Abstract
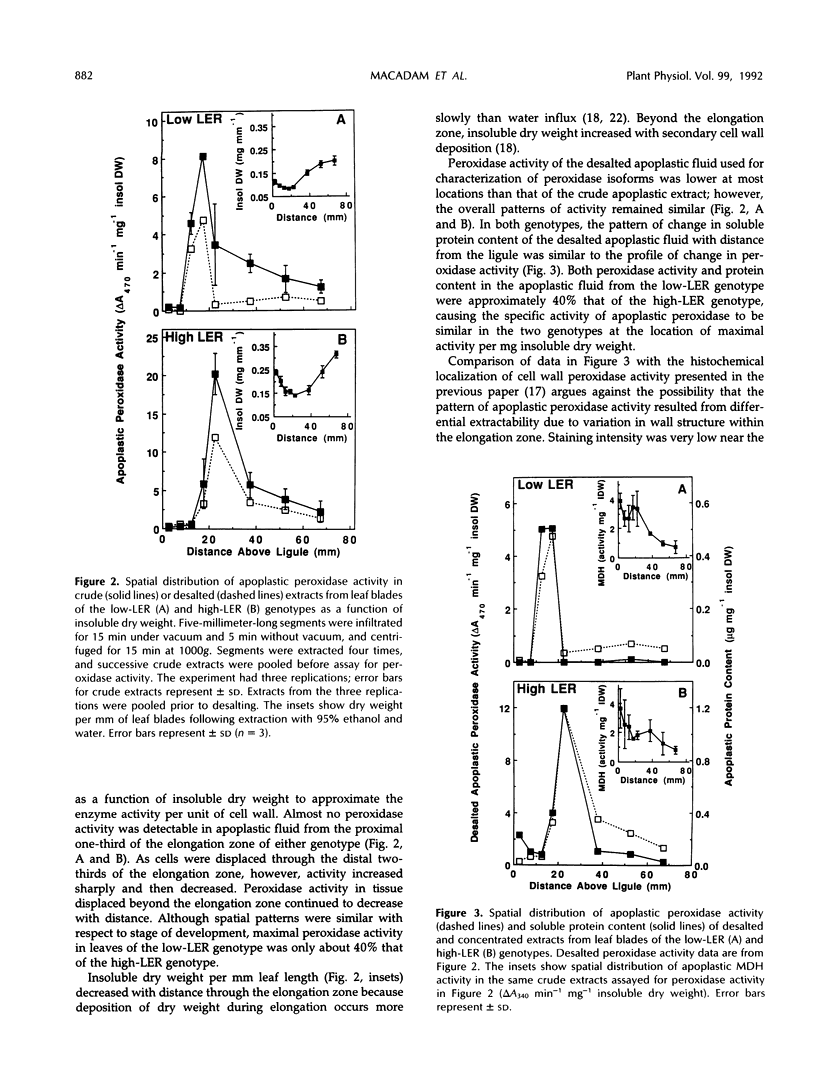
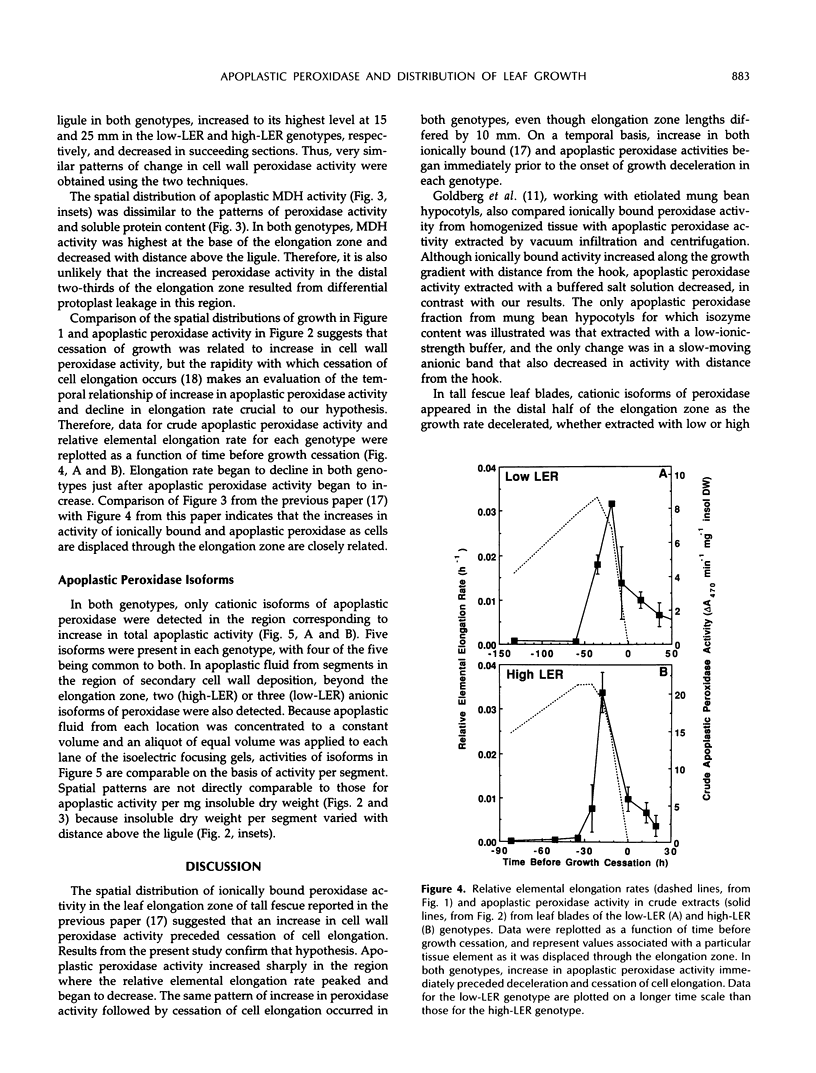
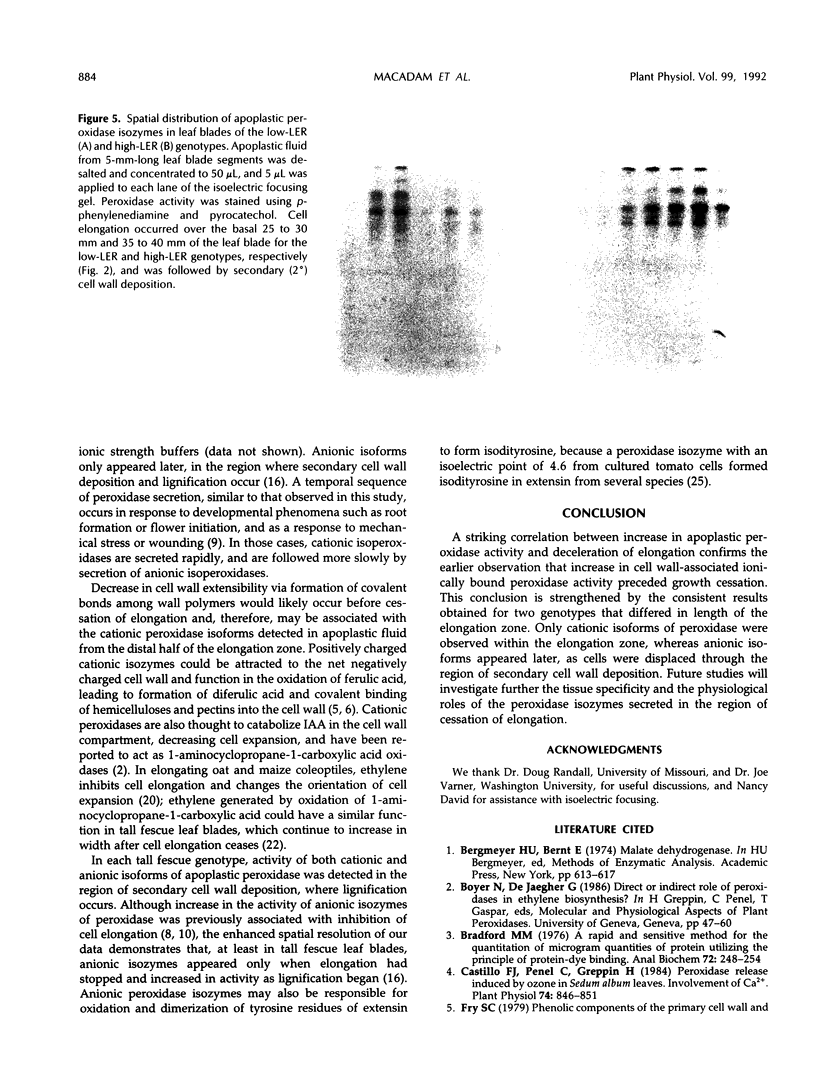
Previous work suggested that cell wall peroxidase activity increased as cells were displaced through the elongation zone in leaf blades of tall fescue (Festuca arundinacea Schreb.). In this study, two genotypes that differ in length of the elongation zone were used to examine the relationship between peroxidase activity in apoplastic fluid of intact leaf blade segments and the spatial distribution of leaf growth. Apoplastic fluid was extracted by vacuum infiltration and centrifugation, and peroxidase activity was assayed spectrophotometrically. Isoelectric focusing was used to characterize the isoforms of apoplastic peroxidase within the region of elongation and in the region of secondary cell wall deposition, which is distal to the elongation zone. A striking correlation was found in each genotype between both the location and timing of increase in apoplastic peroxidase activity and the onset of growth deceleration. Only cationic isoforms of apoplastic peroxidase could be identified in the elongation zone, whereas additional anionic isoforms appeared in the region of secondary cell wall deposition. We conclude that cessation of elongation growth in tall fescue leaf blades is likely to be related to the secretion of cationic isoforms of peroxidase into the cell wall.