Abstract
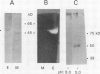
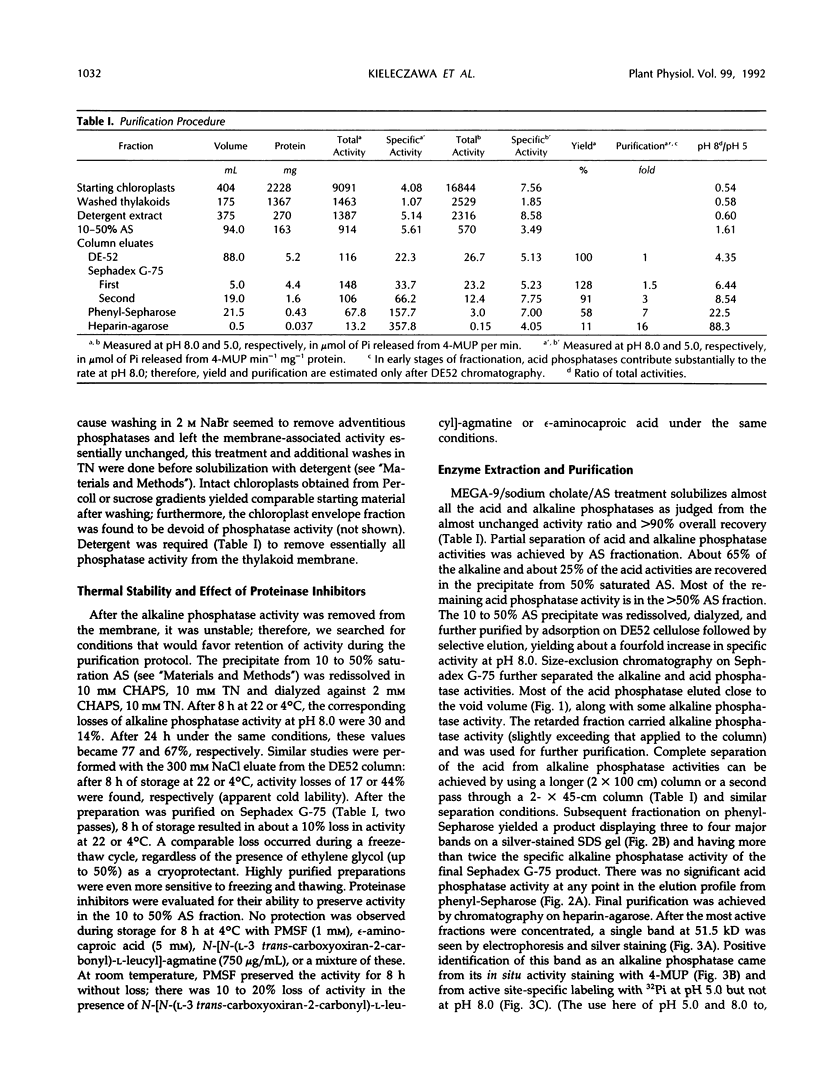
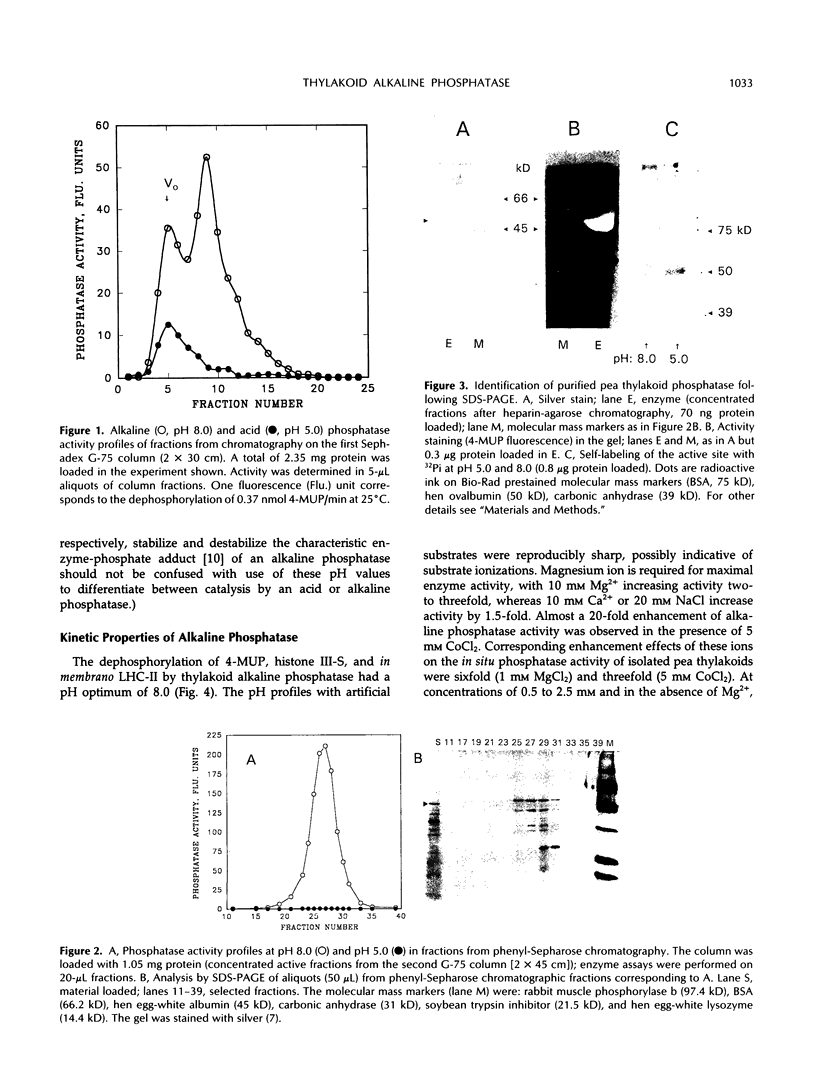
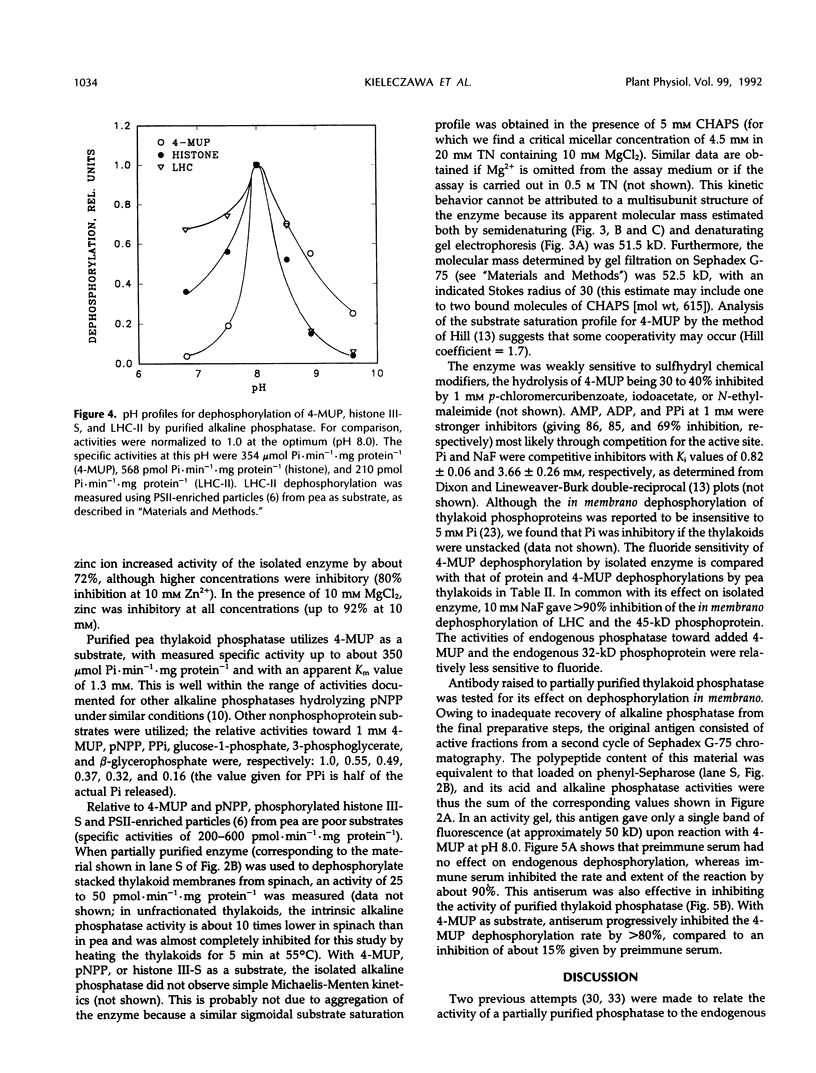
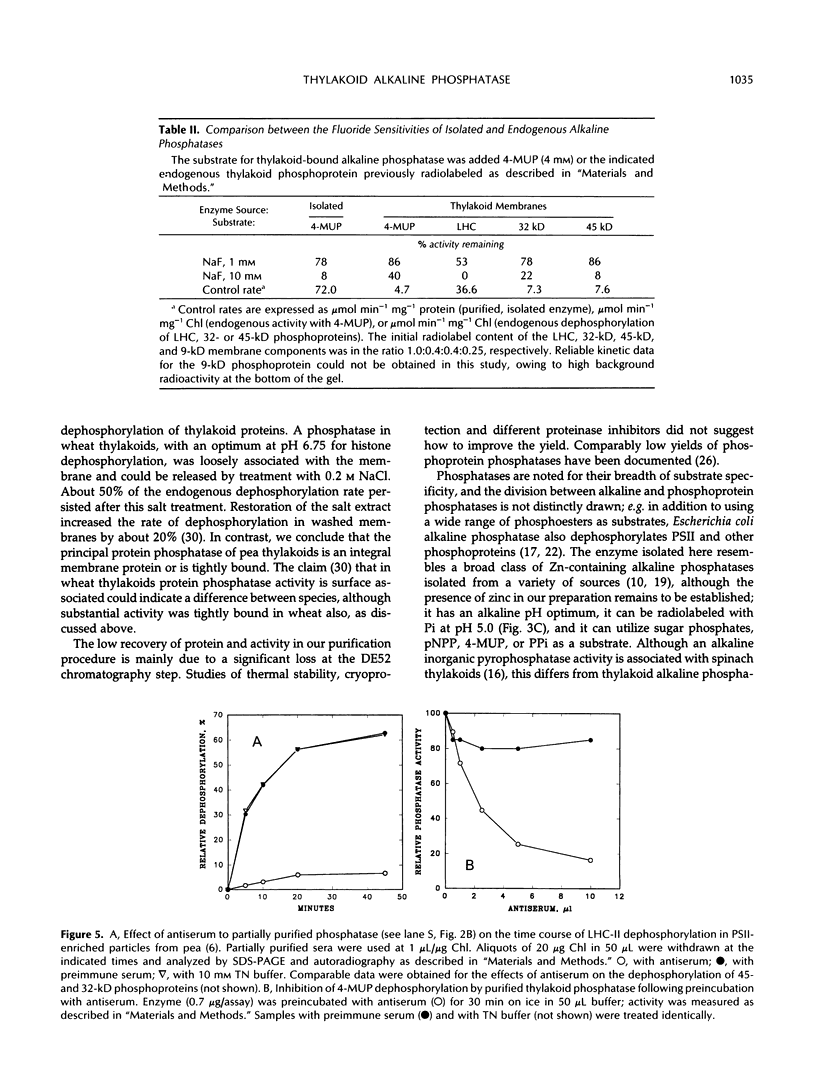
Endogenous dephosphorylation of the light-harvesting chlorophyll-protein complex of photosystem II in pea (Pisum sativum, L. cv Progress 9) thylakoids drives the state 2 to state 1 transition; the responsible enzyme is a thylakoid-bound, fluoride-sensitive phosphatase with a pH optimum of 8.0 (Bennett J [1980] Eur J Biochem 104: 85-89). An enzyme with these characteristics was isolated from well-washed thylakoids. Its molecular mass was estimated at 51.5 kD, and this monomer was catalytically active, although the activity was labile. The active site could be labeled with orthophosphate at pH 5.0. High levels of alkaline phosphatase activity were obtained with the assay substrate, 4-methylumbelliferyl phosphate (350 micromoles per minute per milligram purified enzyme). The isolated enzyme functioned as a phosphoprotein phosphatase toward phosphorylated histone III-S and phosphorylated, photosystem II-enriched particles from pea, with typical activities in the range of 200 to 600 picomoles per minute per milligram enzyme. These activities all had a pH optimum of 8.0 and were fluoride sensitive. The enzyme required magnesium ion for maximal activity but was not dependent on this ion. Evidence supporting a putative function for this phosphatase in dephosphorylation of thylakoid proteins came from the inhibition of this process by a polyclonal