Abstract
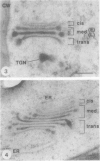
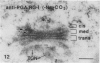
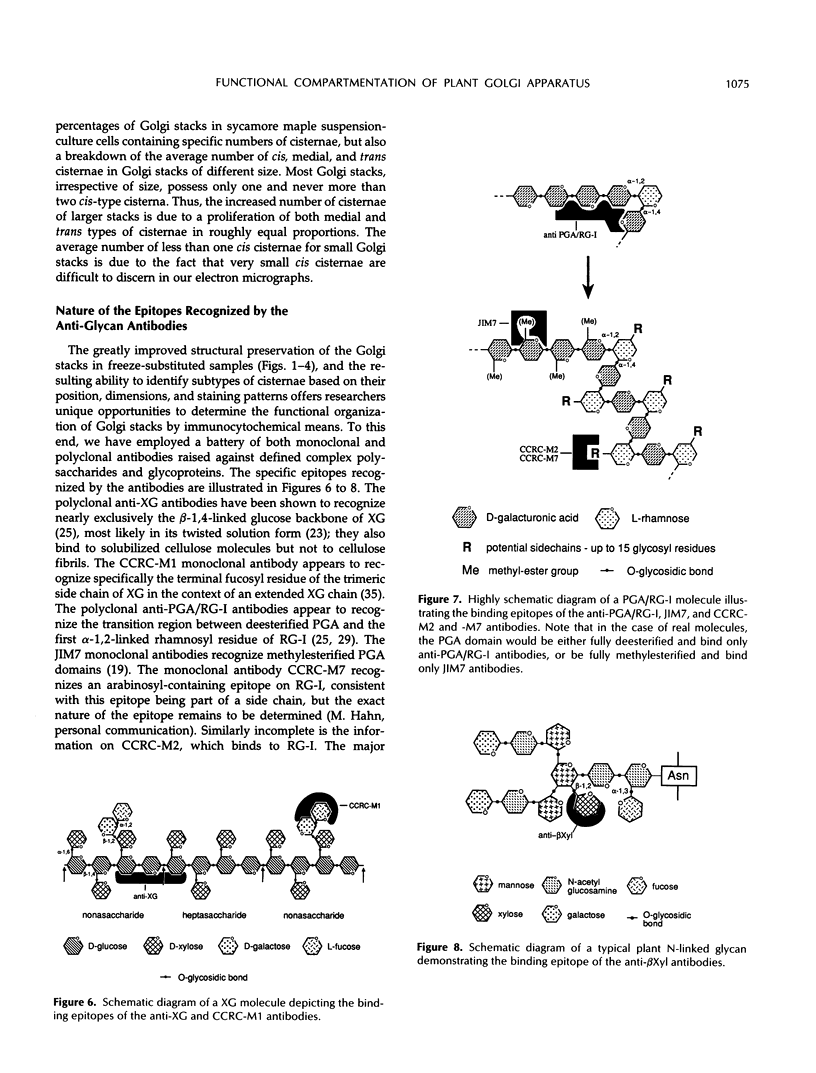
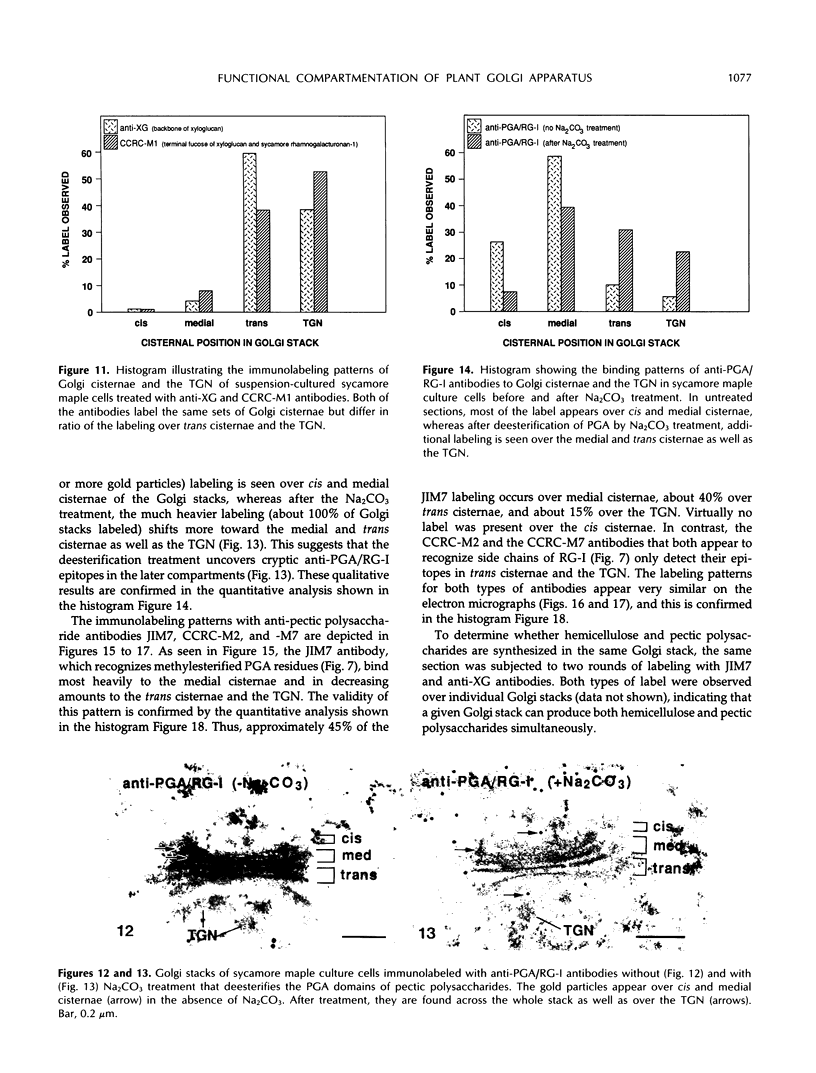
The Golgi apparatus of plant cells is engaged in both the processing of glycoproteins and the synthesis of complex polysaccharides. To investigate the compartmentalization of these functions within individual Golgi stacks, we have analyzed the ultrastructure and the immunolabeling patterns of high-pressure frozen and freeze-substituted suspension-cultured sycamore maple (Acer pseudoplatanus L.) cells. As a result of the improved structural preservation, three morphological types of Golgi cisternae, designated cis, medial, and trans, as well as the trans Golgi network, could be identified. The number of cis cisternae per Golgi stack was found to be fairly constant at approximately 1, whereas the number of medial and trans cisternae per stack was variable and accounted for the varying number of cisternae (3-10) among the many Golgi stacks examined. By using a battery of seven antibodies whose specific sugar epitopes on secreted polysaccharides and glycoproteins are known, we have been able to determine in which types of cisternae specific sugars are added to N-linked glycans, and to xyloglucan and polygalacturonic acid/rhamnogalacturonan-I, two complex polysaccharides. The findings are as follows. The β-1,4-linked d-glucosyl backbone of xyloglucan is synthesized in trans cisternae, and the terminal fucosyl residues on the trisaccharide side chains of xyloglucan are partly added in the trans cisternae, and partly in the trans Golgi network. In contrast, the polygalacturonic/rhamnogalacturonan-I backbone is assembled in cis and medial cisternae, methylesterification of the carboxyl groups of the galacturonic acid residues in the polygalacturonic acid domains occurs mostly in medial cisternae, and arabinose-containing side chains of the polygalacturonic acid domains are added to the nascent polygalacturonic acid/rhamnogalacturonan-I molecules in the trans cisternae. Double labeling experiments demonstrate that xyloglucan and polygalacturonic acid/rhamnogalacturonan-I can be synthesized concomitantly within the same Golgi stack. Finally, we show that the xylosyl residue-linked β-1,2 to the β-linked mannose of the core of N-linked glycans is added in medial cisternae. Taken