Abstract
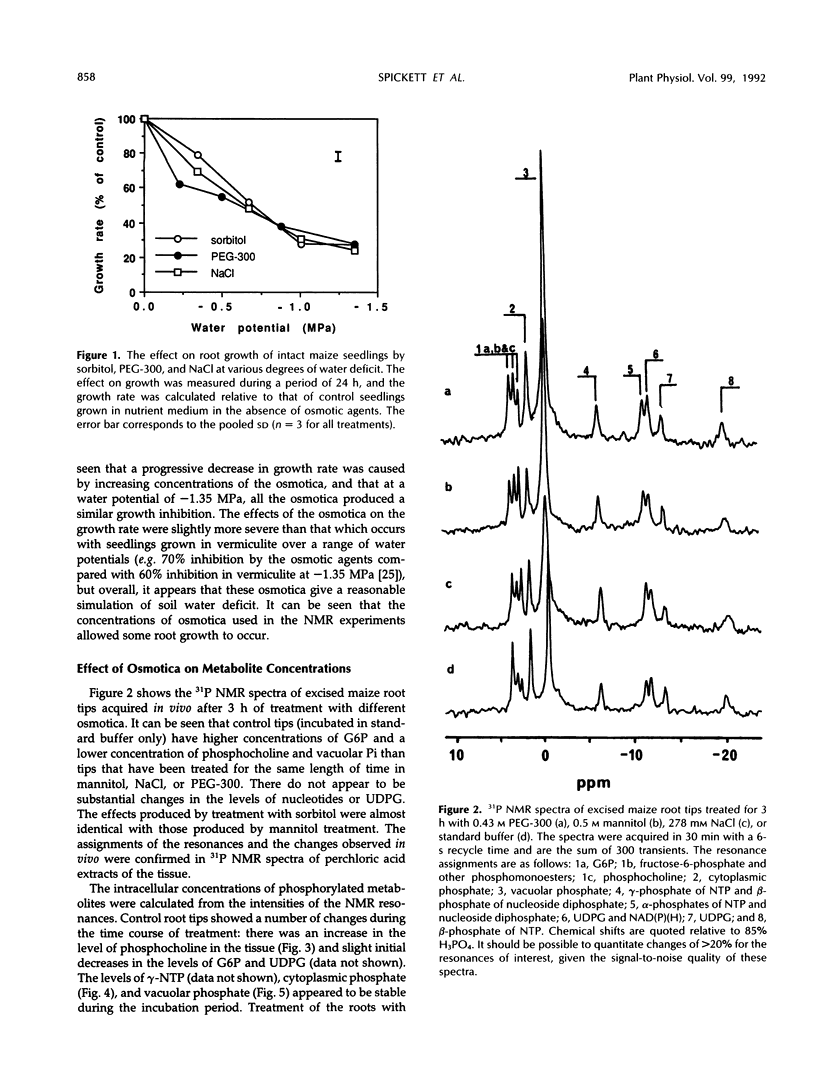
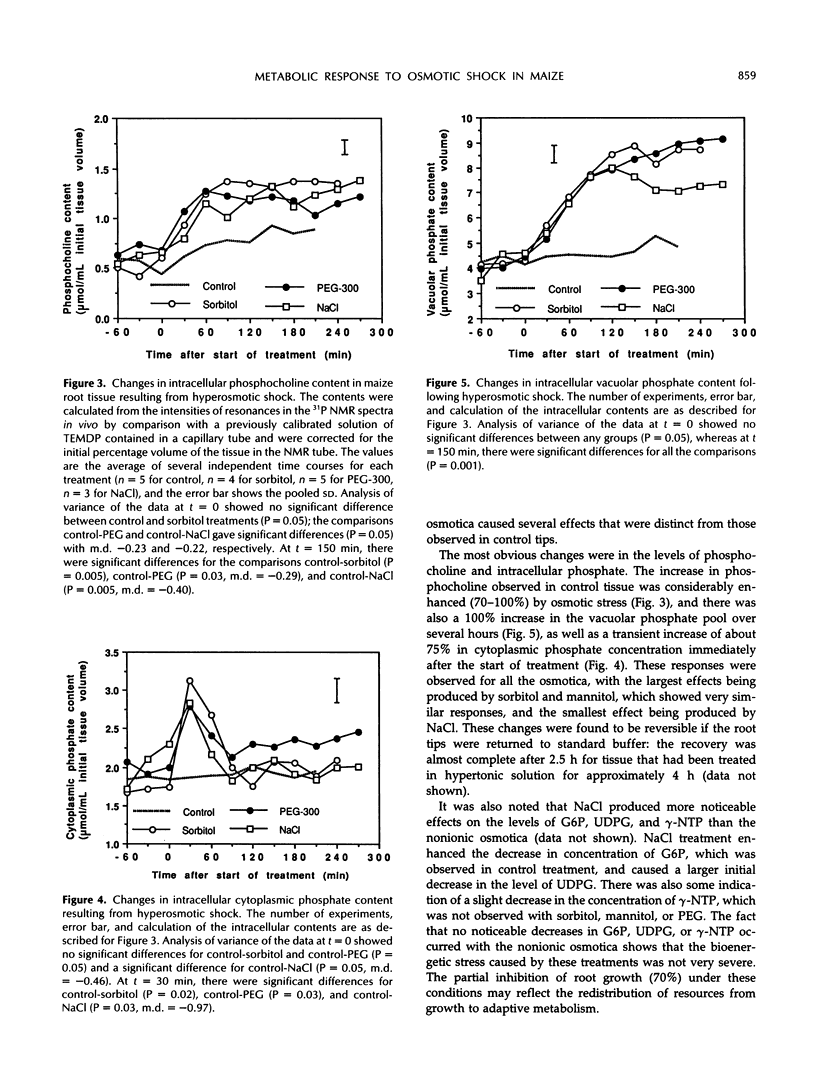
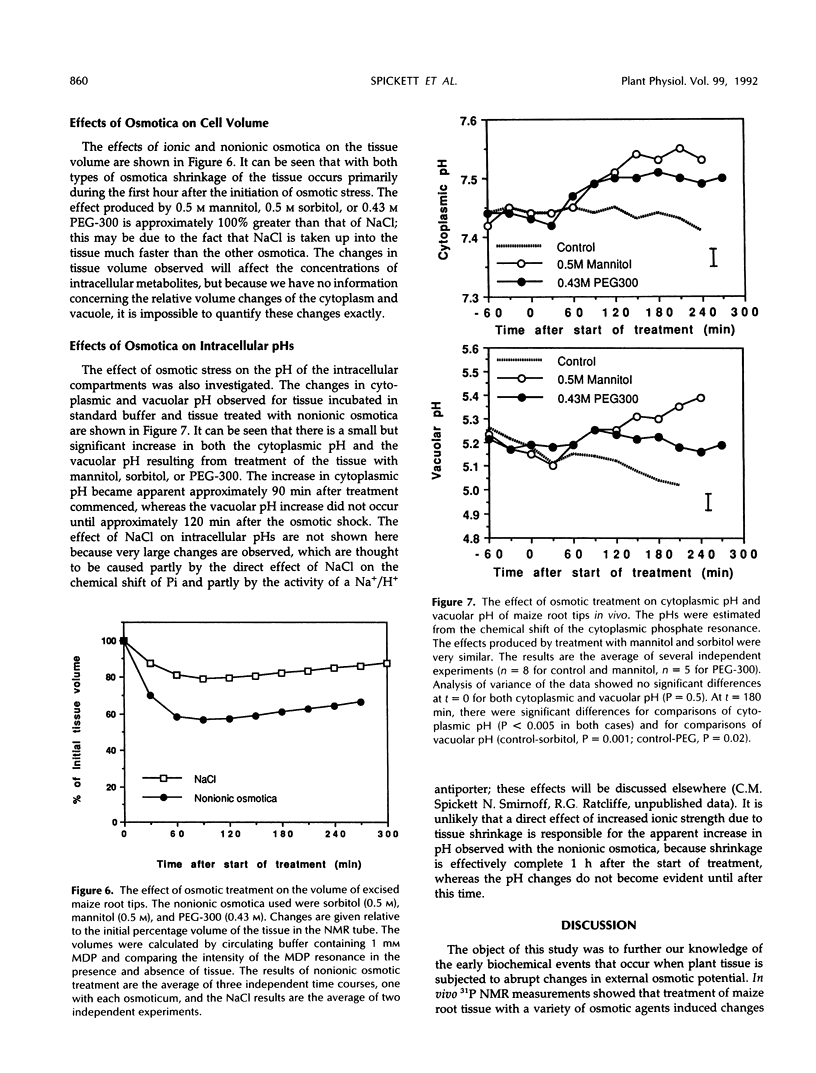
31P nuclear magnetic resonance spectroscopy was used to study the response of maize (Zea mays L.) root tips to hyperosmotic shock. The aim was to identify changes in metabolism that might be relevant to the perception of low soil water potential and the subsequent adaptation of the tissue to these conditions. Osmotic shock was found to result in two different types of response: changes in metabolite levels and changes in intracellular pH. The most notable metabolic changes, which were produced by all the osmotica tested, were increases in phosphocholine and vacuolar phosphate, with a transient increase in cytoplasmic phosphate. It was observed that treatment with ionic and nonionic osmotica produced different effects on the concentrations of bioenergetically important metabolites. It is postulated that these changes are the result of hydrolysis of phosphatidylcholine and other membrane phospholipids, due to differential activation of specific membrane-associated phospholipases by changes in the surface tension of the plasmalemma. These events may be important in the detection of osmotic shock and subsequent acclimatization. A cytoplasmic alkalinization was also observed during hyperosmotic treatment, and this response, which is consistent with the activation of the plasmalemma H+-ATPase, together with the other metabolic changes, may suggest the existence of a complex and integrated mechanism of osmoregulation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bental M., Pick U., Avron M., Degani H. The role of intracellular orthophosphate in triggering osmoregulation in the alga Dunaliella salina. Eur J Biochem. 1990 Feb 22;188(1):117–122. doi: 10.1111/j.1432-1033.1990.tb15378.x. [DOI] [PubMed] [Google Scholar]