antibody preparation raised against the partially purified enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony F. A., Merat D. L., Cheung W. Y. A spectrofluorimetric assay of calmodulin-dependent protein phosphatase using 4-methylumbelliferyl phosphate. Anal Biochem. 1986 May 15;155(1):103–107. doi: 10.1016/0003-2697(86)90232-0. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baykov A. A., Evtushenko O. A., Avaeva S. M. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal Biochem. 1988 Jun;171(2):266–270. doi: 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- Bennett J. Chloroplast phosphoproteins. Evidence for a thylakoid-bound phosphoprotein phosphatase. Eur J Biochem. 1980 Feb;104(1):85–89. doi: 10.1111/j.1432-1033.1980.tb04403.x. [DOI] [PubMed] [Google Scholar]
- Brunette M. G., Chan M., Lebrun M. A microfluorometric method for alkaline phosphatase: application to the various segments of the nephron. Anal Biochem. 1981 Jul 15;115(1):236–242. doi: 10.1016/0003-2697(81)90552-2. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Coleman J. E., Gettins P. Alkaline phosphatase, solution structure, and mechanism. Adv Enzymol Relat Areas Mol Biol. 1983;55:381–452. doi: 10.1002/9780470123010.ch5. [DOI] [PubMed] [Google Scholar]
- Coughlan S. J., Hind G. Purification and characterization of a membrane-bound protein kinase from spinach thylakoids. J Biol Chem. 1986 Aug 25;261(24):11378–11385. [PubMed] [Google Scholar]
- DeGuzman A., Lee E. Y. Preparation of low-molecular-weight forms of rabbit muscle protein phosphatase. Methods Enzymol. 1988;159:356–368. doi: 10.1016/0076-6879(88)59036-5. [DOI] [PubMed] [Google Scholar]