together, our results indicate that in sycamore maple suspension-cultured cells, different types of Golgi cisternae contain different sets of glycosyl transferases, that the functional organization of the biosynthetic pathways of complex polysaccharides is consistent with these molecules being processed in a cis to trans direction like the N-linked glycans, and that the complex polysaccharide xyloglucan is assembled exclusively in trans Golgi cisternae and the trans Golgi network.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Camirand A., Maclachlan G. Biosynthesis of the fucose-containing xyloglucan nonasaccharide by pea microsomal membranes. Plant Physiol. 1986 Oct;82(2):379–383. doi: 10.1104/pp.82.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S., Staehelin L. A. High pressure freezing of intact plant tissues. Evaluation and characterization of novel features of the endoplasmic reticulum and associated membrane systems. Eur J Cell Biol. 1988 Apr;46(1):81–93. [PubMed] [Google Scholar]
- Dunphy W. G., Rothman J. E. Compartmental organization of the Golgi stack. Cell. 1985 Aug;42(1):13–21. doi: 10.1016/s0092-8674(85)80097-0. [DOI] [PubMed] [Google Scholar]
- Farkas V., Maclachlan G. Fucosylation of exogenous xyloglucans by pea microsomal membranes. Arch Biochem Biophys. 1988 Jul;264(1):48–53. doi: 10.1016/0003-9861(88)90568-1. [DOI] [PubMed] [Google Scholar]
- Gordon R., Maclachlan G. Incorporation of UDP-[C]Glucose into Xyloglucan by Pea Membranes. Plant Physiol. 1989 Sep;91(1):373–378. doi: 10.1104/pp.91.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Matsuda K. Biosynthesis of xyloglucan in suspension-cultured soybean cells. Occurrence and some properties of xyloglucan 4-beta-D-glucosyltransferase and 6-alpha-D-xylosyltransferase. J Biol Chem. 1981 Nov 10;256(21):11117–11122. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laurière M., Laurière C., Chrispeels M. J., Johnson K. D., Sturm A. Characterization of a xylose-specific antiserum that reacts with the complex asparagine-linked glycans of extracellular and vacuolar glycoproteins. Plant Physiol. 1989 Jul;90(3):1182–1188. doi: 10.1104/pp.90.