Abstract
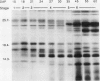
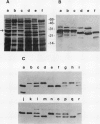
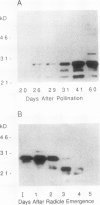
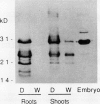
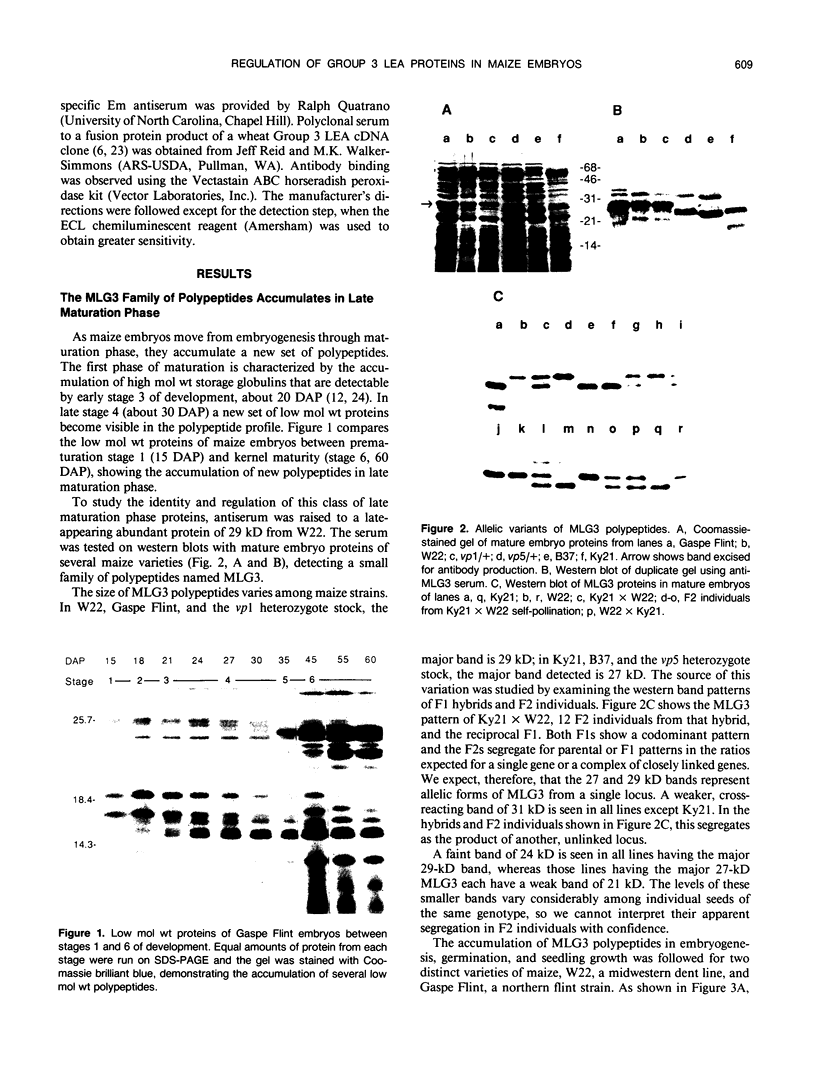
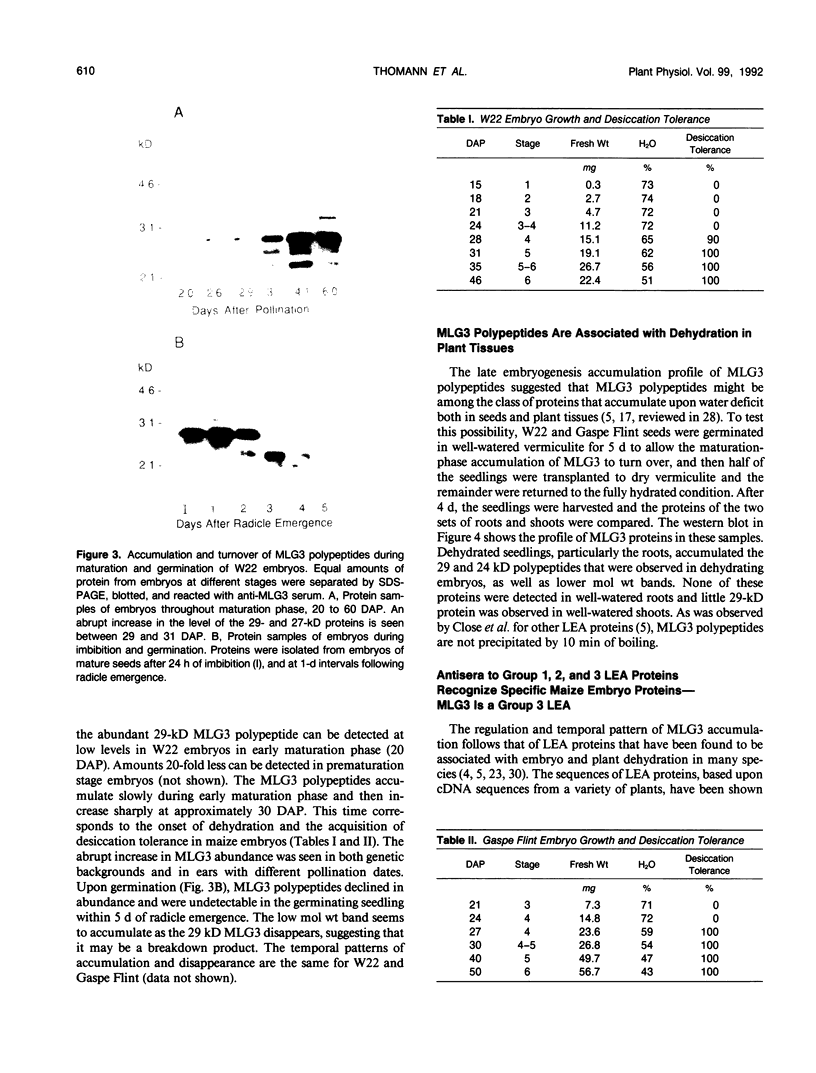
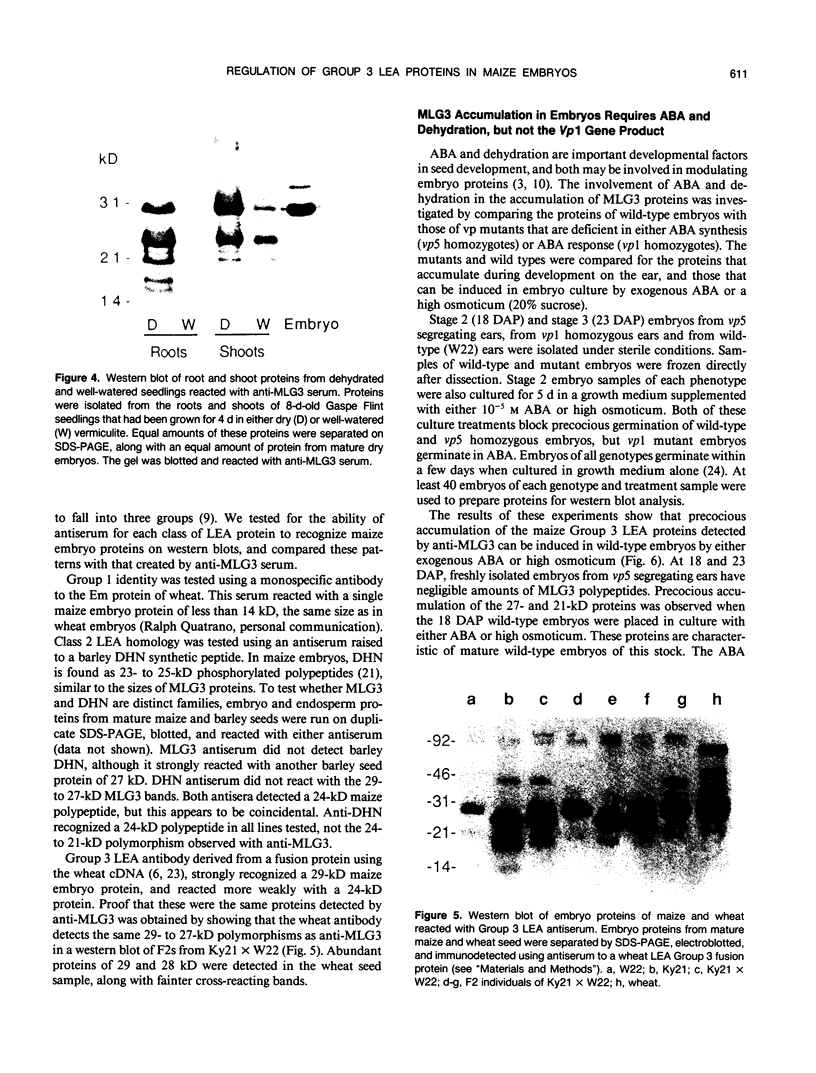
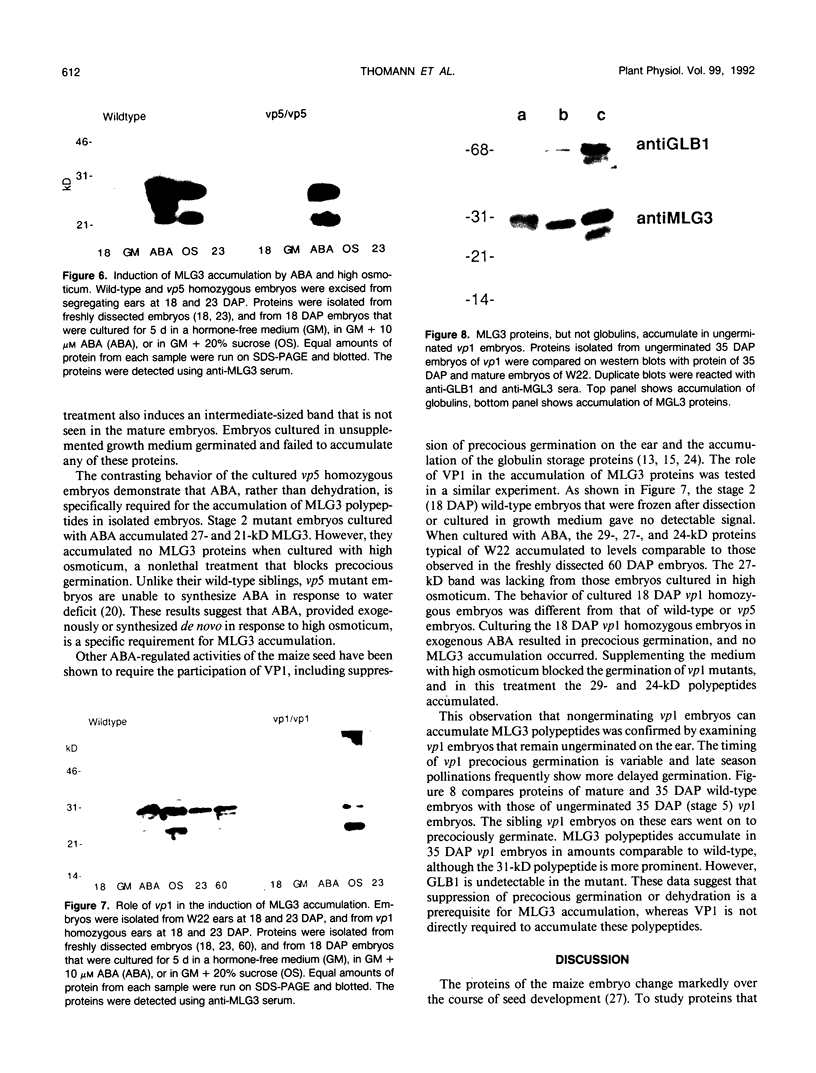
Several different types of proteins that are modulated by abscisic acid (ABA) accumulate in developing embryos of maize (Zea mays L.). Some of these proteins are specific to the developing seed, such as the storage globulin, GLB1, whereas others are involved in general responses to water deficit. Here we describe a maize protein family of this second type, a Group 3 late embryogenesis abundant (MLG3). Like other proteins of this class, MLG3 polypeptides are ABA-responsive. They are found in maturing seeds and in dehydrating plant tissues. Antigenically related proteins are found in other cereals. To distinguish the regulation of developmentally programmed ABA responses from those that are environmentally induced, we compared the ontological pattern and accumulation requirements of MLG3 polypeptides with those we previously described for GLB1. GLB1 accumulation begins early in the maturation phase and specifically requires high levels of ABA and the participation of the Viviparous-1 (Vp1) gene product. Vp1 is required for other ABA-modulated events in maize seed development as well. In experiments using vp1 mutants and