- Cabot M. C., Welsh C. J., Zhang Z. C., Cao H. T., Chabbott H., Lebowitz M. Vasopressin, phorbol diesters and serum elicit choline glycerophospholipid hydrolysis and diacylglycerol formation in nontransformed cells: transformed derivatives do not respond. Biochim Biophys Acta. 1988 Mar 4;959(1):46–57. doi: 10.1016/0005-2760(88)90148-8. [DOI] [PubMed] [Google Scholar]
- Campbell-Burk S. L., Shulman R. G. High-resolution NMR studies of Saccharomyces cerevisiae. Annu Rev Microbiol. 1987;41:595–616. doi: 10.1146/annurev.mi.41.100187.003115. [DOI] [PubMed] [Google Scholar]
- Chitlaru E., Pick U. Regulation of glycerol synthesis in response to osmotic changes in dunaliella. Plant Physiol. 1991 May;96(1):50–60. doi: 10.1104/pp.96.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demel R. A., Geurts van Kessel W. S., Zwaal R. F., Roelofsen B., van Deenen L. L. Relation between various phospholipase actions on human red cell membranes and the interfacial phospholipid pressure in monolayers. Biochim Biophys Acta. 1975 Sep 16;406(1):97–107. doi: 10.1016/0005-2736(75)90045-0. [DOI] [PubMed] [Google Scholar]
- Einspahr K. J., Maeda M., Thompson G. A., Jr Concurrent changes in Dunaliella salina ultrastructure and membrane phospholipid metabolism after hyperosmotic shock. J Cell Biol. 1988 Aug;107(2):529–538. doi: 10.1083/jcb.107.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Money N. P. Osmotic Pressure of Aqueous Polyethylene Glycols : Relationship between Molecular Weight and Vapor Pressure Deficit. Plant Physiol. 1989 Oct;91(2):766–769. doi: 10.1104/pp.91.2.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg P., Liljenberg C. Lipids of plasma membranes prepared from oat root cells : effects of induced water-deficit tolerance. Plant Physiol. 1991 Aug;96(4):1136–1141. doi: 10.1104/pp.96.4.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren-Shamir M., Pick U., Avron M. Involvement of the Plasma Membrane ATPase in the Osmoregulatory Mechanism of the Alga Dunaliella salina. Plant Physiol. 1989 Apr;89(4):1258–1263. doi: 10.1104/pp.89.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren-Shamir M., Pick U., Avron M. Plasma membrane potential of the alga dunaliella, and its relation to osmoregulation. Plant Physiol. 