Abstract
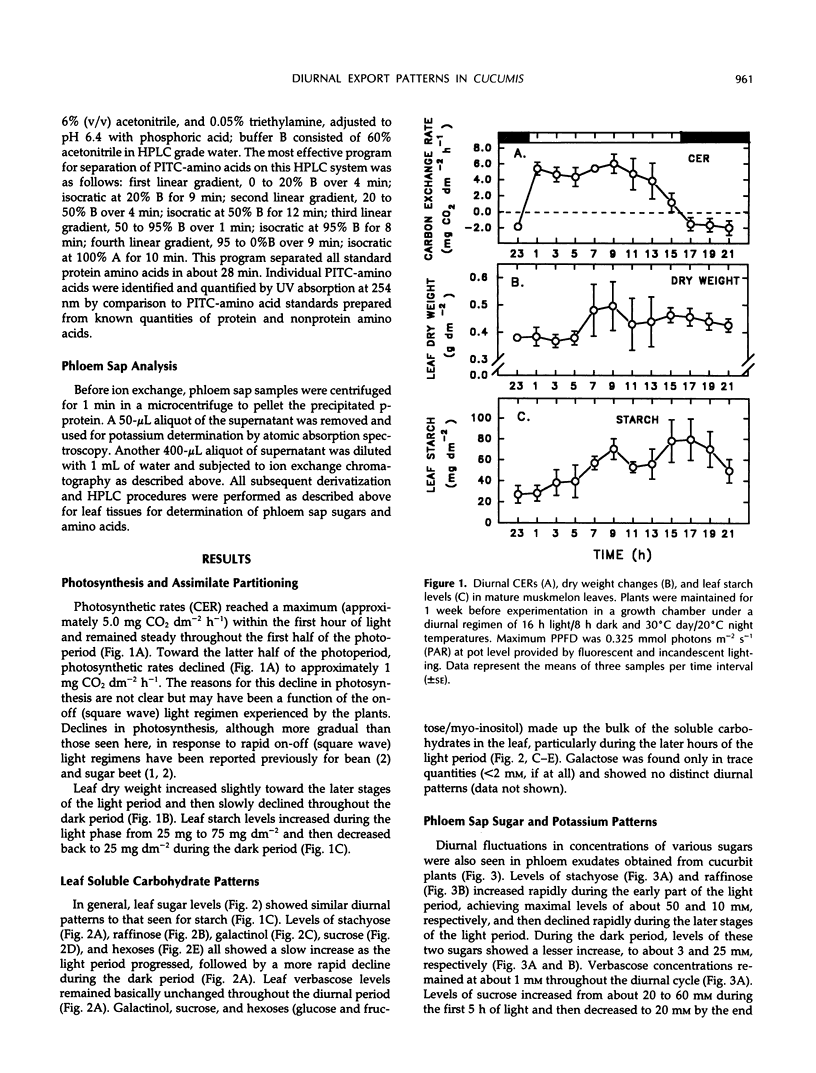
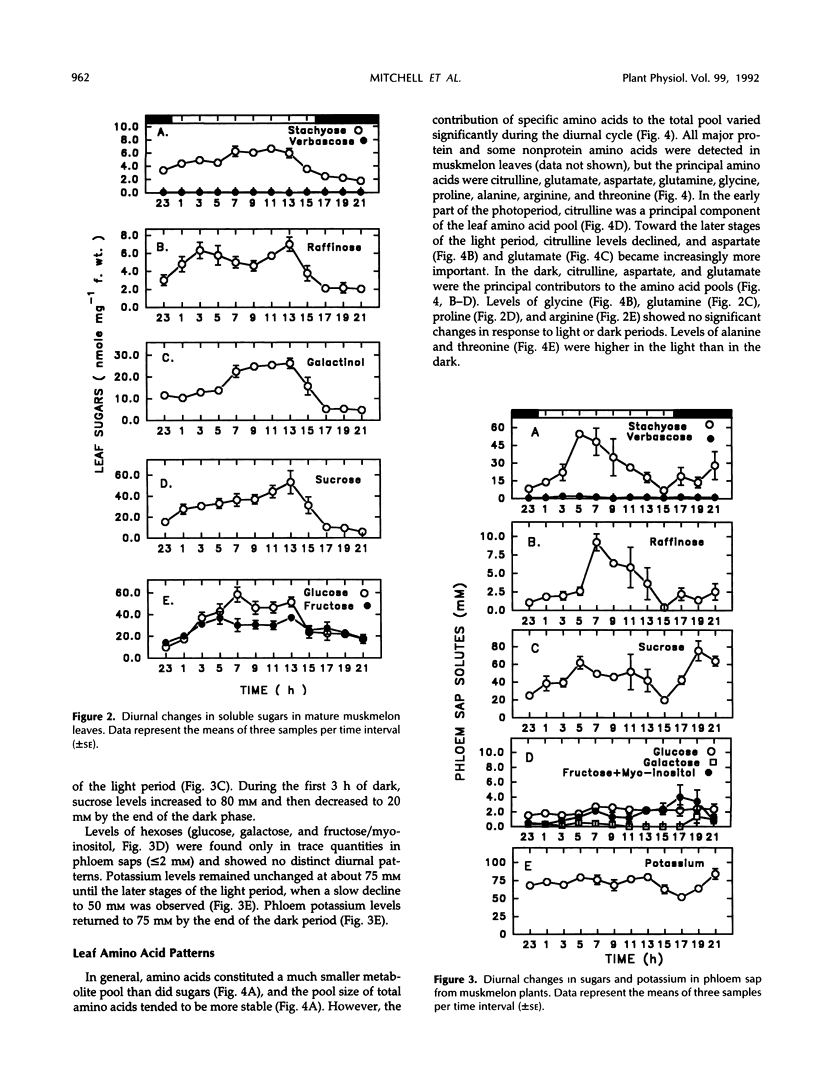
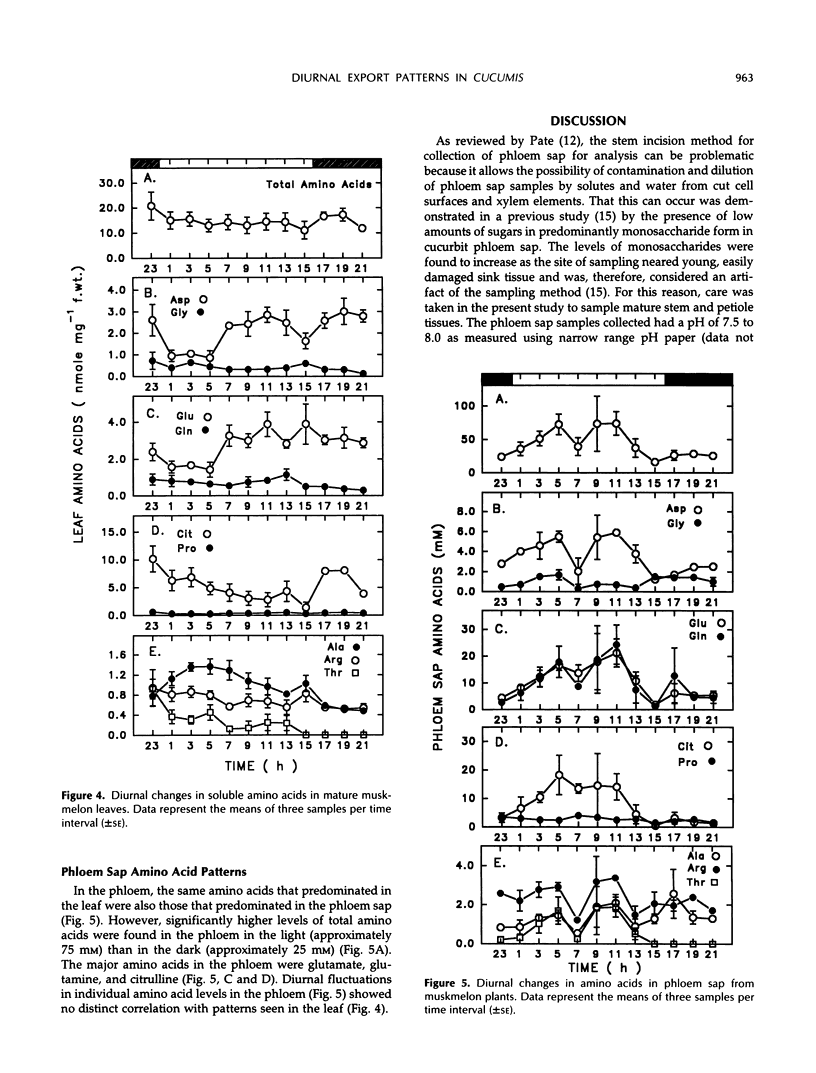
Continuous monitoring of steady-state carbon dioxide exchange rates in mature muskmelon (Cucumis melo L.) leaves showed diurnal patterns of photosynthesis and respiration that were translated into distinct patterns of accumulation and phloem export of soluble sugars and amino acids. Leaf soluble sugar patterns in general followed the pattern of photosynthetic activity observed in the leaf, whereas starch accumulated steadily throughout the light period. Sugar and starch levels declined through the dark phase. Phloem exudate analysis revealed that diurnal levels of the major transport sugars (stachyose and sucrose) in the phloem did not appear to correlate directly with the photosynthetic activity of the leaf but instead were inversely correlated with leaf starch accumulation and degradation. The amino acid pool in leaf tissues remained constant throughout the diurnal period; however, the relative contribution of individual amino acids to the total pool varied with the diurnal photosynthetic and respiratory activity