mutants deficient in ABA synthesis (vp5 mutation), we show that MLG3 accumulation also is dependent upon ABA, but it shows striking differences from GLB1. MLG3 accumulates much later in embryogenesis, coincident with the onset of dehydration. In contrast to GLB1, MLG3 proteins can be induced by de novo ABA synthesis in response to culturing in high osmoticum. Unlike GLB1, MLG3 has no specific requirement for the Vp1 gene product.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Close T. J., Kortt A. A., Chandler P. M. A cDNA-based comparison of dehydration-induced proteins (dehydrins) in barley and corn. Plant Mol Biol. 1989 Jul;13(1):95–108. doi: 10.1007/BF00027338. [DOI] [PubMed] [Google Scholar]
- Curry J., Morris C. F., Walker-Simmons M. K. Sequence analysis of a cDNA encoding a group 3 LEA mRNA inducible by ABA or dehydration stress in wheat. Plant Mol Biol. 1991 Jun;16(6):1073–1076. doi: 10.1007/BF00016078. [DOI] [PubMed] [Google Scholar]
- Dooner H. K. Viviparous-1 mutation in maize conditions pleiotropic enzyme deficiencies in the aleurone. Plant Physiol. 1985 Feb;77(2):486–488. doi: 10.1104/pp.77.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. R., Crouch M. L. Rapeseed embryo development in culture on high osmoticum is similar to that in seeds. Plant Physiol. 1986 Jul;81(3):907–912. doi: 10.1104/pp.81.