1990 Jun;93(2):403–408. doi: 10.1104/pp.93.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M., Taub D. D., Lin Y. S., Rogers T. J. Immunosuppressive activity of staphylococcal enterotoxin B. I. Characterization of staphylococcal enterotoxin-B-induced suppressor cells. Cell Immunol. 1990 Nov;131(1):159–169. doi: 10.1016/0008-8749(90)90243-k. [DOI] [PubMed] [Google Scholar]
- Reinhold L., Seiden A., Volokita M. Is modulation of the rate of proton pumping a key event in osmoregulation? Plant Physiol. 1984 Jul;75(3):846–849. doi: 10.1104/pp.75.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni M., Colombo R., Lerner H. R., Pradet A., Poljakoff-Mayber A. Osmotically induced proton extrusion from carrot cells in suspension culture. Plant Physiol. 1987 Oct;85(2):383–388. doi: 10.1104/pp.85.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Wade-Jardetzky N., Jardetzky O. Intracellular pH measurements by 31P nuclear magnetic resonance. Influence of factors other than pH on 31P chemical shifts. Biochemistry. 1981 Sep 15;20(19):5389–5394. doi: 10.1021/bi00522a006. [DOI] [PubMed] [Google Scholar]
- Roby C., Martin J. B., Bligny R., Douce R. Biochemical changes during sucrose deprivation in higher plant cells. Phosphorus-31 nuclear magnetic resonance studies. J Biol Chem. 1987 Apr 15;262(11):5000–5007. [PubMed] [Google Scholar]
- Sharp R. E., Silk W. K., Hsiao T. C. Growth of the maize primary root at low water potentials : I. Spatial distribution of expansive growth. Plant Physiol. 1988 May;87(1):50–57. doi: 10.1104/pp.87.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P. A. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol. 1983 Dec;61(12):1444–1461. doi: 10.1139/y83-207. [DOI] [PubMed] [Google Scholar]
- Sáez M. J., Lagunas R. Determination of intermediary metabolites in yeast. Critical examination of the effect of sampling conditions and recommendations for obtaining true levels. Mol Cell Biochem. 1976 Nov 30;13(2):73–78. doi: 10.1007/BF01837056. [DOI] [PubMed] [Google Scholar]