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Castillo F. J., Penel C., Greppin H. Peroxidase Release Induced by Ozone in Sedum album Leaves: Involvement of Ca. Plant Physiol. 1984 Apr;74(4):846–851. doi: 10.1104/pp.74.4.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez J., Pérez-Ojeda E., Serrano J. S., Castillo J. R., Serrano M. I. Possible involvement of epinephrine in the cardiovascular effect of naloxone in humans. Clin Ther. 1985;7(4):418–423. [PubMed] [Google Scholar]
- Kim S. H., Terry M. E., Hoops P., Dauwalder M., Roux S. J. Production and characterization of monoclonal antibodies to wall-localized peroxidases from corn seedlings. Plant Physiol. 1988;88:1446–1453. doi: 10.1104/pp.88.4.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. C., McClure J. W., Hagerman A. E. Soluble and Bound Apoplastic Activity for Peroxidase, beta-d-Glucosidase, Malate Dehydrogenase, and Nonspecific Arylesterase, in Barley (Hordeum vulgare L.) and Oat (Avena sativa L.) Primary Leaves. Plant Physiol. 1989 May;90(1):185–190. doi: 10.1104/pp.90.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macadam J. W., Nelson C. J., Sharp R. E. Peroxidase activity in the leaf elongation zone of tall fescue : I. Spatial distribution of ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiol. 1992 Jul;99(3):872–878. doi: 10.1104/pp.99.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macadam J. W., Volenec J. J., Nelson C. J. Effects of nitrogen on mesophyll cell division and epidermal cell elongation in tall fescue leaf blades. Plant Physiol. 1989 Feb;89(2):549–556. doi: 10.1104/pp.89.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder H., Nelson C. J. Growth rates and assimilate partitioning in the elongation zone of tall fescue leaf blades at high and low irradiance. Plant Physiol. 1989 Jul;90(3):1201–1206. doi: 10.1104/pp.90.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticher L., Penel C., Greppin H. Calcium requirement for the secretion of peroxidases by plant cell suspensions. J Cell Sci. 1981 Apr;48:345–353. doi: 10.1242/jcs.48.1.345. [DOI] [PubMed] [Google Scholar]
- Terry M. E., Bonner B. A. An Examination of Centrifugation as a Method of Extracting an Extracellular Solution from Peas, and Its Use for the Study of Indoleacetic Acid-induced Growth. Plant Physiol. 1980 Aug;66(2):321–325. doi: 10.1104/pp.66.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]



