Abstract
Although there have been no cases of serotype 2 wild poliovirus for more than 20 years, transmission of serotype 2 vaccine-derived poliovirus (VDPV2) and associated paralytic cases in several continents represent a threat to eradication. The withdrawal of the serotype 2 component of oral poliovirus vaccine (OPV2) was implemented in April 2016 to stop VDPV2 emergence and secure eradication of all serotype 2 poliovirus. Globally, children born after this date have limited immunity to prevent transmission. Using a statistical model, we estimated the emergence date and source of VDPV2s detected between May 2016 and November 2019. Outbreak response campaigns with monovalent OPV2 are the only available method to induce immunity to prevent transmission. Yet our analysis shows that using monovalent OPV2 is generating more paralytic VDPV2 outbreaks with the potential for establishing endemic transmission. A novel OPV2, for which two candidates are currently in clinical trials, is urgently required, together with a contingency strategy if this vaccine does not materialize or perform as anticipated.
A public health catch-22
In 2016, the serotype 2 component of the oral poliovirus vaccine given to children was withdrawn. This measure was taken to prevent vaccine-associated disease outbreaks caused by mutation in the live attenuated vaccine. Children around the world now have poor immunity to serotype 2 poliovirus because the inactivated vaccine is far less effective and a new oral vaccine is not yet ready. Using a statistical model, Macklin et al. discovered that most current outbreaks of polio in several countries across Asia and sub-Saharan Africa are likely associated with the serotype 2 vaccine strain (see the Perspective by Donlan and Petri). To block transmission when poliovirus outbreaks occur requires deployment of the only tool in the box: the existing live attenuated serotype 2 oral vaccine, which increases the risk of vaccine-derived disease.
Ever since the oral poliovirus vaccine (OPV) was first identified in 2000 as the source of a paralytic poliomyelitis outbreak, vaccine-derived polioviruses (VDPVs) have been a known obstacle to achieving polio eradication (1, 2). Despite the global withdrawal of the serotype 2 component of OPV (OPV2), paralytic poliomyelitis cases associated with serotype 2 VDPV (VDPV2) have been reported in expanding global geographies. This is important because there is now a global cohort of children without immunity against serotype 2 that would prevent transmission, which could result in established endemicity of the virus. The inactivated poliovirus vaccine (IPV) can protect against paralysis but provides limited intestinal immunity to stop transmission (3). Therefore, the method to control VDPV2 transmission is through vaccination campaigns with monovalent OPV2 (mOPV2) (4). However, any use of mOPV2 carries the risk of seeding more VDPV2 (5).
After the eradication of serotype 2 wild poliovirus (WPV), vaccination continued with OPV2 as part of the trivalent vaccine (tOPV, containing serotypes 1, 2, and 3) (fig. S1), resulting in periodic outbreaks of VDPV2 (as well as VDPV1 and VDPV3) and cases of vaccine-associated paralytic poliomyelitis (VAPP) (6). This is because the attenuated virus strains contained in OPV can mutate and reacquire factors associated with causing paralytic disease and transmission (7). Populations with low immunization coverage are particularly at risk of spread (7). Once the eradication of serotype 2 WPV was certified, it was decided to withdraw the OPV2 to prevent paralysis caused by type 2 poliovirus (fig. S1) (6). In April 2016, the Global Polio Eradication Initiative (GPEI) coordinated a globally synchronized switch from tOPV to bivalent OPV (bOPV, containing serotypes 1 and 3) in all routine and supplemental immunization activities, commonly referred to as “the Switch” (fig. S1) (8). As a risk mitigation strategy, countries began to introduce a dose of IPV into routine immunization schedules to protect against paralysis from serotype 2 poliovirus (9). However, an estimated 143 million children have not received IPV since April 2016 because of supply shortages (43 million) and poor routine immunization coverage (100 million) (10).