of the leaf. In contrast, the phloem sap amino acid pool size was substantially larger in the light than in the dark, a result primarily due to enhanced export of glutamine, glutamate, and citrulline during the light period. The results indicate that the sugar and amino acid composition of cucurbit phloem sap is not constant but varies throughout the diurnal cycle in response to the metabolic activities of the source leaf.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fondy B. R., Geiger D. R. Diurnal changes in allocation of newly fixed carbon in exporting sugar beet leaves. Plant Physiol. 1985 Aug;78(4):753–757. doi: 10.1104/pp.78.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy B. R., Geiger D. R., Servaites J. C. Photosynthesis, Carbohydrate Metabolism, and Export in Beta vulgaris L. and Phaseolus vulgaris L. during Square and Sinusoidal Light Regimes. Plant Physiol. 1989 Feb;89(2):396–402. doi: 10.1104/pp.89.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix D. L., Grange R. I. Carbon partitioning and export from mature cotton leaves. Plant Physiol. 1991 Jan;95(1):228–233. doi: 10.1104/pp.95.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore M. A. Carbohydrate Metabolism in Photosynthetic and Nonphotosynthetic Tissues of Variegated Leaves of Coleus blumei Benth. Plant Physiol. 1990 Jun;93(2):617–622. doi: 10.1104/pp.93.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore M., Grodzinski B. Effect of Oxygen Concentration on C-Photoassimilate Transport from Leaves of Salvia splendens L. Plant Physiol. 1984 Nov;76(3):782–786. doi: 10.1104/pp.76.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins N. S., Pharr D. M. Regulation of photosynthetic carbon metabolism in cucumber by light intensity and photosynthetic period. Plant Physiol. 1987 Oct;85(2):592–597. doi: 10.1104/pp.85.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R., Beebe D. U. The evidence for symplastic Phloem loading. Plant Physiol. 1991 Jun;96(2):349–354. doi: 10.1104/pp.96.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. Y., Sepulveda F. I. Separation of phenylthiocarbamyl amino acids by high-performance liquid chromatography on Spherisob octadecylsilane columns. J Chromatogr. 1985 Oct 18;346:413–416. doi: 10.1016/s0021-9673(00)90532-6. [DOI] [PubMed] [Google Scholar]


