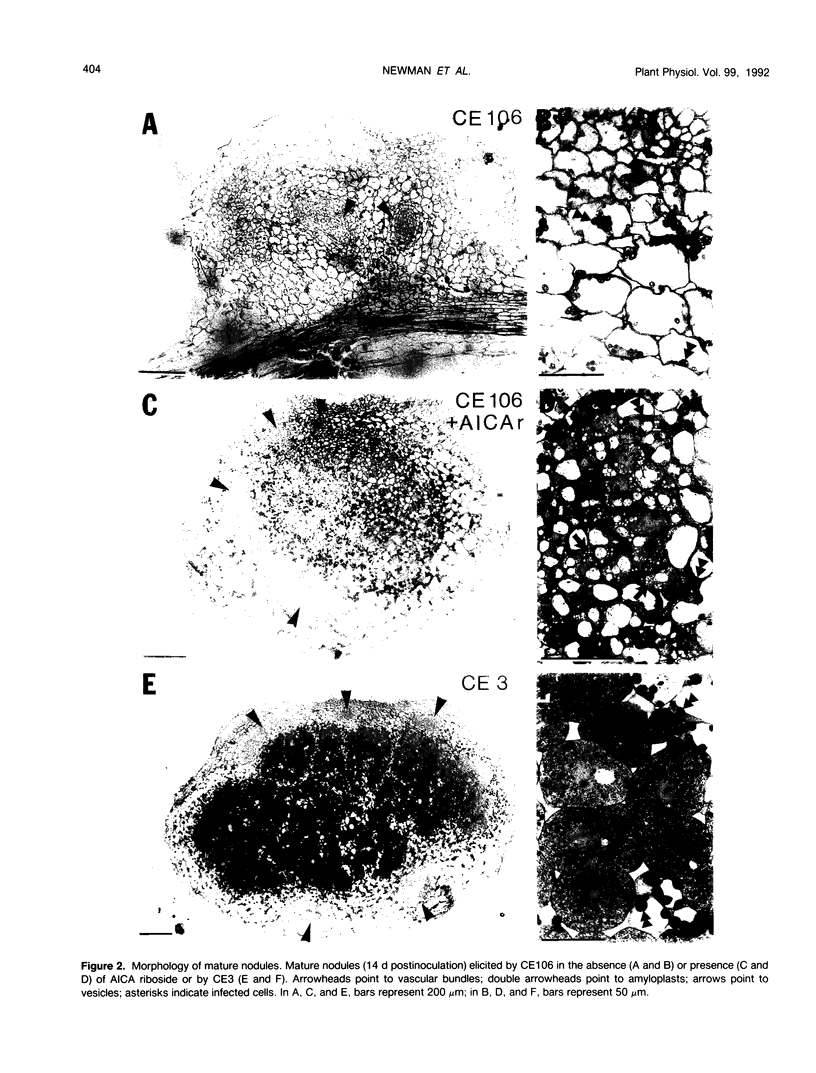
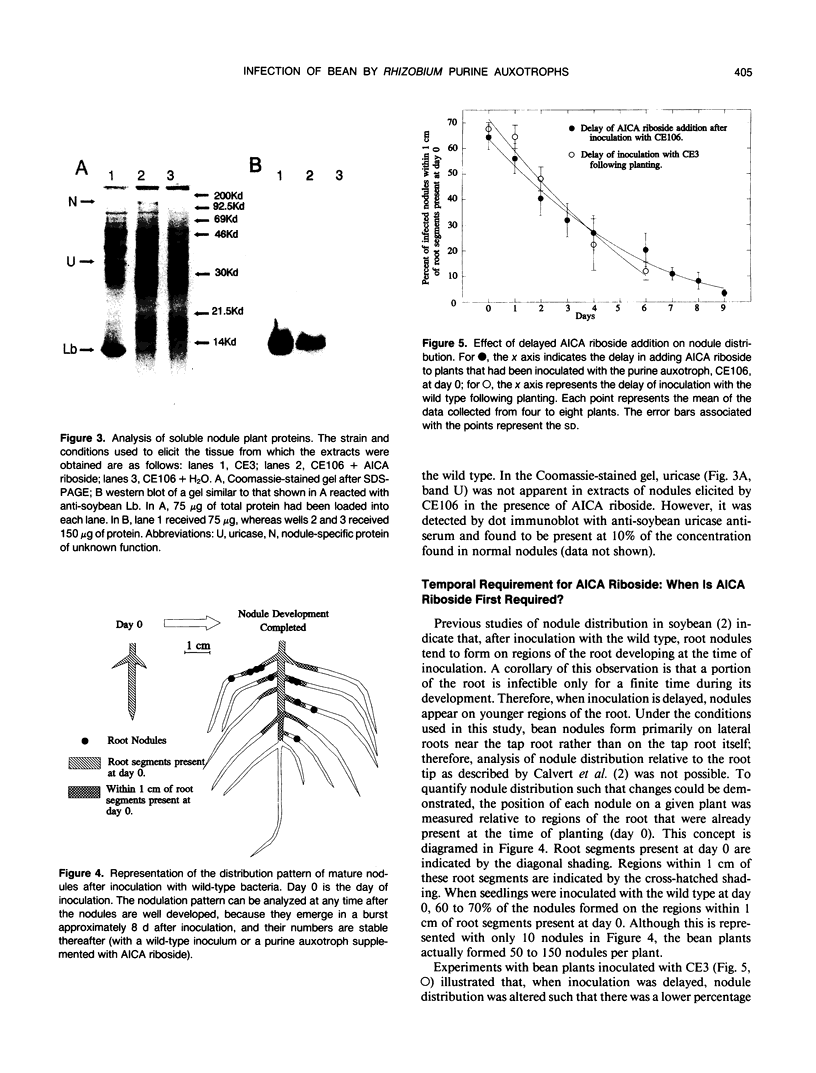
Purine auxotrophs of Rhizobium leguminosarum biovar phaseoli CFN42 elicit uninfected pseudonodules on bean (Phaseolus vulgaris L.). Addition of 4-aminoimidazole-5-carboxamide (AICA) riboside to the root medium during incubation of the plant with these mutants leads to enhanced nodule development, although nitrogenase activity is not detected. Nodules elicited in this manner had infection threads and anatomical features characteristic of normal nodules, such as peripheral vasculature rather than the central vasculature of the pseudonodules that were elicited without AICA riboside supplementation. Although 105 to 106 bacteria could be recovered from these nodules after full development, bacteria were not observed in the interior nodule cells. Instead, large cells with extensive internal membranes were present. Approximately 5% of the normal amount of leghemoglobin and 10% of the normal amount of uricase were detected in these nodules. To promote the development of true nodules rather than pseudonodules, AICA riboside was required no later than the second day through no more than the sixth day following inoculation. After this period, removal of AICA riboside from the root medium did not prevent the formation of true nodules. This observation suggests that there is a critical stage of infection, reached before nodule emergence, at which development becomes committed to forming a true nodule rather than a pseudonodule.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Cock J. M., Mould R. M., Bennett M. J., Cullimore J. V. Expression of glutamine synthetase genes in roots and nodules of Phaseolus vulgaris following changes in the ammonium supply and infection with various Rhizobium mutants. Plant Mol Biol. 1990 Apr;14(4):549–560. doi: 10.1007/BF00027500. [DOI] [PubMed] [Google Scholar]