It was predicted that after the Switch, circulation of serotype 2 polioviruses would steadily disappear. Some VDPV2 outbreaks were expected, largely from prior widespread tOPV use in immunization campaigns (approximately 1.5 billion doses in the 12 months before the Switch) (11, 12). The response to any outbreaks was to conduct campaigns with mOPV2 from a finite global stockpile of vaccine (4). Although the virus disappeared from most geographies, eradication did not occur (13). More recently, outbreaks of VDPV2 have been increasing in frequency and geographic spread (Fig. 1). At present, WHO classifies circulating VDPV2 (cVDPV2) outbreaks as Public Health Emergencies of International Concern (14). Here, we investigated the epidemiology and source of VDPV2 outbreaks through a retrospective analysis of poliovirus surveillance and mOPV2 campaign data between 1 May 2016 and 1 November 2019.
Fig. 1. Geographic location of vaccine-derived poliovirus serotype 2 (VDPV2) isolates detected between 1 May 2016 and 1 November 2019.
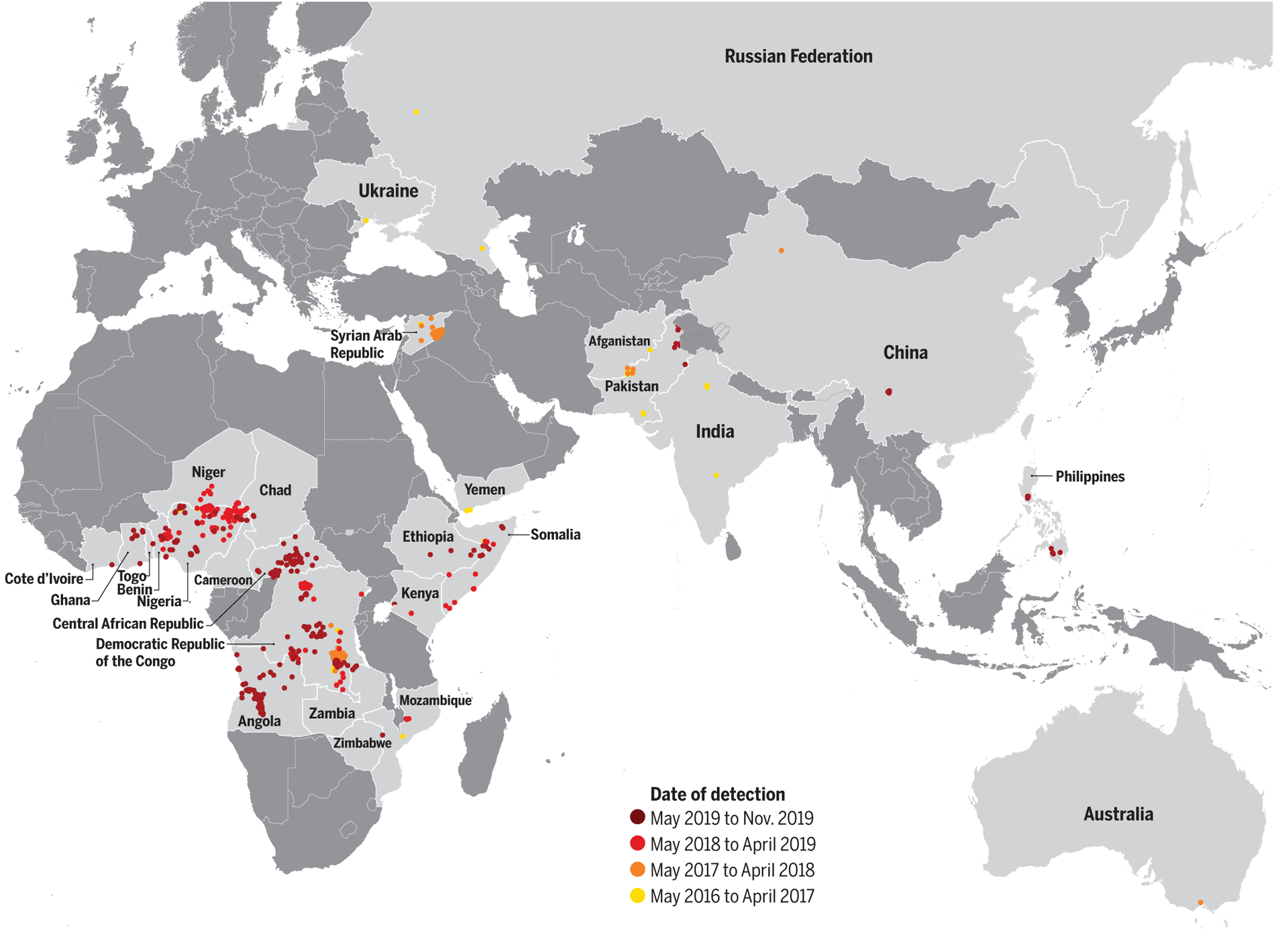
The color of points illustrates the date of isolate detection. Data are as of 1 November 2019.
