Abstract
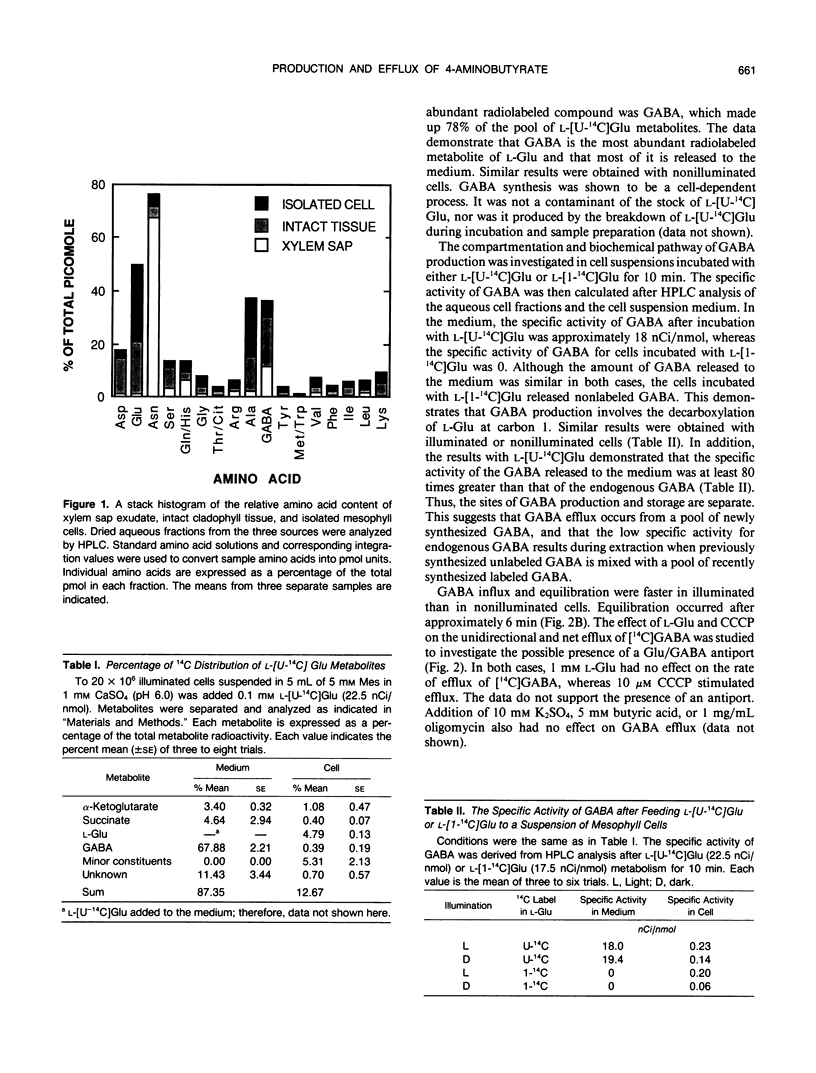
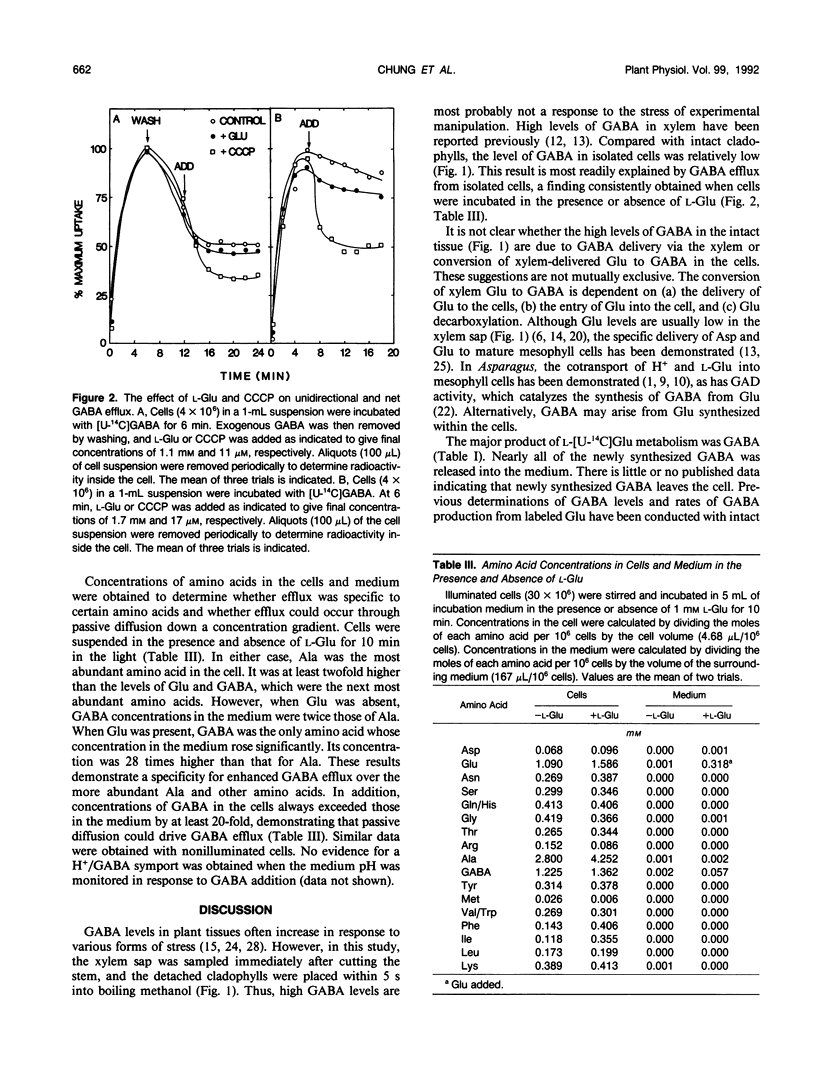
The pathway of 4-aminobutyric acid (GABA) production and efflux was investigated in suspensions of mesophyll cells isolated from asparagus (Asparagus sprengeri Regel) cladophylls. Analysis of free amino acids demonstrated that, on a molar basis, GABA represented 11.4, 19, and 6.5% of the xylem sap, intact cladophyll tissue, and isolated mesophyll cells, respectively. l-Glu, a GABA precursor, was abundant in intact cladophylls and isolated cells but not in xylem sap. When cells were incubated with l-[U-14C]Glu, intracellular GABA contained less than 10% of the radioactivity found in intracellular Glu. However, GABA in the medium contained 78% of the radioactivity found in extracellular l-Glu metabolites. Incubation with l-[1-14C]Glu resulted in the appearance of unlabeled GABA, demonstrating its production through decarboxylation at carbon 1. GABA released to the medium from cells incubated with l-[U-14C]Glu had a specific activity of 18 nanocuries per nanomole, whereas GABA remaining in the cell had a specific activity of 2.25 × 10−1 nanocuries per nanomole. In the presence of exogenous l-Glu, amino acid analysis and cell volume measurements indicated intracellular Ala and GABA concentrations of 4.2 and 1.4 millimolar, respectively. In the medium, however, the corresponding concentrations were 2 and 57 micromolar. The data indicate that l-Glu entering the cell is decarboxylated to GABA, and that specific and passive efflux is from this pool of recently synthesized GABA and not from a previously synthesized unlabeled pool of GABA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Desmaison A. M., Tixier M. Amino Acids Content in Germinating Seeds and Seedlings from Castanea sativa L. Plant Physiol. 1986 Jun;81(2):692–695. doi: 10.1104/pp.81.2.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan B. R., Givan C. V. Effects of Light and Inhibitors on Glutamate Metabolism in Leaf Discs of Vicia faba L: Sources of ATP for Glutamine Synthesis and Photoregulation of Tricarboxylic Acid Cycle Metabolism. Plant Physiol. 1979 Dec;64(6):1043–1047. doi: 10.1104/pp.64.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon S. L., Bown A. W. Evidence for a specific glutamate/h cotransport in isolated mesophyll cells. Plant Physiol. 1987 Mar;83(3):691–697. doi: 10.1104/pp.83.