- Djordjevic S. P., Ridge R. W., Chen H. C., Redmond J. W., Batley M., Rolfe B. G. Induction of pathogenic-like responses in the legume Macroptilium atropurpureum by a transposon-induced mutant of the fast-growing, broad-host-range Rhizobium strain NGR234. J Bacteriol. 1988 Apr;170(4):1848–1857. doi: 10.1128/jb.170.4.1848-1857.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gots J. S., Gollub E. G. SEQUENTIAL BLOCKADE IN ADENINE BIOSYNTHESIS BY GENETIC LOSS OF AN APPARENT BIFUNCTIONAL DEACYLASE. Proc Natl Acad Sci U S A. 1957 Sep 15;43(9):826–834. doi: 10.1073/pnas.43.9.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerppola T. K., Kahn M. L. Symbiotic phenotypes of auxotrophic mutants of Rhizobium meliloti 104A14. J Gen Microbiol. 1988 Apr;134(4):913–919. doi: 10.1099/00221287-134-4-913. [DOI] [PubMed] [Google Scholar]
- Kim C. H., Kuykendall L. D., Shah K. S., Keister D. L. Induction of Symbiotically Defective Auxotrophic Mutants of Rhizobium fredii HH303 by Transposon Mutagenesis. Appl Environ Microbiol. 1988 Feb;54(2):423–427. doi: 10.1128/aem.54.2.423-427.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Noel K. D., Sanchez A., Fernandez L., Leemans J., Cevallos M. A. Rhizobium phaseoli symbiotic mutants with transposon Tn5 insertions. J Bacteriol. 1984 Apr;158(1):148–155. doi: 10.1128/jb.158.1.148-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Vandenbosch K. A., Kulpaca B. Mutations in Rhizobium phaseoli that lead to arrested development of infection threads. J Bacteriol. 1986 Dec;168(3):1392–1401. doi: 10.1128/jb.168.3.1392-1401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth L. E., Stacey G. Cytoplasmic membrane systems involved in bacterium release into soybean nodule cells as studied with two Bradyrhizobium japonicum mutant strains. Eur J Cell Biol. 1989 Jun;49(1):24–32. [PubMed] [Google Scholar]
- Sabina R. L., Holmes E. W., Becker M. A. The enzymatic synthesis of 5-amino-4-imidazolecarboxamide riboside triphosphate (ZTP). Science. 1984 Mar 16;223(4641):1193–1195. doi: 10.1126/science.6199843. [DOI] [PubMed] [Google Scholar]
- Thomas C. B., Arnold W. J., Kelley W. N. Human adenine phosphoribosyltransferase. Purification, subunit structure, and substrate specificity. J Biol Chem. 1973 Apr 10;248(7):2529–2535. [PubMed] [Google Scholar]
- Vandenbosch K. A., Noel K. D., Kaneko Y., Newcomb E. H. Nodule initiation elicited by noninfective mutants of Rhizobium phaseoli. J Bacteriol. 1985 Jun;162(3):950–959. doi: 10.1128/jb.162.3.950-959.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]