- ENGSTROEM L. THE AMINO ACID SEQUENCE AROUND THE REACTIVE SERINE IN CALF-INTESTINAL ALKALINE PHOSPHATASE. Biochim Biophys Acta. 1964 Oct 23;92:79–84. [PubMed] [Google Scholar]
- Fanger B. O. Adaptation of the Bradford protein assay to membrane-bound proteins by solubilizing in glucopyranoside detergents. Anal Biochem. 1987 Apr;162(1):11–17. doi: 10.1016/0003-2697(87)90004-2. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Winget G. D. A membrane-bound alkaline inorganic pyrophosphatase in isolated spinach chloroplasts. Arch Biochem Biophys. 1973 Feb;154(2):606–613. doi: 10.1016/0003-9861(73)90015-5. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Huber J. L. Activation of sucrose-phosphate synthase from darkened spinach leaves by an endogenous protein phosphatase. Arch Biochem Biophys. 1990 Nov 1;282(2):421–426. doi: 10.1016/0003-9861(90)90138-o. [DOI] [PubMed] [Google Scholar]
- Kim E. E., Wyckoff H. W. Reaction mechanism of alkaline phosphatase based on crystal structures. Two-metal ion catalysis. J Mol Biol. 1991 Mar 20;218(2):449–464. doi: 10.1016/0022-2836(91)90724-k. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacKintosh C., Coggins J., Cohen P. Plant protein phosphatases. Subcellular distribution, detection of protein phosphatase 2C and identification of protein phosphatase 2A as the major quinate dehydrogenase phosphatase. Biochem J. 1991 Feb 1;273(Pt 3):733–738. doi: 10.1042/bj2730733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellgren R. L., Slaughter G. R., Thomas J. A. Dephosphorylation of phosphoproteins by Escherichia coli alkaline phosphatase. J Biol Chem. 1977 Sep 10;252(17):6082–6089. [PubMed] [Google Scholar]
