Abstract
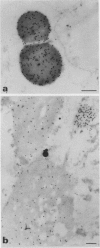
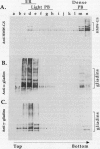
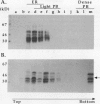
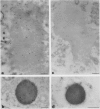
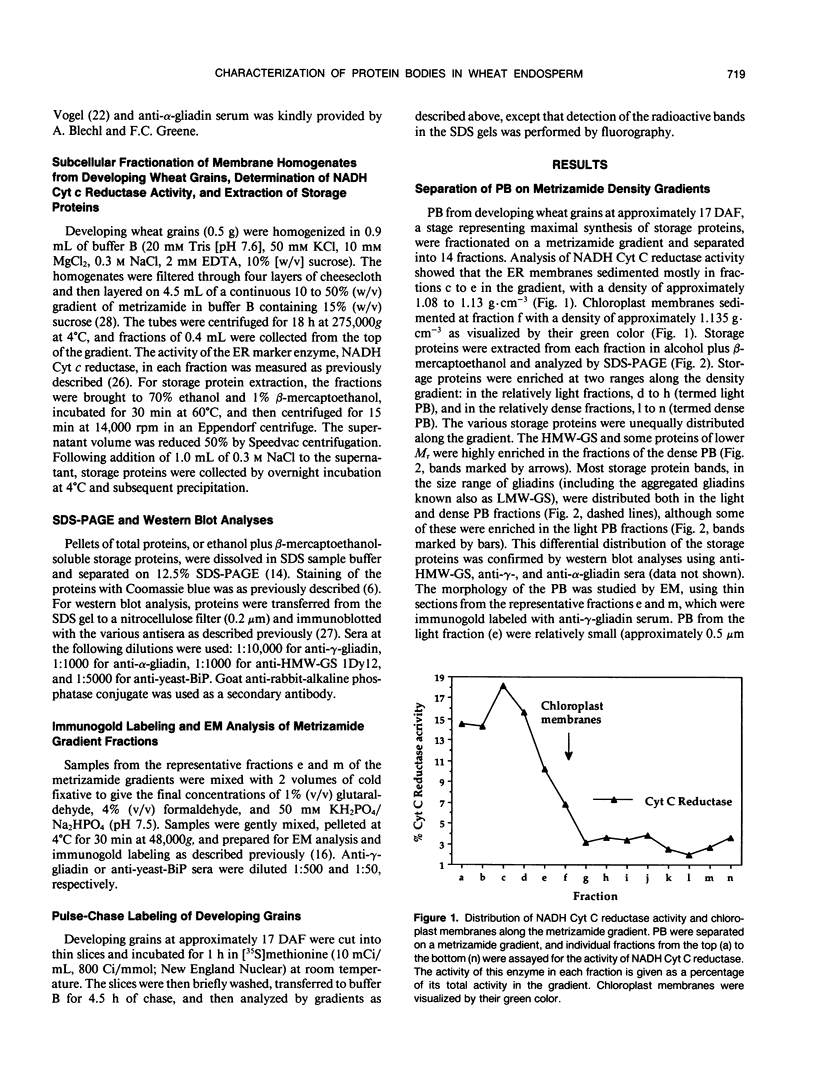
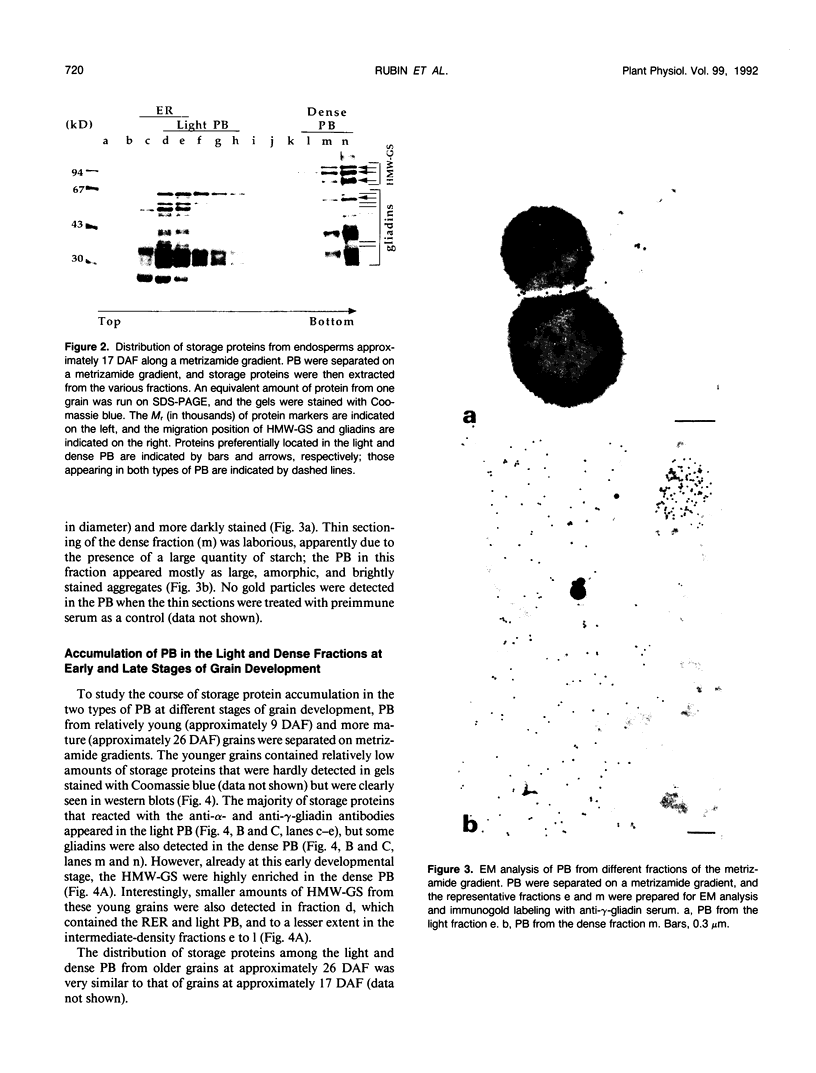
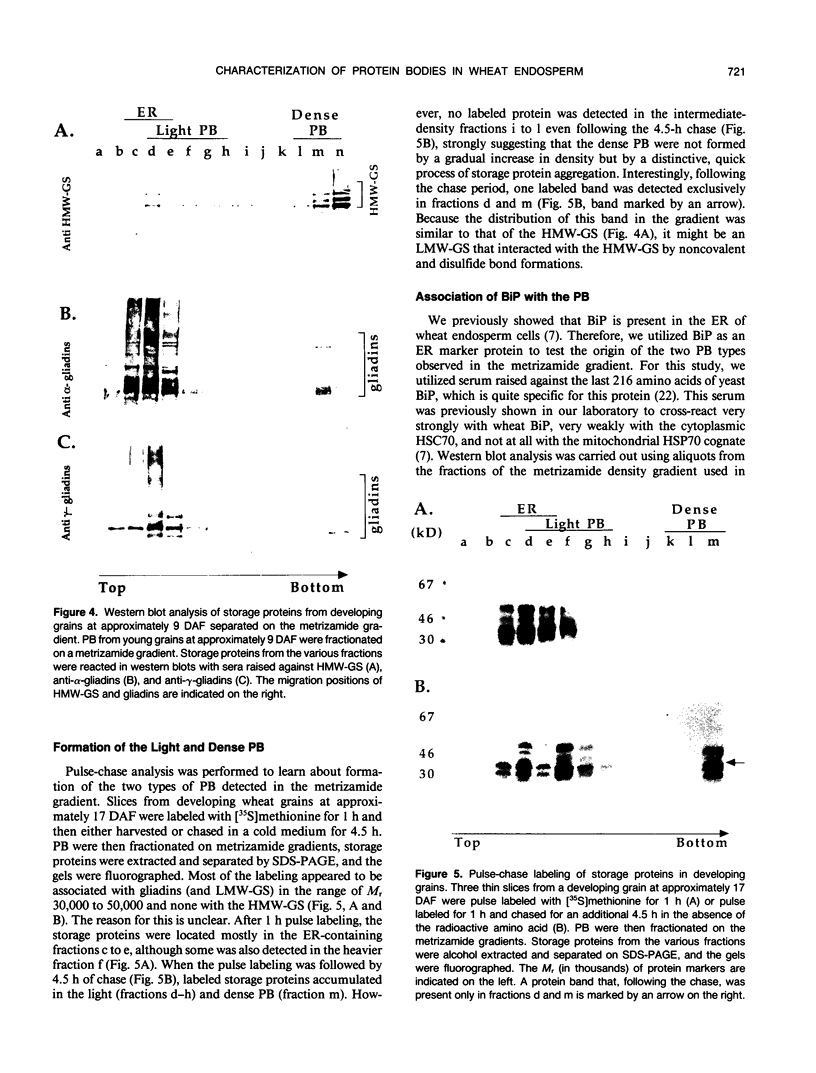
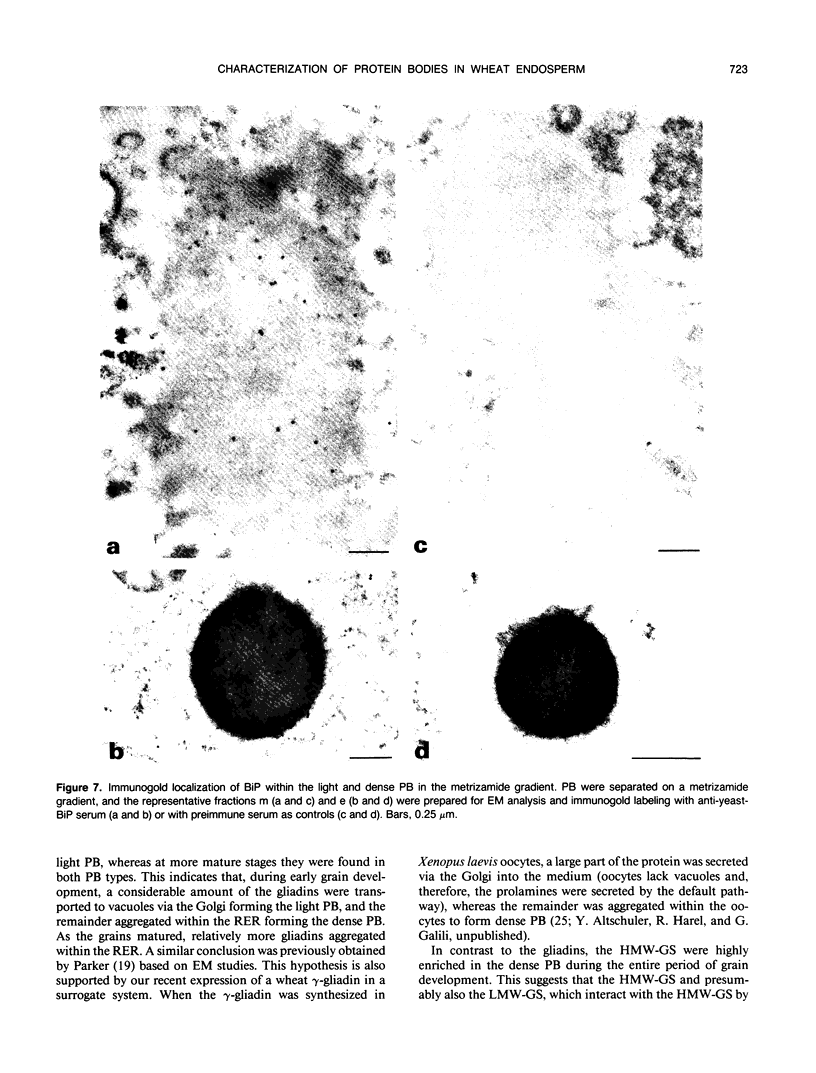
Storage proteins of wheat grains (Triticum L. em Thell) are deposited in protein bodies inside vacuoles. However, the subcellular sites and mechanisms of their aggregation into protein bodies are not clear. In the present report, we provide evidence for two different types of protein bodies, low- and high-density types that accumulate concurrently and independently in developing wheat endosperm cells. Gliadins were present in both types of protein bodies, whereas the high molecular weight glutenins were localized mainly in the dense ones. Pulse-chase experiments verified that the dense protein bodies were not formed by a gradual increase in density but, presumably, by a distinct, quick process of storage protein aggregation. Subcellular fractionation and electron microscopy studies revealed that the wheat