We obtained data on virus isolates from acute flaccid paralysis (AFP) cases and environmental samples through the surveillance network of the Global Polio Laboratory Network (GPLN) on 1 November 2019. We estimated the date of seeding interval (i.e., 95% confidence intervals for the date that the infectious OPV dose was administered) on the basis of the date of detection and the number of nucleotides divergent from the OPV2 virus in the viral protein 1 (VP1) gene (see supplementary materials). We assumed that the first VP1 mutation is instantaneous and that each subsequent mutation follows an average rate, previously estimated at 1.14 × 10–2 nucleotides per site per year, that corresponds to one nucleotide change observed after approximately 35 days (15). The time to each independent mutation was modeled using an exponential distribution, and the sum of waiting times as an Erlang distribution (see supplementary materials).
Global VDPV2 detections and source
Between 1 May 2016 and 1 November 2019, the GPLN had detected 859 isolates of VDPV2 across 26 countries, including 325 cases of AFP (Fig. 1). The AFP cases had a median age of 1.75 years (range 0.2 to 12 years), and 27.0% of cases reported receiving no previous polio vaccine doses.
We calculated that 65.5% (548/837) of sequenced VDPV2 viruses detected since April 2016 had a ≥90% probability of being seeded after the Switch (Fig. 2A). For isolates with a ≥90% probability of being seeded after the Switch, we identified whether a mOPV2 campaign was conducted within the same geographic region during the estimated seeding interval. We found that the source of 71.5% (392/548) of these isolates was consistent with mOPV2 outbreak response campaigns conducted within the country of emergence and that the source of 24.6% (135/548) was consistent with mOPV2 campaigns conducted within a neighboring country (Fig. 2B).