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S., York W. S., Stuike-Prill R., Meyer B., Staehelin L. A. Simulations of the static and dynamic molecular conformations of xyloglucan. The role of the fucosylated sidechain in surface-specific sidechain folding. Plant J. 1991 Sep;1(2):195–215. [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J. G., Schweizer A., Berger E. G., Hauri H. P., Yuan L. C., Klausner R. D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990 Mar 9;60(5):821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Fry S. C., Albersheim P. Structure and function of the primary cell walls of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- Moore P. J., Darvill A. G., Albersheim P., Staehelin L. A. Immunogold localization of xyloglucan and rhamnogalacturonan I in the cell walls of suspension-cultured sycamore cells. Plant Physiol. 1986 Nov;82(3):787–794. doi: 10.1104/pp.82.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. J., Swords K. M., Lynch M. A., Staehelin L. A. Spatial organization of the assembly pathways of glycoproteins and complex polysaccharides in the Golgi apparatus of plants. J Cell Biol. 1991 Feb;112(4):589–602. doi: 10.1083/jcb.112.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Roth J. Subcellular organization of glycosylation in mammalian cells. Biochim Biophys Acta. 1987 Oct 5;906(3):405–436. doi: 10.1016/0304-4157(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Roth J., Taatjes D. J., Weinstein J., Paulson J. C., Greenwell P., Watkins W. M. Differential subcompartmentation of terminal glycosylation in the Golgi apparatus of intestinal absorptive and goblet cells. J Biol Chem. 1986 Oct 25;261(30):14307–14312. [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Movement of proteins through the Golgi stack: a molecular dissection of vesicular transport. FASEB J. 1990 Mar;4(5):1460–1468. doi: 10.1096/fasebj.4.5.2407590. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A., Giddings T. H., Jr, Kiss J. Z., Sack F. D. Macromolecular differentiation of Golgi stacks in root tips of Arabidopsis and Nicotiana seedlings as visualized in high pressure frozen and freeze-substituted samples. Protoplasma. 1990;157(1-3):75–91. doi: 10.1007/BF01322640. [DOI] [PubMed] [Google Scholar]
- Sturm A., Johnson K. D., Szumilo T., Elbein A. D., Chrispeels M. J. Subcellular localization of glycosidases and glycosyltransferases involved in the processing of N-linked oligosaccharides. Plant Physiol. 1987 Nov;85(3):741–745. doi: 10.1104/pp.85.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]