homolog of immunoglobulin heavy-chain-binding protein, an endoplasmic reticulum-resident protein, was present within the dense protein bodies, implying that these were formed by aggregation of storage proteins within the endoplasmic reticulum. The present results suggest that a large part of wheat storage proteins aggregate into protein bodies within the rough endoplasmic reticulum. Because these protein bodies are too large to enter the Golgi, they are likely to be transported directly to vacuoles. This route may operate in concert with the known Golgi-mediated transport to vacuoles in which the storage proteins apparently condense into protein bodies at a postendoplasmic reticulum location. Our results further suggest that although gliadins are transported by either one of these routes, the high molecular weight glutenins use only the Golgi bypass route.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Denecke J., Goldman M. H., Demolder J., Seurinck J., Botterman J. The tobacco luminal binding protein is encoded by a multigene family. Plant Cell. 1991 Sep;3(9):1025–1035. doi: 10.1105/tpc.3.9.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili G. Heterologous expression of a wheat high molecular weight glutenin gene in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7756–7760. doi: 10.1073/pnas.86.20.7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick K. G., Lewis M. J., Semenza J., Dean N., Pelham H. R. ERD1, a yeast gene required for the retention of luminal endoplasmic reticulum proteins, affects glycoprotein processing in the Golgi apparatus. EMBO J. 1990 Mar;9(3):623–630. doi: 10.1002/j.1460-2075.1990.tb08154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Hurkman W. J. Synthesis and deposition of zein in protein bodies of maize endosperm. Plant Physiol. 1978 Aug;62(2):256–263. doi: 10.1104/pp.62.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci. 1990 Dec;15(12):483–486. doi: 10.1016/0968-0004(90)90303-s. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Misra L. M., Vogel J. P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989 Jun 30;57(7):1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- Shani N., Steffen-Campbell J. D., Anderson O. D., Greene F. C., Galili G. Role of the amino- and carboxy-terminal regions in the folding and oligomerization of wheat high molecular weight glutenin subunits. Plant Physiol. 1992 Feb;98(2):433–441. doi: 10.1104/pp.98.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R., Altschuler Y., Rubin R., Galili G. Two Closely Related Wheat Storage Proteins Follow a Markedly Different Subcellular Route in Xenopus laevis Oocytes. Plant Cell. 1990 Sep;2(9):941–950. doi: 10.1105/tpc.2.9.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E. Isolation of subcellular organelles of metabolism on isopycnic sucrose gradients. Methods Enzymol. 1974;31:734–746. doi: 10.1016/0076-6879(74)31077-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOYCHIK J. H., BOUNDY J. A., DIMLER R. J. Starch gel electrophoresis of wheat gluten proteins with concentrated urea. Arch Biochem Biophys. 1961 Sep;94:477–482. doi: 10.1016/0003-9861(61)90075-3. [DOI] [PubMed] [Google Scholar]
- Wallace J. C., Galili G., Kawata E. E., Cuellar R. E., Shotwell M. A., Larkins B. A. Aggregation of lysine-containing zeins into protein bodies in Xenopus oocytes. Science. 1988 Apr 29;240(4852):662–664. doi: 10.1126/science.2834822. [DOI] [PubMed] [Google Scholar]