Fig. 2. Incidence of vaccine-derived poliovirus serotype 2 (VDPV2) isolates detected between 1 May 2016 and 1 November 2019.
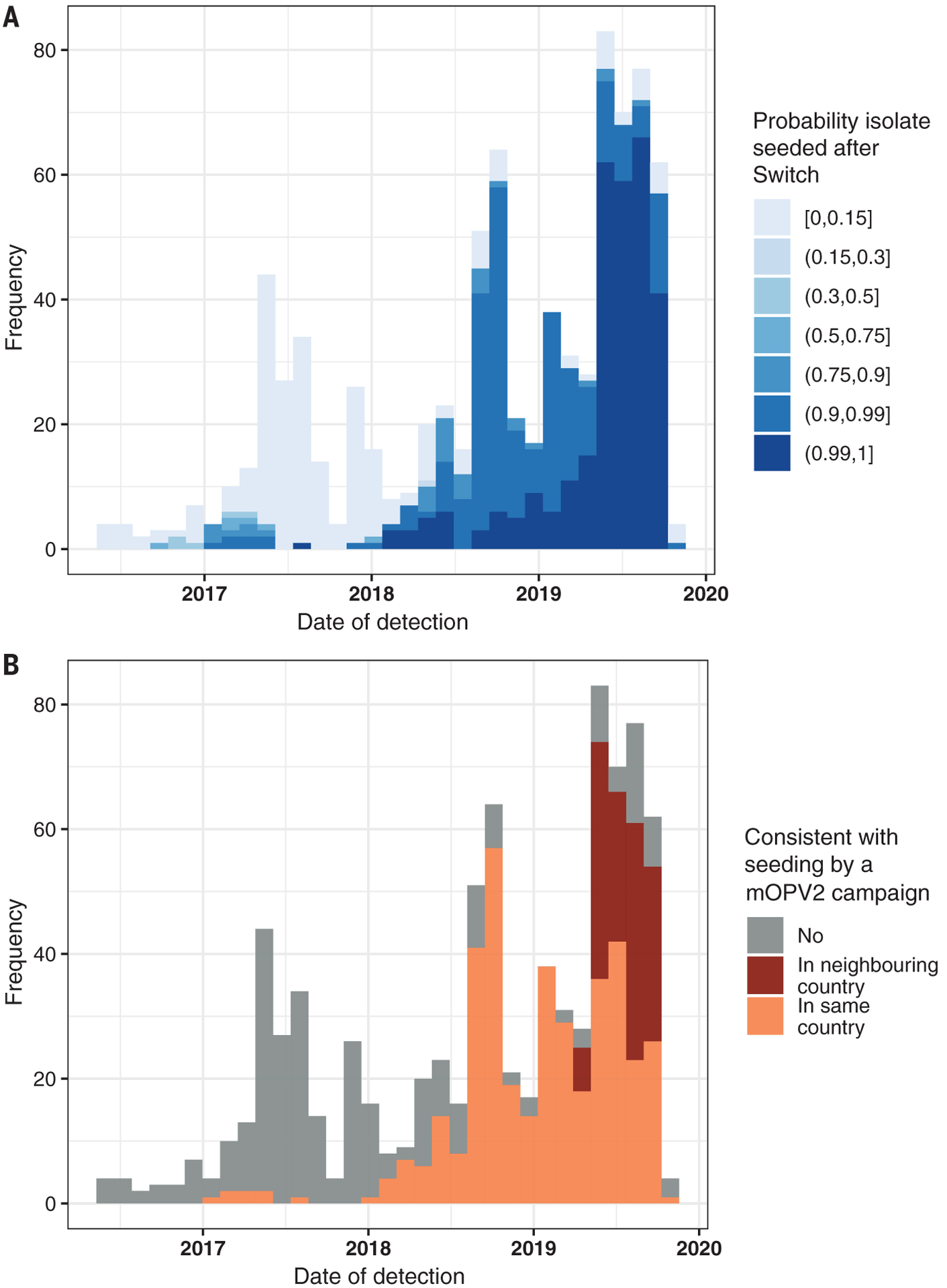
(A) The probability that isolate was seeded after the Switch (1 May 2016) was calculated according to the 95% confidence intervals of the estimated seeding date, estimated by the number of nucleotides of divergence from the poliovirus vaccine strain, in the viral protein 1 gene of the position, assuming a model for the mutation rate (see supplementary materials). (B) For all isolates with >0.9 probability of post-Switch seeding, the color demonstrates whether there was a corresponding mOPV2 campaign within estimated dates of seeding and the same or adjacent country.
Circulating VDPV2 (cVDPV2) outbreaks
VDPV2 emergences are classified as cVDPV2 when there is evidence of person-to-person transmission (isolates are genetically linked to a previously detected isolate), or as ambiguous VDPV2 (aVDPV2) events, when there is no evidence of transmission and after ruling out primary immunodeficiency in infected individuals (16, 17).
We identified 62 aVDPV2 events and 41 independent cVDPV2 outbreaks that have occurred since the Switch (Fig. 3 and table S1). The 41 cVDPV2 outbreaks emerged in Angola (n = 7), Central African Republic (n = 6), China (n = 1), Democratic Republic of the Congo (DRC) (n = 10), Mozambique (n = 1), Nigeria (n = 9), Pakistan (n = 3), Philippines (n = 1), Somalia (n = 1), Syrian Arab Republic (Syria) (n = 1), and Zambia (n = 1). International spread of cVDPV2s has led to transmission in Benin, Cameroon, Chad, Côte d’Ivoire, Ethiopia, Ghana, Kenya, and Togo. The countries where these outbreaks occur are mainly characterized by suboptimal health systems with low routine immunization coverage, inaccessible or active conflict-affected areas, and low sanitation and hygiene (table S1).
