Abstract
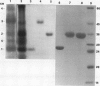
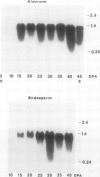
Barley (Hordeum vulgare L.) seeds contain at least five proteins with chitinase (CH) activity. Two of these (CH1 and CH2) are found primarily in the aleurone and endosperm tissues, and the other three (CH3, CH4, and CH5) are enriched in the embryo. From the bran fraction, three of these CHs (CH1, CH2, and CH3) were purified to apparent homogeneity. These three CHs have apparent molecular masses of 27, 34, and 35 kilodaltons and isoelectric points of 9.3, 9.2, and 8.7, respectively. CH2 and CH3 have amino terminal sequences resembling a portion of the chitin-binding domain of lectins and other plant defense proteins. CH1 lacks this domain. All three CHs exhibit antifungal activity and inhibit the mycelial growth of some species of trichoderma and Fusarium in vitro. During the early period of imbibition by seeds, two of the embryo-associated CHs are selectively released into the surrounding aqueous medium.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B., Bosshart R. P., Forrence L. E., Habig W. H. Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiol. 1971 Jan;47(1):129–134. doi: 10.1104/pp.47.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J. Systems for polyacrylamide gel electrophoresis. Methods Enzymol. 1984;104:237–255. doi: 10.1016/s0076-6879(84)04093-3. [DOI] [PubMed] [Google Scholar]
- Conrads-Strauch J., Dow J. M., Milligan D. E., Parra R., Daniels M. J. Induction of Hydrolytic Enzymes in Brassica campestris in Response to Pathovars of Xanthomonas campestris. Plant Physiol. 1990 May;93(1):238–243. doi: 10.1104/pp.93.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. A., Bell J. N., Boller T., Lamb C. J. Chitinase cDNA cloning and mRNA induction by fungal elicitor, wounding, and infection. Plant Physiol. 1988 Jan;86(1):182–186. doi: 10.1104/pp.86.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leah R., Tommerup H., Svendsen I., Mundy J. Biochemical and molecular characterization of three barley seed proteins with antifungal properties. J Biol Chem. 1991 Jan 25;266(3):1564–1573. [PubMed] [Google Scholar]
- Mauch F., Hadwiger L. A., Boller T. Antifungal Hydrolases in Pea Tissue : I. Purification and Characterization of Two Chitinases and Two beta-1,3-Glucanases Differentially Regulated during Development and in Response to Fungal Infection. Plant Physiol. 1988 Jun;87(2):325–333. doi: 10.1104/pp.87.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch F., Mauch-Mani B., Boller T. Antifungal Hydrolases in Pea Tissue : II. Inhibition of Fungal Growth by Combinations of Chitinase and beta-1,3-Glucanase. Plant Physiol. 1988 Nov;88(3):936–942. doi: 10.1104/pp.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkind M., Keegstra K., Palevitz B. A. Distribution of wheat germ agglutinin in young wheat plants. Plant Physiol. 1980 Nov;66(5):950–955. doi: 10.1104/pp.66.5.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molano J., Durán A., Cabib E. A rapid and sensitive assay for chitinase using tritiated chitin. Anal Biochem. 1977 Dec;83(2):648–656. doi: 10.1016/0003-2697(77)90069-0. [DOI] [PubMed] [Google Scholar]
- Molano J., Polacheck I., Duran A., Cabib E. An endochitinase from wheat germ. Activity on nascent and preformed chitin. J Biol Chem. 1979 Jun 10;254(11):4901–4907. [PubMed] [Google Scholar]
- Neale A. D., Wahleithner J. A., Lund M., Bonnett H. T., Kelly A., Meeks-Wagner D. R., Peacock W. J., Dennis E. S. Chitinase, beta-1,3-glucanase, osmotin, and extensin are expressed in tobacco explants during flower formation. Plant Cell. 1990 Jul;2(7):673–684. doi: 10.1105/tpc.2.7.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby D., Broglie K., Cressman R., Biddle P., Chet I. L., Broglie R. Activation of a Bean Chitinase Promoter in Transgenic Tobacco Plants by Phytopathogenic Fungi. Plant Cell. 1990 Oct;2(10):999–1007. doi: 10.1105/tpc.2.10.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinshi H., Mohnen D., Meins F. Regulation of a plant pathogenesis-related enzyme: Inhibition of chitinase and chitinase mRNA accumulation in cultured tobacco tissues by auxin and cytokinin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):89–93. doi: 10.1073/pnas.84.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinshi H., Neuhas J. M., Ryals J., Meins F., Jr Structure of a tobacco endochitinase gene: evidence that different chitinase genes can arise by transposition of sequences encoding a cysteine-rich domain. Plant Mol Biol. 1990 Mar;14(3):357–368. doi: 10.1007/BF00028772. [DOI] [PubMed] [Google Scholar]
- Stanford A., Bevan M., Northcote D. Differential expression within a family of novel wound-induced genes in potato. Mol Gen Genet. 1989 Jan;215(2):200–208. doi: 10.1007/BF00339718. [DOI] [PubMed] [Google Scholar]
- Trudel J., Asselin A. Detection of chitinase activity after polyacrylamide gel electrophoresis. Anal Biochem. 1989 May 1;178(2):362–366. doi: 10.1016/0003-2697(89)90653-2. [DOI] [PubMed] [Google Scholar]