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon S. L., Ciccarelli B. W., Chung I., Shelp B., Bown A. W. l-Glutamate-Dependent Medium Alkalinization by Asparagus Mesophyll Cells : Cotransport or Metabolism? Plant Physiol. 1988 Dec;88(4):1042–1047. doi: 10.1104/pp.88.4.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayan V., Nair P. M. Purification and characterization of glutamate decarboxylase from Solanum tuberosum. Eur J Biochem. 1985 Jul 1;150(1):53–60. doi: 10.1111/j.1432-1033.1985.tb08987.x. [DOI] [PubMed] [Google Scholar]
- Secor J., Schrader L. E. Characterization of amino Acid efflux from isolated soybean cells. Plant Physiol. 1984 Jan;74(1):26–31. doi: 10.1104/pp.74.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocum R. D., Kaur-Sawhney R., Galston A. W. The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys. 1984 Dec;235(2):283–303. doi: 10.1016/0003-9861(84)90201-7. [DOI] [PubMed] [Google Scholar]
- Snedden W. A., Chung I., Pauls R. H., Bown A. W. Proton/l-Glutamate Symport and the Regulation of Intracellular pH in Isolated Mesophyll Cells. Plant Physiol. 1992 Jun;99(2):665–671. doi: 10.1104/pp.99.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G., Thompson J. F. In Vivo and In Vitro Studies on gamma-Aminobutyric Acid Metabolism with the Radish Plant (Raphanus sativus, L.). Plant Physiol. 1972 Apr;49(4):579–584. doi: 10.1104/pp.49.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmann T. C., Dickson R. E., Larson P. R. Comparative Distribution and Metabolism of Xylem-Borne Amino Compounds and Sucrose in Shoots of Populus deltoides. Plant Physiol. 1985 Feb;77(2):418–428. doi: 10.1104/pp.77.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. J. Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol. 1979 Jul;64(1):88–93. doi: 10.1104/pp.64.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K. A., Givan C. V., Keys A. J. Glutamic Acid metabolism and the photorespiratory nitrogen cycle in wheat leaves: metabolic consequences of elevated ammonia concentrations and of blocking ammonia assimilation. Plant Physiol. 1984 May;75(1):60–66. doi: 10.1104/pp.75.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W., Secor J., Schrader L. E. Rapid Accumulation of gamma-Aminobutyric Acid and Alanine in Soybean Leaves in Response to an Abrupt Transfer to Lower Temperature, Darkness, or Mechanical Manipulation. Plant Physiol. 1984 May;75(1):170–175. doi: 10.1104/pp.75.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]