Fig. 3. Timeline of circulating VDPV2 outbreaks reported between 1 May 2016 and 1 November 2019, ordered by the date of first isolate detection.
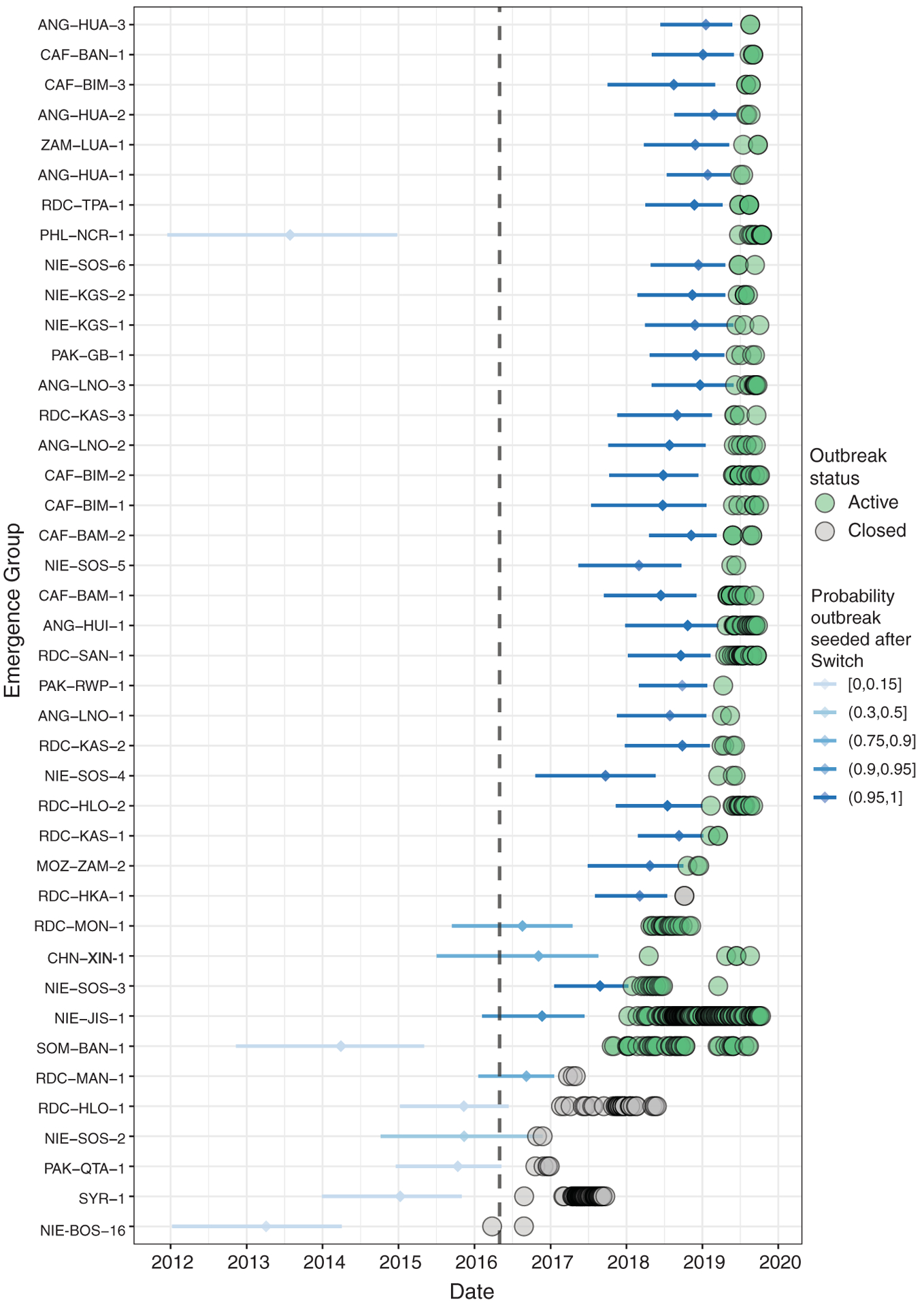
The estimated seeding date (i.e., the date that an infectious OPV dose was administered) and 95% confidence intervals are given by horizontal bars, colored by the probability that the date of seeding was after the Switch on 1 May 2016 (dashed black line denotes date of Switch). Detected virus isolates are shown by colored circles, with the color indicating whether the outbreak is assumed to be active (detection within previous 12 months) or closed (no detection in previous 12 months). Data are as of 1 November 2019. NIE-BOS-16: This outbreak was genetically linked to a cVDPV2 emergence originating in Chad in 2012.