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galau G. A., Bijaisoradat N., Hughes D. W. Accumulation kinetics of cotton late embryogenesis-abundant mRNAs and storage protein mRNAs: coordinate regulation during embryogenesis and the role of abscisic acid. Dev Biol. 1987 Sep;123(1):198–212. doi: 10.1016/0012-1606(87)90442-8. [DOI] [PubMed] [Google Scholar]
- Kriz A. L. Characterization of embryo globulins encoded by the maize Glb genes. Biochem Genet. 1989 Apr;27(3-4):239–251. doi: 10.1007/BF02401804. [DOI] [PubMed] [Google Scholar]
- Kriz A. R., Wallace M. S., Paiva R. Globulin Gene Expression in Embryos of Maize viviparous Mutants : Evidence for Regulation of the Glb1 Gene by Abscissic Acid. Plant Physiol. 1990 Feb;92(2):538–542. doi: 10.1104/pp.92.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte W. R., Jr, Russell S. H., Quatrano R. S. Abscisic acid-responsive sequences from the em gene of wheat. Plant Cell. 1989 Oct;1(10):969–976. doi: 10.1105/tpc.1.10.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty D. R., Carson C. B., Stinard P. S., Robertson D. S. Molecular Analysis of viviparous-1: An Abscisic Acid-Insensitive Mutant of Maize. Plant Cell. 1989 May;1(5):523–532. doi: 10.1105/tpc.1.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty D. R., Hattori T., Carson C. B., Vasil V., Lazar M., Vasil I. K. The Viviparous-1 developmental gene of maize encodes a novel transcriptional activator. Cell. 1991 Sep 6;66(5):895–905. doi: 10.1016/0092-8674(91)90436-3. [DOI] [PubMed] [Google Scholar]