- Moss D. W., Eaton R. H., Smith J. K., Whitby L. G. Association of inorganic-pyrophosphatase activity with human alkaline-phosphatase preparations. Biochem J. 1967 Jan;102(1):53–57. doi: 10.1042/bj1020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polya G. M., Haritou M. Purification and characterization of two wheat-embryo protein phosphatases. Biochem J. 1988 Apr 15;251(2):357–363. doi: 10.1042/bj2510357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanik A. M., Bingsmark S., Lindahl M., Baltscheffsky H., Baltscheffsky M., Andersson B. Inorganic-pyrophosphate-dependent phosphorylation of spinach thylakoid proteins. Eur J Biochem. 1991 May 23;198(1):183–186. doi: 10.1111/j.1432-1033.1991.tb15999.x. [DOI] [PubMed] [Google Scholar]
- Rengasamy A., Selvam R., Gnanam A. Isolation and properties of an acid phosphatase from thylakoid membranes of Sorghum vulgare. Arch Biochem Biophys. 1981 Jun;209(1):230–236. doi: 10.1016/0003-9861(81)90275-7. [DOI] [PubMed] [Google Scholar]
- Shenolikar S., Nairn A. C. Protein phosphatases: recent progress. Adv Second Messenger Phosphoprotein Res. 1991;23:1–121. [PubMed] [Google Scholar]
- Sun G., Bailey D., Jones M. W., Markwell J. Chloroplast thylakoid protein phosphatase is a membrane surface-associated activity. Plant Physiol. 1989 Jan;89(1):238–243. doi: 10.1104/pp.89.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]