A total of 126 post-Switch mOPV2 campaigns have been conducted in response to these outbreaks, using more than 300 million doses of the mOPV2 vaccine (table S2), primarily in Nigeria (59%) and DRC (15%). These campaigns are consistent with seeding 27 of 41 outbreaks (table S2).
Evolving situation over time
In the first year after the Switch (May 2016 to April 2017), our analysis shows that there were six cVDPV2 outbreaks, seeded before (n = 5) or close to the time of the Switch (n = 1), likely through immunization with tOPV (Fig. 3 and table S1). This was consistent with the predictions made, including from mathematical modeling groups (11, 18). These outbreaks, which occurred in Nigeria (n = 2), DRC (n = 2), Pakistan (n = 1), and Syria (n = 1), were rapidly controlled through mOPV2 use (table S1) (19).
Interestingly, we observed that no virus was detected later than 6 months after the Switch in the American, European, and South-East Asian regions of WHO: No cVDPV2 outbreaks occurred, and the rare detection of aVDPV2 in the first 6 months in these regions was limited, likely because of generally high pre-Switch intestinal mucosal immunity, good sanitation standards, and post-Switch IPV use (13, 20).
In the second year after the Switch (May 2017 to April 2018), five more outbreaks emerged (table S1). We calculate that one of these was seeded before the Switch and that the other four were seeded after the Switch (Fig. 2). In two of these outbreaks (SOM-BAN-1 and NIE-JIG-1 emergences), failure to control the virus has resulted in spread across national borders to establish transmission in neighboring countries: from Somalia to Kenya and Ethiopia and from Nigeria to Niger, Cameroon, Ghana, Benin, Chad, Togo, and Côte d’Ivoire (table S1). These two outbreaks, which have not yet been controlled, are the longest in duration, with transmission detected for periods of 22 and 21 months, respectively (table S1).
In the third and fourth years after the Switch (May 2018 to November 2019), it was expected (and planned) that there would be a substantial reduction in the number of outbreaks (18). However, we demonstrate that the highest frequency of outbreaks has been in this period: 10 outbreaks emerged between May 2018 and April 2019 and 20 in the period from May 2019 to November 2019 alone. Our analysis shows that all except one of these emergences were seeded after the Switch (Fig. 1).
There has been a shift in epidemiology observed over this period, characterized by the emergence of several cVDPV2s in 2019 with low nucleotide divergence in geographies without preceding mOPV2 use (Fig. 3). There have been six cVDPV outbreaks in the Central African Republic and seven in Angola (table S1), which are consistent with seeding from mOPV2 responses in the neighboring DRC. Additionally, two low-divergence cVDPV2s have emerged in Pakistan, a country where mOPV2 had not been used in outbreak response for more than 1 year prior to the estimated seeding date. Ongoing investigations are exploring hypotheses of outbreak source, including multiple international importations from mOPV2-using areas and inadvertent mOPV2/tOPV use. However, established transmission of cVDPV2 now exists in these populations, and as such, the geographic scope of detections is expanding rapidly (Fig. 2).
The detection of two highly divergent cVDPV2s in China and the Philippines in 2019 confirms transmission in the Western Pacific Region of WHO (table S1). In the Philippines, the cVDPV2 was first detected in an AFP case in June 2019 with a 64-nucleotide divergence from OPV2, which suggests that the virus was seeded in 2014 (Fig. 3). Subsequently, an individual with primary immunodeficiency was detected excreting virus genetically linked to the outbreak; however, the role of this case in the outbreak is not clear. It seems unlikely that the virus would circulate undetected for 5 years, although serotype 2 poliovirus is thought to have approximately 2000 infections for every paralytic case, yet these examples emphasize the need for continuing high-quality surveillance and expanding environmental surveillance (21).