- Mundy J., Chua N. H. Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J. 1988 Aug;7(8):2279–2286. doi: 10.1002/j.1460-2075.1988.tb03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla M., Goday A., Vilardell J., Gómez J., Pagès M. Differential regulation of ABA-induced 23-25 kDa proteins in embryo and vegetative tissues of the viviparous mutants of maize. Plant Mol Biol. 1989 Oct;13(4):385–394. doi: 10.1007/BF00015550. [DOI] [PubMed] [Google Scholar]
- Rivin C. J., Grudt T. Abscisic Acid and the developmental regulation of embryo storage proteins in maize. Plant Physiol. 1991 Feb;95(2):358–365. doi: 10.1104/pp.95.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. S. The Genetics of Vivipary in Maize. Genetics. 1955 Sep;40(5):745–760. doi: 10.1093/genetics/40.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver K., Mundy J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell. 1990 Jun;2(6):503–512. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Martínez D., Puigdomènech P., Pagès M. Regulation of Gene Expression in Developing Zea mays Embryos: Protein Synthesis during Embryogenesis and Early Germination of Maize. Plant Physiol. 1986 Oct;82(2):543–549. doi: 10.1104/pp.82.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardell J., Goday A., Freire M. A., Torrent M., Martínez M. C., Torné J. M., Pagès M. Gene sequence, developmental expression, and protein phosphorylation of RAB-17 in maize. Plant Mol Biol. 1990 Mar;14(3):423–432. doi: 10.1007/BF00028778. [DOI] [PubMed] [Google Scholar]