Using logistic regression, we demonstrate the probability that a new VDPV2 emergence (i) was seeded after the Switch, is increasing over time (regression coefficient = 1.99, P < 0.001, intercept = −1.66); and (ii) establishes person-to-person transmission, is increasing over time (regression coefficient = 0.88, P < 0.001, intercept = −2.27).
At this juncture, we show that polio eradication is battling both the new emergences of cVDPV outbreaks seeded after the Switch—largely through mOPV2 use in response to outbreaks—and outbreaks seeded before the Switch that had delayed detection. In 2019, we have observed the largest numbers of outbreaks and countries experiencing cVDPV2 transmission to date. We conclude that the GPEI is in a paradoxical situation: On one hand, it is not currently possible to control the outbreaks without inducing intestinal mucosal immunity through mOPV2 use, but on the other hand, the use of mOPV2 is generating VDPV2. The risk of VDPV2 circulation is increasing over time as the immunity of the global population rapidly decreases (5).
Policy perspective
Since the Switch more than 4 years ago, the epidemiology of serotype 2 poliovirus has developed in directions that were neither expected nor planned. This has policy implications for polio. Although the Switch has largely eliminated the incidence of serotype 2 VAPP and immunodeficiency-related VDPV cases, it has not achieved the major objective—that is, the eradication of the last serotype 2 polioviruses (those originating from the oral poliovirus vaccine) in all populations. The question remains as to what the GPEI should do next.
In 2010, the GPEI initiated the development of two candidates for serotype 2 novel oral poliovirus vaccine (nOPV2), which are currently completing phase II clinical trials (21). The nOPV2s are designed to provide intestinal immunity similar to that of the current OPV while being more genetically stable. Therefore, the major advantage of nOPV2 use in outbreak control would be a lower risk of seeding new VDPV2 (and cVDPV2 outbreaks). In 2020, there are efforts to rapidly accelerate the clinical development of one candidate for this vaccine and pursue World Health Organization regulatory approval though the Emergency Use Listing procedure (22).
A strategy for the response to cVDPV2s has been developed for 2020–2021. In the time before nOPV2 is available, the approach is to conduct enhanced outbreak response campaigns with the current mOPV2 to contain cVDPV2 spread. Capacity to conduct aggressive, rapid, and high-quality campaigns is essential: Persistent delays and pockets of low coverage will continually hinder the impact of outbreak responses with any vaccine, whether the nOPV2 or mOPV2. Strengthening routine administration of IPV and strategic vaccination with remaining available IPV doses (to ensure that missed children in areas at high risk are reached) will be used as a paralysis prevention method.
When the nOPV2 vaccine becomes available in sufficient quantities, it will be rolled out to eventually replace mOPV2 in outbreak response. In the situation that nOPV2 does not materialize or perform as anticipated, or incurs substantial delays, the GPEI would have to implement a contingency plan (under preparation). The reintroduction of preventive vaccination with mOPV2 or tOPV, either through preventive campaigns or routine immunization, would have to be considered. However, this approach would require quantities of mOPV2 or tOPV doses that are currently not available.
It is critical that cVDPV2 outbreaks be managed as national public health emergencies in line with the declaration of a Public Health Emergency of International Concern by WHO (14). All GPEI partners, member state governments, and agencies must fully operationalize their emergency frameworks to prevent the reestablishment of endemic transmission of serotype 2 poliovirus in the form of cVDPV2. It remains clear that OPV removal is essential to stop all cases of paralytic poliomyelitis. However, the epidemiology that has evolved since OPV2 removal has implications for existing strategies outlined for total OPV cessation, which need urgent attention (23).
Data and materials availability:
Data used in this study are the property of the individual countries and are available on the Polio Information System (PolIS), https://extranet.who.int/polis/. Data access was provided through the Global Polio Eradication Initiative Data Sharing Agreement.
Supplementary Material
ACKNOWLEDGMENTS
This article was submitted on behalf of the Strategy Committee of the Global Polio Eradication Initiative (GPEI). Members of the committee include M. Zaffran (World Health Organization), J. Wenger (Bill & Melinda Gates Foundation), R. Martin (Centers for Disease Control and Prevention), A. Iyer (UNICEF), A. Nguyen (Gavi, the Vaccine Alliance), and C. Pandak (Rotary International). We acknowledge the personnel at the 144 laboratories of the WHO Global Polio Laboratory Network (GPLN) in all six WHO regions, as well as personnel from Virus Isolation, Intratypic Differentiation, and Sequencing Laboratories, for providing the data used in this study. We also acknowledge the contribution of discussions with the Institute for Disease Modelling and Kid Risk Inc. The results and conclusions in this article are those of the authors and do not necessarily represent the official position or policies of the U.S. Centers for Disease Control and Prevention.
Funding:
Supported by a Ph.D. scholarship from the UK Medical Research Council and funding from the Bill and Melinda Gates Foundation (OPP1191821) and WHO. N.C.G. acknowledges joint Centre funding from the UK Medical Research Council and Department for International Development.
Footnotes
Competing interests:
The authors declare no competing interests.
SUPPLEMENTARY MATERIALS
View the article online
REFERENCES AND NOTES
- 1.Kew O et al. , Science 296, 356–359 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Fine PE, Oblapenko G, Sutter RW, Bull. World Health Organ 82, 47–52 (2004). [PMC free article] [PubMed] [Google Scholar]
- 3.Macklin GR et al. , Lancet Infect. Dis 19, 1121–1128 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Global Polio Eradication Initiative, Standard Operating Procedures: Responding to a Poliovirus Event or Outbreak (2019); http://polioeradication.org/wp-content/uploads/2016/07/sop-polio-outbreak-response-version-20193101.pdf.
- 5.McCarthy KA, Chabot-Couture G, Famulare M, Lyons HM, Mercer LD, BMC Med. 15, 175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention, MMWR Morb. Mortal. Wkly. Rep 50, 222–224 (2001).11300627 [Google Scholar]
- 7.Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA, Annu. Rev. Microbiol 59, 587–635 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Hampton LM et al. , MMWR Morb. Mortal. Wkly. Rep 65, 934–938 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Wkly. Epidemiol. Rec 88, 1–16 (2013). [PubMed] [Google Scholar]
- 10.Zipursky S et al. , J. Infect. Dis 216 (suppl. 1), S15–S23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pons-Salort M et al. , PLOS Pathog. 12, e1005728 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duintjer Tebbens RJ, Pallansch MA, Wassilak SGF, Cochi SL, Thompson KM, BMC Infect. Dis 16, 137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blake IM et al. , N. Engl. J. Med 379, 834–845 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarocostas J, Lancet 392, 2425 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Burns CC et al. , J. Virol 87, 4907–4922 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Global Polio Eradication Initiative, Classification and Reporting of Vaccine-Derived Polioviruses (VDPV) (2016); http://polioeradication.org/wp-content/uploads/2016/09/Reporting-and-Classification-of-VDPVs_Aug2016_EN.pdf.
- 17.Macklin G et al. , Front. Immunol 8, 1103 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson KM, Duintjer Tebbens RJ, J. Infect. Dis 210 (suppl. 1), S475–S484 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Mbaeyi C et al. , MMWR Morb. Mortal. Wkly. Rep 67, 690–694 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kew OM et al. , Bull. World Health Organ 82, 16–23 (2004). [PMC free article] [PubMed] [Google Scholar]
- 21.Nathanson N, Kew OM, Am. J. Epidemiol 172, 1213–1229 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organisation, Emergency Use Listing Procedure, Version 9 (January 2020); https://extranet.who.int/prequal/sites/default/files/documents/EUL_Procedure_Jan2020.pdf.
- 23.Global Polio Eradication Initiative, Polio Post-Certification Strategy (2018); http://polioeradication.org/wp-content/uploads/2018/04/polio-post-certification-strategy-20180424-2.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


